Abstract
In response to the current pandemic caused by the novel SARS-CoV-2, identifying and validating effective therapeutic strategies is more than ever necessary. We evaluated the in vitro antiviral activities of a shortlist of compounds, known for their cellular broad-spectrum activities, together with drugs that are currently under evaluation in clinical trials for COVID-19 patients. We report the antiviral effect of remdesivir, lopinavir, chloroquine, umifenovir, berberine and cyclosporine A in Vero E6 cells model of SARS-CoV-2 infection, with estimated 50% inhibitory concentrations of 0.99, 5.2, 1.38, 3.5, 10.6 and 3 μM, respectively. Virus-directed plus host-directed drug combinations were also investigated. We report a strong antagonism between remdesivir and berberine, in contrast with remdesivir/diltiazem, for which we describe high levels of synergy, with mean Loewe synergy scores of 12 and peak values above 50. Combination of host-directed drugs with direct acting antivirals underscore further validation in more physiological models, yet they open up interesting avenues for the treatment of COVID-19.
Keywords: COVID-19, Antivirals, Drug combination, Remdesivir, Berberine, Diltiazem
Despite unprecedented global efforts during the last months in the fight of the COVID-19 pandemic, which include more than 500 randomized clinical trials (“Covid-19 living Data,” n.d.), neither a preventive vaccine nor an effective treatment as a potential complement to standard supportive care have yet been validated. This situation is more problematic in the case of severe patients, provided increasing clinical reports account for a rather biphasic pathophysiology. In fact, the initial phase of the disease is characterized by a typical virus-driven response, which might subsequently (by day 7–10 after symptom onset) shunt into an acute immunopathologic phase with an associated hypercytokinemia (Huang et al., 2020; Mehta et al., 2020; Zhu et al., 2020), hence adding more complexity to the choice of a potentially effective treatment. However, such “cytokine storm” is not independent of viral replication but frequently an attempt to clear the viral aggression. In that regard, recent data suggest that high virus titers during the first week of infection could be correlated with a higher probability of moderate to severe clinical outcome (Liu et al., 2020b; Zheng et al., 2020). This highlights the value of identifying and validating molecules and/or combination of molecules with a strong SARS-CoV-2 antiviral activity for an early and effective reduction of viral load that could ultimately contribute to a more favorable clinical prognosis.
In this context, we sought to assess the in vitro antiviral activity of a shortlist of compounds known for their relative broad-spectrum antiviral activities that in our view have a rationale for a putative inhibitory effect against SARS-CoV-2 and, on top of that, could be readily available for further pre-clinical and clinical evaluation. Favipiravir (T-705; 6-fluoro-3-hydroxy-2-pyrazinecarboxamine) is a pyrazine derivative that has demonstrated potent antiviral activity against multiple RNA viruses, being currently approved in Japan for the treatment of influenza infections (Furuta et al., 2013, 2002). The ribonucleoside analog ribavirin (1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide) is also known to show broad-spectrum antiviral activity and is used in combination with interferon-α to treat hepatitis C virus infection (Crotty et al., 2000; Sidwell et al., 1972). Umifenovir (Arbidol) is a small indole-derivative molecule, licensed in Russia and China for the prophylaxis and treatment of influenza and other respiratory viral infections (Blaising et al., 2014). Berberine is a plant-derived isoquinoline alkaloid, described as having broad antiviral activity, notably against influenza and alphaviruses (Varghese et al., 2016a; Yan et al., 2018). Cyclosporine A is a widely used immunosuppressant drug also known for its ability to inhibit the replication of several viruses, including several coronaviruses (de Wilde et al., 2011). Lastly, diltiazem is a voltage gated Ca2+ channel blocker commonly used as anti-hypertensive, which we have recently repurposed as a host-directed inhibitor of influenza virus (Pizzorno et al., 2019).
