Abstract
Background
The long-term pulmonary function and related physiological characteristics of COVID-19 survivors have not been studied in depth, thus many aspects are not understood.
Methods
COVID-19 survivors were recruited for high resolution computed tomography (HRCT) of the thorax, lung function and serum levels of SARS-CoV-2 IgG antibody tests 3 months after discharge. The relationship between the clinical characteristics and the pulmonary function or CT scores were investigated.
Findings
Fifty-five recovered patients participated in this study. SARS-CoV-2 infection related symptoms were detected in 35 of them and different degrees of radiological abnormalities were detected in 39 patients. Urea nitrogen concentration at admission was associated with the presence of CT abnormalities (P = 0.046, OR 7.149, 95% CI 1.038 to 49.216). Lung function abnormalities were detected in 14 patients and the measurement of D-dimer levels at admission may be useful for prediction of impaired diffusion defect (P = 0.031, OR 1.066, 95% CI 1.006 to 1.129). Of all the subjects, 47 of 55 patients tested positive for SARS-CoV-2 IgG in serum, among which the generation of Immunoglobulin G (IgG) antibody in female patients was stronger than male patients in infection rehabilitation phase.
Interpretation
Radiological and physiological abnormalities were still found in a considerable proportion of COVID-19 survivors without critical cases 3 months after discharge. Higher level of D-dimer on admission could effectively predict impaired DLCO after 3 months discharge. It is necessary to follow up the COVID-19 patients to appropriately manage any persistent or emerging long-term sequelae.
Funding
Key Scientific Research Projects of Henan Higher Education Institutions
Key words: Covid-19, Recovered patients, Follow-up study, Pulmonary function, Serum marker
Research in the context.
Evidence before this study
We searched PubMed without language restriction for studies published form database until June 8, 2020, using the keywords “2019 novel coronavirus”, “2019-nCoV”, “SARS-CoV-2”, “Wuhan coronavirus” AND “long term follow-up” OR “pulmonary function” OR “sequelae”. Although it has been reported that short term radiological outcomes and abnormal lung function found in patients when discharged from the hospitals, the long-time follow-up survey of COVID-19 survivors has not been reported yet.
Added value of this study
We report that the long term effects on changes in both pulmonary function and HRCT imaging. Although critical pneumonia has been excluded from our study, residual abnormalities of pulmonary function and chest radiography were still observed in three quarters of the cohort at 3 months after discharge. There have been several risk factors at admission were risk factors of in-hospital death for adult patients with COVID-19, we found the level of D-dimer at admission was an important factor for abnormal carbon monoxide diffusion capacity (DLCO). In our study, eight patients showed negative results in the SARS-CoV-2 IgG antibody test for at least two times 3 months after discharge.
Implications of all the available evidence
Thus, for patients who have markable raised D-dimer, pulmonary rehabilitation should are also needed subsequently even in the absence of other severity respiratory symptoms. It is necessary to follow-up these patients to detect and appropriately manage any persistent long-term sequelae in lung caused due to SARS-CoV-2.
Alt-text: Unlabelled box
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by a novel coronavirus, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. From December 2019, it has rapidly spread across China and many other countries [2], [3], [4], [5]. By 8nd June 2020, accumulative 6931,000 confirmed cases including 400,857 deaths were reported globally [6]. Person-to-person transmission of SARS-CoV-2 has gained global attention and extensive measures to effectively control the outbreak and treatment of COVID-19. COVID-19 due to SARS-CoV-2 involves multiple organs and lung injury is one of the most clinical manifestations. The entry route of SARS-CoV-2 into the human cells is mainly facilitated by the angiotensin-converting enzyme 2 (ACE2) receptors, which seem to be expressed by type 2 pneumocytes [7]. The binding of SARS-CoV-2 to the ACE2 receptors could arise into acute systemic inflammatory responses and cytokine storm, consequentially leading to lung-resident dentritic cells (rDCs) activation, and to T lymphocytes production and release antiviral cytokines into the alveolar septa and interstitial compartments [8]. However, the knowledge about the sequelae of SARS-CoV-2 infection remains limited.
Although it has been reported that short term radiological outcomes and abnormal lung function found in patients when discharged from the hospitals [9,10], the long-time follow-up survey of COVID-19 survivors has not been reported yet. In addition, a study of the serum levels of the specific IgG antibody against COVID-19 is needed to be investigated, because it plays a vital role in better understanding of the immune response to aid in protection and recovery from repeated infections with SARS-CoV-2.
From the results of all the studies, patients appeared to have stabilized in their symptoms and resolution of chest HRCT abnormalities at 3 months follow-up after hospital discharge, thus we investigated the patient population at the uniform time-point after hospital discharge. Here we studied the pulmonary function, HRCT scan of the thorax and SARS-CoV-2 IgG in serum in COVID-19 patients 3 months after their hospital discharged. Additionally, the potential serum biomarkers related to the pulmonary function were also investigated.
2. Materials and methods
2.1. Case definitions and case identification
In China, all laboratory-confirmed COVID-19 patients were reported through a national influenza surveillance system. All COVID-19 patients in this study were identified through the national influenza surveillance system and confirmed with SARS-CoV-2 virus infection by RT-PCR. According to the WHO interim guidance and the guidance from China, all participants were categorized as mild illness (mild clinical symptoms without pneumonia manifestations in imaging), pneumonia (having symptoms and pneumonia manifestations in imaging, with no requirement for supplemental oxygen), and severe pneumonia (having radiographic evidence of pneumonia, meeting any of the following: respiratory rate ≥ 30 breaths/min; oxygen saturations ≤ 93% at a rest state; severe respiratory distress; > 50% lesions progression within 24 to 48 h in lung imaging) [11,12].
2.2. Participants and design of study
This retrospective multi-center cohort study was approved by the Institutional Review Board of the Relevant Centers. The written informed consent was obtained from all the patients. All the adult patients who were diagnosed with COVID-19 according to World Health Organization (WHO) interim guidance were consecutively enrolled from Jan 20, 2020 to Feb 24, 2020 in 3 tertiary hospitals of Henan Province, including the First Affiliated Hospital of Zhengzhou University, Guangshan People's Hospital and Xixian People's Hospital. All the patients were hospitalized in these designated hospitals and taken care by a multidisciplinary team. Critical cases were excluded from the study.
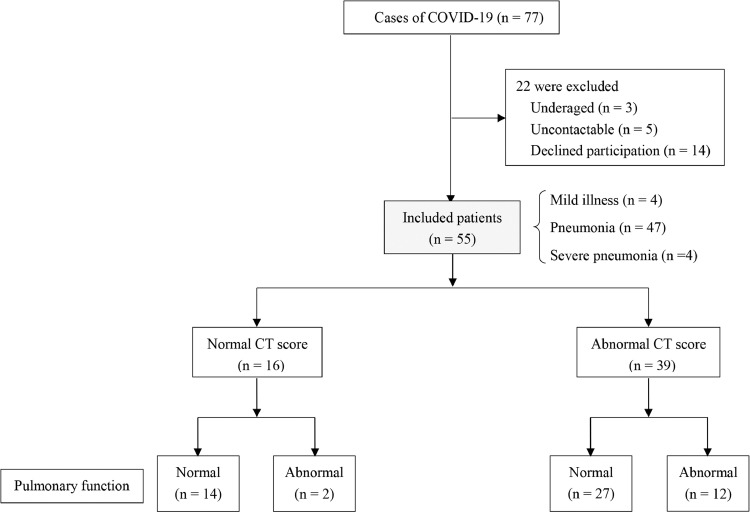
Fig. 1 shows the flowchart of the study. Over the 3-month recruitment period, 55 consecutively eligible patients were enrolled. Of 55 patients, including 4 mild (7.27%), 47 moderate (85.45%) and 4 severe (7.27%) cases, 39 had residual abnormalities in chest CT scans and 16 had normal CT images. Supplementary Table 1 presented the differences exist in mild, moderate and severe cases. Of 39 cases with abnormal CT manifestations, 12 cases (30.77%) found abnormality in pulmonary function. In normal CT group, 2 out of 14 cases (12.50%) had abnormal lung function. The final follow-up evaluation was carried out at the time ranged from 64 to 93 days after discharge from hospitals.
Fig. 1.
Enrolment of patients and follow-up at 3 months after hospital discharge. COVID-19: Coronavirus Disease 2019.
2.3. Ethical approval statement
This study has been approved by the Institutional Review Board of the Relevant Centers. The written informed consent was obtained from all the patients.
2.4. Data collection
The medical records of patients were analyzed by the research team of the Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Zhengzhou University. All the clinical data on epidemiological, demographic, medical history, under comorbidities, clinical, laboratory, chest CT scans, treatment and outcome were checked by 2 physicians. The date of disease onset was defined as the day when the first symptom was noticed.
The real-time reverse transcriptase polymerase chain-reaction (RT-PCR) test was performed using nasal and pharyngeal swab specimens at the Zhengzhou or Xinyang Municipal Center for Disease Prevention and Control (CDC).
For all discharged COVID-19 survivors, chest CT scan, pulmonary function test and SARS-CoV-2 IgG test were done.
2.5. Chest CT protocols
All examinations represented the initial and follow-up CT scans for every patient. All CT images were acquired at the end of inhalation using a 64-row CT scanner (Somatom Definition AS 128, Siemens Health System, Forcheinm, Germany) with a detector configuration of 64 × 0.6 mm or using a 16-row CT scanner (Philips Brilliance 16, Philips Healthcare, Amsterdam, the Netherlands) with a detector collimation of 16 × 0.75 mm. Other acquisition parameters for these two scanners were set as follows: tube voltage of 120 kV, automatic tube current modulation of 100–300 mA, pitch of 0.3 to 1.1 mm, matrix = 512 × 512, rotation time ranging 300 to 500 ms, slice thickness = 1.0–1.5 mm. All images were then reconstructed with a slice of 1.0–2.0 mm with the same increment.
2.6. Image analysis and quantification
Two radiologists, who were blinded to the clinical data, reviewed all the chest CT images and decided the final conclusions via a view console. When there was a difference of opinion, the third radiologist with over 10 years of experience in interpreting chest CT was consulted.
The distribution features such as ground-glass opacity (GGO), consolidation, interstitial thickening, bronchiectasis, crazy paving, air bronchogram, irregular interface, coarse reticular pattern, parenchymal band, lymphadenopathy, and pleural effusion, as well as the involving lung lobes were recorded. The main analysis criteria were the number of affected lung lobes. The presence of ground-glass opacity (GGO), consolidation, interstitial thickening, fibrosis and air trapping and were analyzed quantitatively using a radiologic scoring system ranging from 0 to 25 points, which has been previously used to described idiopathic pulmonary fibrosis caused by SARS [13], [14], [15]. There were 5 lung lobes and each was evaluated 0–5 points on the basis of the area involved, with score 0 for normal performance, 1 for ground glass opacity involving less than 5% of lobe, 2 for involving up to 25%, 3 for involving 25–49%, 4 for involving 50–75%, 5 for more than 75%. Individual segmental scores were added together as a total score in the statistical analysis.
2.7. Pulmonary function testing
Pulmonary function tests were performed by technicians in the Pulmonary Function laboratory, the First Affiliated Hospital of Zhengzhou University. Spirometry and pulmonary diffusion capacity test was conducted using the spirometry (Masterscreen PFT, Jaeger, Germany) and the procedure was followed by the ATS-ERS guidelines [16]. The following parameters were measured: forced vital capacity (FVC), forced expiratory capacity at the first second of exhalation (FEV1), total lung capacity (TLC), and diffusion capacity of the lung for carbon monoxide (DLCO) measured by means of the single-breath test. The hemoglobin value was also taken for correcting the DLCO. If obstruction was presented, the spirometry measurements were repeated for analysis after the administration of a bronchodilator (2 puffs of salbutamol). All pulmonary function test measurements were expressed as percentages of predicted normal values. Diffusion deficit was considered as DLCO < 80% of predicted value.
2.8. Immunoglobulin G test for SARS-CoV-2
Serum was separated by centrifugation at 2500 g for 5 min within 12 h of collection. The SARS-CoV-2 IgG chemiluminescence immunoassay (CLIA) kits (C86095G/C86095G) used in this study were purchased from Shenzhen YHLO Biological Technology Co., Ltd,China [17,18]. In all patients, IgG antibodies against the SARS-CoV-2 envelope (E) protein and the cut-off value for a positive result was 10 AU/mL, samples with values more than or equal to 10 AU/mL were considerate as positive results.
2.9. Statistical analysis
Continuous variables were described using mean with standard deviation (SD) or median with interquartile range(IQR), followed by unpaired t-test or Mann-Whitney test. Categorical variables were described as percentage and compared using the Chi-square test. Logistic regression analysis was used to study the independent covariates for the presence of HRCT abnormalities or abnormal pulmonary function. The correlation of different variables was analyzed using the Spearman's correlation. The conventional level of statistical significance of 0.05 was used for all analyses. Statistical analyses were performed using SPSS Version 21.0.
2.10. Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the Article, or the decision to submit for publication. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
Fifty-five of the 77 COVID-19 survivors completed the study. Their mean (SD) age was 47.74 (15.49), among which 41.82% were female. 9 patients (16.36%) had underlying co-morbidities, while 2 patients (3.64%) had 2 or more comorbidities. Common comorbidities included hypertension (6 cases, 10.91%), diabetes mellitus (2 cases, 3.64%) and cardiovascular diseases (2 cases, 3.64%). No underlying pulmonary diseases were observed on admission. Only 4 patients were smokers, 2 had quit tobacco among them. Except for the standard therapy, the traditional Chinese medicine Lianhua qingwen granules were used in 21 patients (38.18%), and low-dose corticosteroids were used in 7 patients (12.73%); 14 patients required additional oxygen therapy, and no one required mechanical ventilation. Supplementary Table 2 and 3 summarize the demographics, clinical characteristics at admission, and the treatment for the 55 patients.
Symptoms were also systematically recorded. On the day of follow up after 3 months, the presenting symptoms included gastrointestinal (GI) symptoms (30.91%), headache (18.18%), fatigue (16.36%), exertional dyspnea (14.55%), as well as cough and sputum (1.81%). Of the 55 patients, 6 patients with COVID-19 experienced olfactory and gustatory dysfunctions during infection period. Although there was a significant improvement in self-rating of severity of olfactory and gustatory loss, 2 female patients still experienced a decrease sense of taste during follow-up period. All 55 patients had returned to their original work. Meanwhile, the mean body mass index of the group was 24.62 ± 3.31.
3.1. Comparison of clinical characteristics between normal and abnormal HRCT scanning of the thorax
Three months after discharge, the degrees of radiological abnormalities were detected in 39 patients (70.91%), and their HRCT scan images were viewed. The median number of segments involved was 1 (IQR of 0.00–2.00) and the median total score was 1 (IQR of 0.00–2.00). In half of patients (54.55%), 1–3 segments were involved. Thirteen patients (23.64%) showed bilateral involvement on chest HRCT scans. The lower right lobe was involved in 23 patients (41.82%), while lower left lobe and upper left lobe were involved in 12 patients (21.82%) and 11 patients (20%), respectively. As shown in Fig. 2, typical features such as pure GGO, interstitial thickening and crazy paving were almost resolved, but evidence of fibrosis, such as interstitial thickening were observed. From the HRCT scans of the latest follow-up patients after discharge, pure GGO (7 of 55, 7.27%), interstitial thickening (15 of 55, 27.27%), and crazy paving (3 of 55, 5.45%) were the most common CT features found.
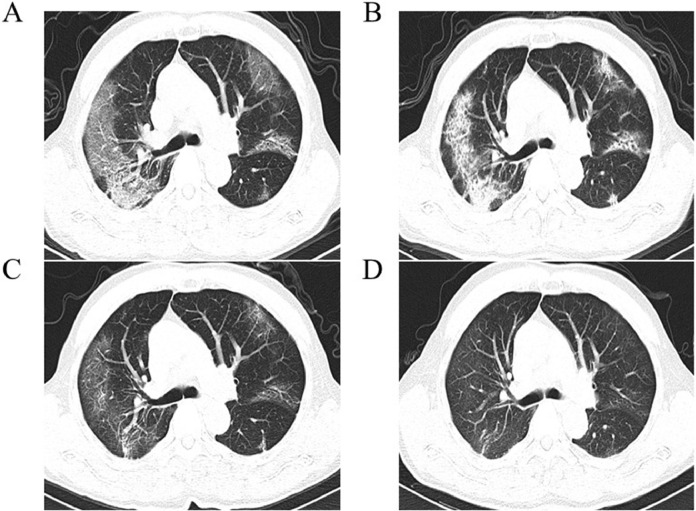
Fig. 2.
Follow-up thin-section CT imaging of 63-year-old man with confirmed COVID-19 pneumonia with dry cough. (A) First thin-section chest CT in hospital on February 2, 2020 (7 days after symptoms onset). CT imaging shows GGO associated with smooth interlobular and intralobular septal thickeing (crazy paving). (B) Crazy paving with some consolidations were observed over 7 days. (C) On March 4, 2020, scans showed that the previous lesion was absorbed and parenchymal bands with residual GGO were observed. (D) On May 2, 2020, intenstitial thickeing and residual GGO were observed. CXR: chest radiography.
Laboratory examinations varied widely between the 2 groups (Table 1 and Supplementary Table 4). In compared with the 16 survivors with normal CT, patients in the abnormal CT group were significantly older (52.05 ± 15.05 vs. 37.13 ± 11.73, P = 0.001), longer incubation period (6.00 [4.00–9.00] vs. 4.50 [2.50–6.00], P = 0.046) and with higher CXR peak score (8.30 ± 5.00 vs. 5.00 ± 3.40, P = 0.019). Compared with survivors with normal CT, on admission, survivors with abnormal CT had lower albumin level (41.76 ± 3.31 vs. 44.64 ± 3.83, P = 0.007), lower serum sodium concentration (140.60 [137.10–142.00] vs. 141.80 [137.10–142.00], P = 0.038), higher urea nitrogen level (4.73 [3.96–5.32] vs. 3.86 [3.03–4.23], P = 0.000). Admission values of glucose, hsCRP, and D-dimer concentration were also significantly higher in the COVID-19 survivors with abnormal CT. Based on these variables, further multivariate analysis using the forward method was performed, and it was found that the increase of urea nitrogen was the independent risk factor associated with the presence of CT abnormalities (P = 0.046, OR 7.149, 95% CI 1.038 to 49.216; Table 2).
Table 1.
Univariate analysis of predictors of abnormal CT scores.
| Parameters | Normal range | Normal CT (n = 16) | Abnormal CT (n = 39) | P value |
|---|---|---|---|---|
| Age, years | ≥ 18 | 37.13 ± 11.73 | 52.05 ± 15.05 | 0.001 |
| Sex, (% female) | 37.50% | 43.59% | 0.643 | |
| Incubation period, d | 4.50 (2.50–6.00) | 6.00 (4.00–9.00) | 0.046 | |
| Temperature, °C | 37.83 (36.90–38.50) | 37.74 (37.20–38.30) | 0.926 | |
| CXR peak score | 5.00 ± 3.44 | 8.32 ± 5.00 | 0.019 | |
| Comorbidities | ||||
| Hypertension | 0 | 6 | 0.236 | |
| Coronary heart disease | 0 | 2 | 0.897 | |
| Diabetes mellitus | 0 | 2 | 0.897 | |
| Signs and symptoms at admission | ||||
| Fever | 12 (75%) | 25 (64.10%) | 0.641 | |
| Cough | 7 (43.75%) | 23 (58.97%) | 0.303 | |
| Feeble | 4 (25%) | 14 (7.27%) | 0.641 | |
| Laboratory data | ||||
| Blood routine | ||||
| Leucocyte count (× 109/L) | 4–10 | 5.26 ± 1.92 | 5.81 ± 1.84 | 0.331 |
| Neutrophil count(× 109/L) | 2–7 | 3.51 ± 1.52 | 3.92 ± 1.76 | 0.417 |
| Lymphocyte count (× 109/L) | 0.8–4.0 | 1.37 (0.98–1.69) | 1.41 (1.08–1.77) | 0.767 |
| NLR | 2.56 (2.10–3.11) | 2.84 (1.71–3.97) | 0.711 | |
| Hemoglobin concentration (g/L) | 110–160 | 145.63 ± 19.46 | 139.62 ± 20.43 | 0.320 |
| Platelet count (× 109/L) | 100–300 | 184.00 (128.00–217.25) | 167.00 (143.00–210.00) | 0.926 |
| Blood Biochemistry | ||||
| ALT, U/L | 0–40 | 22.75 ± 7.33 | 27.35 ± 8.85 | 0.071 |
| AST, U/L | 0–40 | 16.00 (8.68–28.13) | 22.60 (14.40–30.40) | 0.159 |
| Albumin, g/L | 40–53 | 44.64 ± 3.83 | 41.76 ± 3.31 | 0.007 |
| TP, g/L | 64–83 | 66.38 ± 4.41 | 64.32 ± 5.37 | 0.180 |
| GGT, U/L | 7–50 | 16.45 (13.18–24.30) | 24.80 (16.40–44.00) | 0.062 |
| Total bilirubin, μmol/L | 3.42–20.50 | 9.10 (7.28–13.98) | 9.20 (7.60–12.80) | 0.487 |
| Urea nitrogen, mmol/L | 1.43–7.14 | 3.86 (3.03–4.23) | 4.73 (3.96–5.32) | 0.000 |
| UA, μmol/L | 170–390 | 304.24 ± 82.90 | 265.64 ± 99.64 | 0.178 |
| Glucose, mmol/L | 3.89–6.11 | 4.84 (4.82–5.44) | 5.73 (4.93–6.62) | 0.006 |
| TG, mmol/L | 0.00–1.71 | 0.98 (0.88–1.30) | 1.18 (1.03–1.73) | 0.059 |
| Infection associated | ||||
| hsCRP, mg/L | 1.04 (0.36–9.65) | 6.43 (0.93–15.00) | 0.041 | |
| Myocardial injury markers | ||||
| CK, U/L | 25–200 | 83.45 (51.45–120.20) | 66.70 (42.90–103.10) | 0.420 |
| LDH, U/L | 0.00–3.10 | 198.21 ± 49.61 | 205.00 ± 77.29 | 0.747 |
| Blood coagulation | ||||
| Prothrombin time, s | 10–13.5 | 11.10 (10.65–14.98) | 12.70 (10.80–14.90) | 0.383 |
| Thrombin time, s | 10–18 | 15.90 (13.88–17.48) | 16.60 (14.80–18.10) | 0.321 |
| Fibrinogen, g/L | 2.00–4.00 | 3.19 (2.97–3.55) | 3.56 (3.00–4.67) | 0.097 |
| D-dimer, mg/L | 0–0.55 | 0.16 ± 0.01 | 0.30 ± 0.04 | 0.006 |
| Treatment | ||||
| Low-dose corticosteroids | 2 (12.50%) | 5 (12.82%) | 0.974 | |
| Hospital period, d | 14.06 ± 4.80 | 15.87 ± 6.84 | 0.340 |
Data are expressed as mean± SD, median (IQR) and No. (%). Comparisons were determined by Student's test, Mann-Whitney U test or χ2 test as appropriate.
Abbreviations: NLR, Neutrophil-lymphocyte ratio. ALT, Alanine aminotransferase. AST, Aspartate aminotransferase. TP, Total protein. GGT, Gamma-Glutamyl Transferase. UA, Uric acid. TG, Triglyceride. hsCRP, High-sensitivity c-reactive protein. CK, Creatine kinase. LDH, Lactate dehydrogenase.
Table 2.
Multivariate analysis of predictors of abnormal CT score.
| β | P value | OR (95% CI) | P value | OR (95% CI) a | P value a | |
|---|---|---|---|---|---|---|
| Age | 0.009 | 0.817 | 1.009 (0.933 to 1.093) | 0.817 | 1.033 (0.978–1.099) | 0.315 |
| Incubation period | 0.115 | 0.488 | 1.122 (0.811 to 1.553) | 0.488 | 1.254 (0.951–1.654) | 0.108 |
| CXR peak score | 0.026 | 0.832 | 1.027 (0.806 to 1.307) | 0.832 | 1.051 (0.888–1.243) | 0.565 |
| Albumin | −0.421 | 0.051 | 0.657 (0.430 to 1.002) | 0.051 | 0.730 (0.564–0.944) | 0.016 |
| Urea nitrogen | 1.967 | 0.046 | 7.149 (1.038 to 49.216) | 0.046 | 2.364 (1.038–5.385) | 0.041 |
| Glucose | 0.151 | 0.711 | 1.164 (0.523 to 2.590) | 0.711 | 1.392 (0.551–3.516) | 0.485 |
| hsCRP | 0.025 | 0.417 | 1.025 (0.966 to 1.088) | 0.417 | 1.015 (0.972–1.059) | 0.482 |
| D-dimer | 0.005 | 0.268 | 1.005 (0.996 to 1.013) | 0.268 | 1.006 (0.999–1.012) | 0.115 |
Abbreviations: CI, confidence interval. a Logistic regression analysis adjusted for sex, the level of CREA, UA P values.
3.2. Characteristics of anomalies on follow-up lung function
Spirometry was completed in all patients. Even though most patients were free of respiratory symptoms at follow, lung function abnormalities were detected in 14 patients (25.45%). Anomalies were noted in TLC of 4 patients (7.27%), FEV1 of 6 patients (10.91%), FVC of 6 patients (10.91%), DLCO of 9 patients (16.36%), and small airway function in 7 patients (12.73%).
As the pulmonary function in COVID-19 patients on the day of discharge, DLCO anomalies was the most common symptom appeared [9]. We therefore analyzed the correlation between DLCO and other characteristics. For all demographic data, clinical presentation, and laboratory examinations at admission presented in Table 3 and Supplementary Table 5, we initially evaluated each variable in difference between DLCO-impaired group and DLCO-normal group, using unpaired t-test or Mann-Whitney test univariate analysis. The median TBIL concentration in the abnormal DLCO group (13.20 [9.65–16.35]) was obviously higher than the normal DLCO group (8.90 [7.28–13.38]) (P = 0.048). Levels of urea nitrogen (abnormal DLCO group: 5.14 [4.68–6.91] vs. normal DLCO group: 4.25 [3.73–4.97]), D-dimer (abnormal DLC group: (0.42 ± 0.21) vs. normal DLCO group (0.23 ± 0.17) were higher in the DLCO-impaired group than in the DLCO-normal group (P < 0.05). In DLCO-impaired group, blood level of prothrombin time (15.20 [11.40–16.35]) was higher compared with that in DLCO-normal group (12.25 [10.75–14.55]). Levels of ALB were significantly decreased in abnormal DLCO group (40.38 ± 3.12) g/L compared with normal DLCO group (43.03 ± 3.66) g/L. No other significant differences were found between patients with normal and abnormal lung function. By analyzing the complete data for all variables in the multivariable logistic regression model, it was found that higher level of d-dimer at admission were associated with DLCO% predicted < 80% (P = 0.031, OR 1.066, 95% CI 1.006 to 1.129; Table 4). These results indicated that some of recovered COVID-19 patients still had significant impaired lung function symptom 3 months after discharge, and d-dimer might be a potential biomarker to predict DLCO of these patients.
Table 3.
Univariate analysis of predictors of abnormal DLCO% predicted.
| Parameters | Normal range | DLCO normal group (n = 46) | DLCO impaired group (n = 9) | P value |
|---|---|---|---|---|
| Age, years | ≥ 18 | 44.99 ± 14.70 | 52.57 ± 18.91 | 0.095 |
| Sex, (% female) | 19 (41.30%) | 4 (44.44%) | 0.861 | |
| Incubation period, d | 6.00 (4.00–7.25) | 6.00 (4.50–7.50) | 0.503 | |
| Temperature, °C | 37.80 (36.70–38.43) | 38.00 (37.60–38.35) | 0.600 | |
| CXR peak score | 7.22 ± 4.66 | 8.06 ± 5.82 | 0.638 | |
| Comorbidities | ||||
| Hypertension | 5 (10.87%) | 1 (11.11%) | 0.983 | |
| Coronary heart disease | 2 (4.35%) | 0 (0%) | 1.000 | |
| Diabetes mellitus | 1 (2.17%) | 1 (11.11%) | 0.737 | |
| Signs and symptoms at admission | ||||
| Fever | 28 (60.87%) | 9 (100%) | 0.057 | |
| Cough | 22 (47.83%) | 8 (88.89%) | 0.058 | |
| Feeble | 18 (39.13%) | 0 (0%) | 0.057 | |
| Laboratory data | ||||
| Blood Routine | ||||
| Leucocyte count (× 109/L) | 4–10 | 5.62 ± 1.75 | 5.81 ± 2.50 | 0.774 |
| Neutrophil count(× 109/L) | 2–7 | 3.74 ± 1.48 | 4.11 ± 2.63 | 0.556 |
| Lymphocyte count (× 109/L) | 0.8–4.0 | 1.42 (1.08–1.73) | 1.22 (0.98–1.87) | 0.601 |
| NLR | 2.79 (1.89–3.66) | 2.11 (1.73–4.46) | 0.716 | |
| Hemoglobin concentration (g/L) | 110–160 | 143.59 ± 18.73 | 130.00 ± 24.44 | 0.064 |
| Platelet count (× 1012/L) | 100–300 | 175.67 ± 55.40 | 179.89 ± 75.67 | 0.845 |
| Blood Biochemistry | ||||
| ALT, U/L | 0–40 | 24.90 ± 7.42 | 31.63 ± 12.30 | 0.146 |
| AST, U/L | 0–40 | 20.45 (13.98–30.10) | 21.30 (11.50–38.90) | 0.991 |
| Albumin, g/L | 40–53 | 43.03 ± 3.66 | 40.38 ± 3.12 | 0.047 |
| TP, g/L | 64–83 | 65.12 ± 4.92 | 63.39 ± 6.49 | 0.518 |
| GGT, U/L | 7–50 | 20.60 (15.05–38.13) | 25.90 (16.00–52.35) | 0.460 |
| Total bilirubin, μmol/L | 3.42–20.50 | 8.90 (7.28–13.38) | 13.20 (9.65–16.35) | 0.048 |
| Urea nitrogen, mmol/L | 1.43–7.14 | 4.25 (3.73–4.97) | 5.14 (4.68–6.91) | 0.012 |
| Creatinine, mmol/L | 44–97 | 65.61 ± 15.63 | 77.63 ± 23.97 | 0.060 |
| UA, μmol/L | 170–390 | 276.96 ± 84.70 | 276.37 ± 147.67 | 0.987 |
| Glucose, mmol/L | 3.89–6.11 | 5.40 (4.82–5.95) | 5.79 (5.14–8.33) | 0.187 |
| Inflammatory markers | ||||
| ESR, mm/h | 0–20 | 26.50 (7.00–45.50) | 52.00 (20.00–86.50) | 0.050 |
| Myocardial injury markers | ||||
| CK, U/L | 25–200 | 76.20 (49.13–104.83) | 54.00 (36.50–117.90) | 0.290 |
| LDH, U/L | 100–240 | 191.90 (164.25–230.43) | 219.00 (139.00–322.60) | 0.345 |
| Blood coagulation | ||||
| Prothrombin time, s | 10.0–13.5 | 12.25 (10.75–14.55) | 15.20 (11.40–16.35) | 0.043 |
| D-dimer, mg/L | 0–0.55 | 0.23 ± 0.17 | 0.42 ± 0.21 | 0.006 |
| Treatment | ||||
| low-dose corticosteroids | 6 (13.04%) | 1 (11.11%) | 1.000 | |
| Hospital period (d) | 15.50 (10.00–18.00) | 17.00 (11.50–19.50) | 0.600 |
Data are expressed as mean± SD, median (IQR) and No. (%). Comparisons were determined by Student's test, Mann-Whitney U test or χ2 test as appropriate.
Abbreviations: NLR, Neutrophil-lymphocyte ratio. ALT, Alanine aminotransferase. AST, Aspartate aminotransferase. TP, Total protein. GGT, Gamma-Glutamyl Transferase. UA, Uric acid. TG, Triglyceride. HDL, High-density lipoprotein. ESR, Erythrocyte sedimentation rate. CK, Creatine kinase. LDH, Lactate dehydrogenase.
Table 4.
Multivariate analysis of predictors of abnormal DLCO.
| β | P value | OR (95% CI) | P value a | OR (95% CI) a | |
|---|---|---|---|---|---|
| Albumin | −0.181 | 0.251 | 0.834 (0.612 to 1.136) | 0.054 | 0.711 (0.503–1.006) |
| Total bilirubin | 0.092 | 0.246 | 1.096 (0.938 to 1.281) | 0.515 | 1.048 (0.910–1.207) |
| Urea nitrogen | 0.494 | 0.166 | 1.640 (0.815 to 3.298) | 0.332 | 1.434 (0.692–2.973) |
| Prothrombin time | 0.335 | 0.097 | 1.398 (0.941 to 2.077) | 0.163 | 1.449 (0.861–2.438) |
| D-dimer | 0.064 | 0.031 | 1.066 (1.006 to 1.129) | 0.047 | 1.011 (1.001–1.023) |
Abbreviations: CI, confidence interval. a Logistic regression analysis adjusted for sex, age, history of smoking, the level of CREA P values.
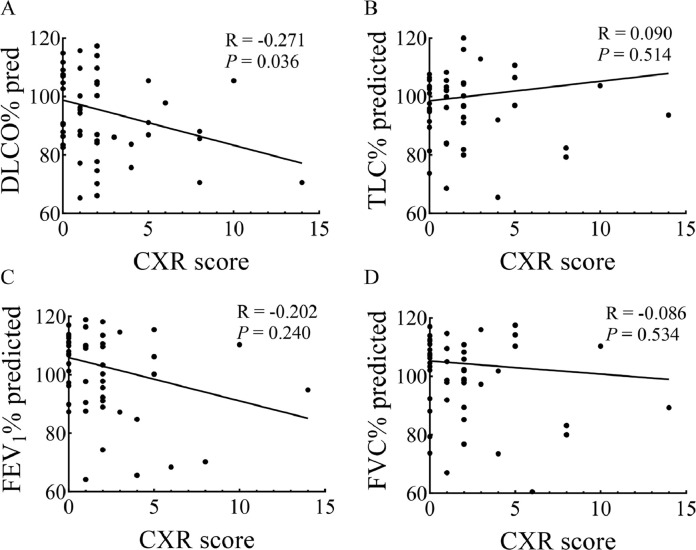
Finally, the correlation between predicted FVC%, predicted TLC%, predicted DLCO%, predicted FEV1% and CXR in recovered subjects were analyzed. There was a significant negative correlation between the value of predicted DLCO% and CXR (R = −0.271, P = 0.036), whereas the value of predicted TLC%, predicted FEV1%, and predicted FVC% showed no correlation with CXR (Fig. 3).
Fig. 3.
Spearman's correlation analysis for CXR score 3 month after discharge with pulmonary function: DLCO% predicted (A), TLC% predicted (B), FEV1% predicted (C), and FVC% predicted (D).
3.3. Comparison of IgG antibodies with nucleic acid test
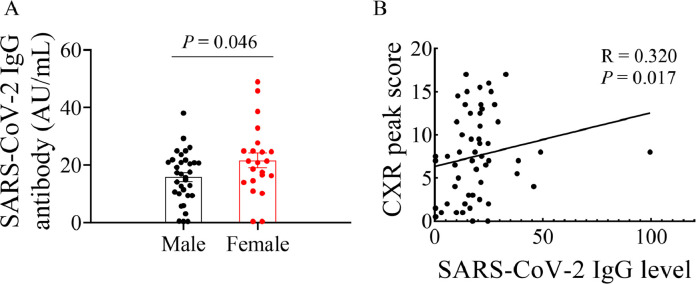
Nuclei acid test and IgG antibody test were performed at least twice. IgG antibody levels were summarized in Table 5. Of the rehabilitating COVID-19 patients, as expected, all samples were tested negative for the viral RNA. In the SARS-COV-2 IgG antibody test, positive results were obtained in 47 patients (85.45%) and negative results were seen in 8 patients (14.55%). Of note, 6 negative SARS-CoV-2 IgG antibody patients were male without comorbidities, and the other 2 were female. The results indicated that there was a possibility to be repeatedly infected among the recovered COVID-19 patients. As shown in Fig. 4, the concentration of SARS-CoV-2 IgG antibody in female patients was higher compared with male patients. These data suggest that the concentration of SARS-CoV-2 IgG antibody in female recovered patients tended to be higher than male recovered patients 3 months after discharge. In addition, the correlation between the corresponding SARS-CoV-2 IgG and CXR peak score in each patient was analyzed. it was found that there was a strong correlation between IgG levels and CXR peak score (R = 0.320, P = 0.017. Fig. 4).
Table 5.
Comparison of IgG antibodies with nucleic acid test.
| Nucleic acid detection | IgG | ||
|---|---|---|---|
| Negative 55 (100%) | + | – | |
| Normal CT group | 13 (81.25%) | 3 (18.75%) | |
| Abnormal CT group | 34 (87.18%) | 5 (12.82%) |
The data are presented as n (%). N is the number of patients with available data. The cut-off value for a positive result was 10 AU/mL.
Fig. 4.
Comparison of SARS-CoV-2 IgG antibody concentration between male and female patients in recovering status (A). Spearman's correlation analysis for CXR peak score with SARS-CoV-2 IgG antibody in recovered COVID-19 patients (B).
4. Discussion
After the SARS outbreak, plenty of patients recovered and there was an important question for hospitals and doctors: will patients recovered from SARS have any clinical sequelae? According to a previous report, 33 patients (30%) had abnormal CT manifestation and 17 patients (15.5%) had impaired DLCO at 6 months after recovered from SARS [19]. David et al. analyzed 97 patients who recovered from SARS and found that after 1-year, abnormal CT findings and DLCO anomalies were still present [20]. A follow-up study of H7N9 demonstrated that lesions persisted in patients up to 64-month after illness onset, with restrictive ventilation dysfunction and dyspnea [21]. In our retrospective multi-center cohort study, we found that a high percentage of COVID-19 patients had abnormalities on chest CT scans persisted 3 months after discharge (Supplementary Table 6). In this situation, doctors should overtake their responsibility for longer follow-up time survey to detect persistent lung damage and long-term pulmonary dysfunction.
To the best of our knowledge, few reports have described the sequelae of COVID-19 survivors [9,22,23], and this project was the first time to investigate the long term effects on changes in both pulmonary function and HRCT imaging. In our studies, we presented the results of lung function tests and HRCT of the chest in these patients with COVID-19 3 months after their hospital discharge.
Patients with COVID-19 are known to have fever, cough, headache, loss of smell and deterioration of GI system in general [24]. Even with such a huge mortality rate, a large number of the COVID-19 patients still be able to recover from this deadly situation. It is highly beneficial for offering follow-up checks and investigating reinfection possibilities of COVID-19 recovered patients. Since high ACE2 expression found in the GI tract, patients with GI symptoms was diagnosed as 3.6~11.4% of COVID-19 patients according to the virus mutation [25,26]. In our study, 30.91% of the cohort still showed GI symptoms even after 3 months discharge, indicating the severe GI injury of the SAR-CoV-2 caused by transmissibility, virulence and multi-organ infection. Previously, many patients recovered from SARS in the early rehabilitation phase complained of limitation in general physical function and/or shortness of breath [27]. Taken together, although patients who have recovered from COVID-19 have been noted to manifest radiological, functional and psychological abnormalities to varying degrees, they felt performing household tasks or general work was moderately or severely impaired, and it is important to follow up these patients.
The rate of radiological abnormalities (74.55%) is lower than that reported in an earlier study (83%) over 7 days after admission [28,29], which suggested that radiological abnormalities caused by SARS-CoV-2 might get better over time. A similar rate of residual radiographic changes was also identified in survivors with other viral pneumonias, including specifically SARS, H1N1, and H7N9 pneumonia [30], [31], [32]. SARS-CoV-2 differs from the original SARS-CoV-1 by 380 amino acid substitutions, which results to the differences in five of the six vital amino acids in the receptor-binding domain between the viral spike (S) protein with angiotensin converting enzyme 2 (ACE2) [33]. The binding affinity of SARS-CoV-2 with ACE2 seems stronger than SARS-CoV-1, with a higher spread ability than SARS-CoV-1, which may explain the considerably larger global influence of COVID-19 than the initial SARS [34]. Therefore, COVID-19 may not be analogous with others as its unique characteristic is different from others. As shown in Supplementary Table 6, patients who survived severe illness from virus might have persistent lung damage and long-term pulmonary function. Clinically, patients with abnormal HRCT scans were generally older than those with normal chest HRCT score, which implied that higher chest radiological scores was mostly obtained in elder patients [35]. Patients in the abnormal CT group had longer incubation period and higher CXR peak score than those in the normal CT group, indicating that patients with residual lesions in chest radiology after discharge had more severe side effects. Additionally, it was found that the levels of ALT, urea nitrogen, hsCRP and D-dimer indicated multi-organ damage caused by COVID-19, and triggered deteriorations to general functions, which was similar to other reports [36], [37], [38]. The baseline characteristics (including sex, comorbidities) and laboratory indexes of patients in our cohort showed no statistical difference. Urea nitrogen is a key element reflecting the intricate interrelation between nutritional status, protein metabolism, and renal situation. Higher levels of urea nitrogen in COVID-19 patients suggesting that there is the existence of persistent inflammation-immunosuppression and catabolism syndrome. Urea nitrogen has been reported to be a risk factor for severe COVID-19 patients [39]. Furthermore, the level of urea nitrogen was found to be an independent factor associated with radiographic changes, which was consistent with the previous studies. Therefore, the level of urea nitrogen can be a parameter to predict patients with COVID-19 infection whether they are at a higher risk of developing residual radiographic changes after discharge, thereby, it enable better to centralize management and recovery treatment of severe patients.
At 3-months after discharge, residual abnormalities of pulmonary function were observed in 25.45% of the cohort, mostly demonstrated diffusion reductions in DLCO. This was lower than the abnormal pulmonary function in COVID-19 patients when discharge [9]. Abnormalities in DLCO indicated pulmonary fibrosis or a late phase in the course of recovery. In the following-up studies for the patients rehabilitating from SARS, impaired lung function could last for months or even years [19,20,40]. D-dimer elevation has reported as an important laboratory finding noted in COVID-19 patients which requires extra attention. Several studies have reported that D-dimer on admission was the independent predictor of in-hospital death for patients with COVID-19 [25,41,42]. We also found the level of D-dimer was an important prognostic factor for abnormal DLCO. Thus, for patients who have marked raised d-dimer, pulmonary rehabilitation should need subsequently even in the absence of severity respiratory symptoms.
Despite negative nucleic acid test results, 8 individuals (14.55%) showed negative results in the SARS-CoV-2 IgG antibody test for at least 2 tests, suggesting that these patients may be infected again. This research also identified IgG antibody presented a stronger production in female patients 3 months after discharge. Most of the female patients produced a high level of SARS-CoV-2 IgG antibody in the severe status over the first 2 to 4 weeks [41,43]. Considering the protective role of SARS-CoV-2 IgG antibody, we believe that the different concentration of SARS-CoV-2 IgG antibody between male and female allows to explain the different radiological and physiological outcomes. Our study proposed that convalescent plasma should be used in advance to prevent diseases from progressing long-term sequelae.
However, there were several limitations in this study. Firstly, only 55 patients with confirmed SARS-CoV-2 infection were enrolled in this study. Larger sample size would be more ideal for the study. Secondly, since the patients included in this study were non-critical, the value of pulmonary function test and HRCT scanning in critical patients need to be assessed. Further study should be conducted to involve objective measurements of functional status (such as the exercise lung function test or 6-minute walk test) and the physiological and radiological defects.
In conclusions, this research has demonstrated that significant radiographic and physiological abnormalities still existed in a high proportion of COVID-19 patients 3 months after discharge. SARS-CoV-2 IgG antibody has vanished in several patients. It is necessary to follow up these patients, performing comprehensive assessment and early rehabilitation exercise for detection and appropriate management of any persistent or emerging long-term sequelae in the radiological and physiological domains.
Declaration of Competing Interest
The authors have no interests to declare.
Acknowledgments
Acknowledgements
The authors’ work was supported by the Key Scientific Research Projects of Henan Higher Education Institutions (under grant 20B320032). We thank our patients and their families, who have sought to help others understand this disease. We are also grateful for the support of the Department of Infection at Guangshan People's Hospital and Xixian People's Hospital. Finally, we would like to acknowledge Dr Chuansong Hu at the Guangshan People's Hospital, for his expertise and guidance in this work.
Data sharing statement
Request for access to the data should be made to the corresponding author at aiguoxu@zzu.edu.cn. Data could be made available provided the applicant has appropriate ethics approval and approval from the authors.
Author contribution
XAG, GYF and LH had the idea for and designed the study and had full access to all the date in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. GYF, ZYM and SYM drafted the paper. AXG, GYF, ZYM, SYM, SWB, XQF, JJL and LLM did the analysis, and all authors critically revised the manuscript for important intellectual content and gave final approve for the version to be published. ZYM, SYM, SWB, LH and GYF collected the data. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100463.
Contributor Information
Hong Luo, Email: lh7201@163.com.
Yan-feng Gao, Email: gaoyf@zzu.edu.cn.
Ai-guo Xu, Email: aiguoxu@hotmail.com.
Appendix. Supplementary materials
References
- 1.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du Toit A. Outbreak of a novel coronavirus. Nat Rev Microbiol. 2020;18(3):123. doi: 10.1038/s41579-020-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holshue M.L., DeBolt C., Lindquist S. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassetti M., Vena A., Giacobbe D.R. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Invest. 2020;50(3):e13209. doi: 10.1111/eci.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. COVID-19 dashboard. 2019. Accessed June 8, 2020. https://covid19.who.int/.
- 7.Verdecchia P., Cavallini C., Spanevello A., Angeli F. COVID-19: ACE2centric infective disease? Hypertension. 2020 doi: 10.1161/HYPERTENSIONAHA.120.15353. [DOI] [PubMed] [Google Scholar]
- 8.Zhu H., Rhee J.W., Cheng P. Cardiovascular Complications in Patients with COVID-19: consequences of Viral Toxicities and Host Immune Response. Curr Cardiol Rep. 2020;22(5):32. doi: 10.1007/s11886-020-01292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mo X., Jian W., Su Z. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J. 2020 doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu M., Liu Y., Xu D., Zhang R., Lan L., Xu H. Prediction of the Development of Pulmonary Fibrosis Using Serial Thin-Section CT and Clinical Features in Patients Discharged after Treatment for COVID-19 Pneumonia. Korean J Radiol. 2020;21(6):746–755. doi: 10.3348/kjr.2020.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Clinical management of severe acute respiratory infection when COVID-19 disease is suspected: interim guidance, 13 March 2020. https://www.who.int/publications/i/item/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- 12.National Health Commission of the People's Republic of China. Chinese management guideline for COVID-19 (Chinese version, Version 7.0). Accessed March 4. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
- 13.Chung M., Bernheim A., Mei X. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV) Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonio G.E., Wong K.T., Hui D.S. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. 2003;228(3):810–815. doi: 10.1148/radiol.2283030726. [DOI] [PubMed] [Google Scholar]
- 15.Dai H., Zhang X., Xia J. High-resolution Chest CT Features and Clinical Characteristics of Patients Infected with COVID-19 in Jiangsu, China. Int J Infect Dis. 2020;95:106–112. doi: 10.1016/j.ijid.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham B.L., Steenbruggen I., Miller M.R. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu J., Wu C., Li X. Profile of IgG and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin Y., Wang M., Zuo Z. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int J Infect Dis. 2020;94:49–52. doi: 10.1016/j.ijid.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hui D.S., Joynt G.M., Wong K.T. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hui D.S., Wong K.T., Ko F.W. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128(4):2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q., Jiang H., Xie Y. Long-term clinical prognosis of human infections with avian influenza A(H7N9) viruses in China after hospitalization. EClinicalMedicine. 2020;20 doi: 10.1016/j.eclinm.2020.100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson R., Robinson L. Rehabilitation After Critical Illness in People With COVID-19 Infection. Am J Phys Med Rehabil. 2020;99(6):470–474. doi: 10.1097/PHM.0000000000001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei J., Lei P., Yang H. Analysis of thin-section CT in patients with coronavirus disease (COVID-19) after hospital discharge. Clin Imaging. 2020 doi: 10.1016/j.clinimag.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balachandar V., Mahalaxmi I., Subramaniam M. Follow-up studies in COVID-19 recovered patients - is it mandatory? Sci Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.139021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan W.J., Ni Z.Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan K.S., Zheng J.P., Mok Y.W. SARS: prognosis, outcome and sequelae. Respirology. 2003;8 doi: 10.1046/j.1440-1843.2003.00522.x. Suppl:S36-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong Y., Sun D., Liu Y. Clinical and High-Resolution CT Features of the COVID-19 Infection: comparison of the Initial and Follow-up Changes. Invest Radiol. 2020;55(6):332–339. doi: 10.1097/RLI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han X., Cao Y., Jiang N. Novel Coronavirus Pneumonia (COVID-19) Progression Course in 17 Discharged Patients: comparison of Clinical and Thin-Section CT Features During Recovery. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng C.K., Chan J.W., Kwan T.L. Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors. Thorax. 2004;59(10):889–891. doi: 10.1136/thx.2004.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mineo G., Ciccarese F., Modolon C., Landini M.P., Valentino M., Zompatori M. Post-ARDS pulmonary fibrosis in patients with H1N1 pneumonia: role of follow-up CT. Radio Med. 2012;117(2):185–200. doi: 10.1007/s11547-011-0740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q., Zhang Z., Shi Y., Jiang Y. Emerging H7N9 influenza A (novel reassortant avian-origin) pneumonia: radiologic findings. Radiology. 2013;268(3):882–889. doi: 10.1148/radiol.13130988. [DOI] [PubMed] [Google Scholar]
- 33.Wu A., Peng Y., Huang B. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gheblawi M., Wang K., Viveiros A. Angiotensin-Converting Enzyme 2: sARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: celebrating the 20th Anniversary of the Discovery of ACE2. Cir Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Das K.M., Lee E.Y., Singh R. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 2017;27(3):342–349. doi: 10.4103/ijri.IJRI_469_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng Z., Peng F., Xu B. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J., Wang X., Jia X. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Y., Sun L.J., Xu M. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. J Zhejiang Univ Sci B. 2020;21(5):378–387. doi: 10.1631/jzus.B2000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong J., Ou J., Qiu X. A Tool to Early Predict Severe Corona Virus Disease 2019 (COVID-19): a Multicenter Study using the Risk Nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ngai J.C., Ko F.W., Ng S.S., To K.W., Tong M., Hui D.S. The long-term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15(3):543–550. doi: 10.1111/j.1440-1843.2010.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L., Yan X., Fan Q. d-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng F., Dai C., Cai P. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J Med Virol. 2020 doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.