Molecules were obtained from Sigma-Aldrich and 10 mM stock solutions were prepared in DMSO (favipiravir, lopinavir, remdesivir, umifenovir), ethanol (berberine, cyclosporine A) or water (chloroquine, diltiazem, ribavirin). We therefore performed experimental infections in Vero E6 cells at a multiplicity of infection (MOI) of 0.01 using the BetaCoV/France/IDF0571/2020 SARS-CoV-2 strain, previously isolated from one of the first COVID-19 cases in France. One-hour post infection (hpi), the viral inoculum was removed, and cells were treated with serial dilutions of the candidate molecules in infection media. Dilutions of the corresponding solvents in infection media were used for mock-treated controls. Supernatants were collected 48 hpi for subsequent viral titration by RT-qPCR, as described elsewhere (Pizzorno et al., 2020). Noteworthy, we included the active principles of the three drugs evaluated in the WHO's COVID-19 Solidarity Trial (”‘Solidarity 2020’ clinical trial for COVID-19 treatments,” 2020) as comparators: i) remdesivir, an experimental adenosine nucleotide analog prodrug previously evaluated in the context of the 2018 Kivu Ebola epidemic (Mulangu et al., 2019); ii) lopinavir, a protease inhibitor antiretroviral approved for the treatment of HIV infections (Chu, 2004); iii) chloroquine, an aminoquinolone derivative mainly used for the treatment of malaria but also for certain chronic inflammatory diseases such as rheumatoid arthritis and lupus erythematosus (Al-Bari, 2017).
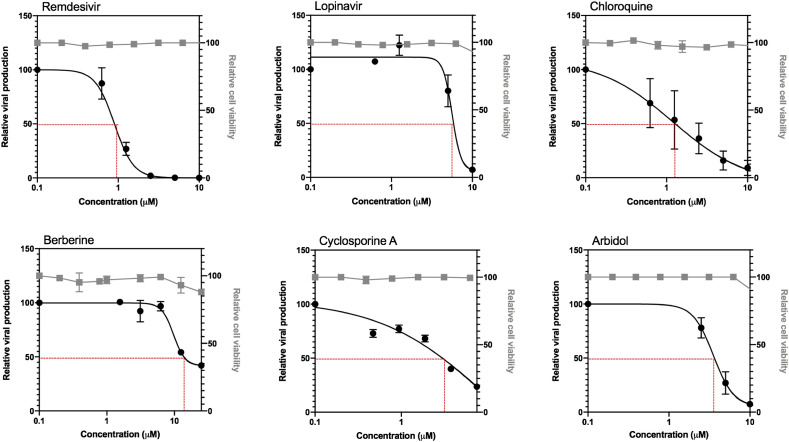
Table 1 and Fig. 1 recapitulate the main features of the evaluated compounds, including the calculated 50% inhibitory concentration (IC50), 50% cytotoxic concentration (CC50) and selectivity index (SI) values as well as the dose-response curves of the compounds with such an effect. As expected, calculated IC50 values for lopinavir, remdesivir and chloroquine were in the low micromolar range (5.2, 0.99 and 1.38 μM, respectively), with the latter two showing a very favorable SI (278 and 172, respectively), in good agreement with recent reports (Choy et al., 2020; Wang et al., 2020). On the other hand, although their efficacy against influenza viruses was previously validated (data not shown), neither diltiazem nor ribavirin or favipiravir showed antiviral activity against SARS-CoV-2 in the conditions tested. This is not surprising in the case of diltiazem, considering its recently described antiviral properties are mostly based on the capacity to induce type I and III interferon (IFN) antiviral responses (Pizzorno et al., 2019), which are partially abrogated in the Vero E6 cell line (Prescott et al., 2010). However, we would a priori have expected that broad spectrum inhibitors of viral RNA synthesis ribavirin and favipiravir show at least mild antiviral activity against SARS-CoV-2, notably in the context of preliminary clinical data suggesting a putative benefit of favipiravir for the treatment of COVID-19 patients in comparison with umifenovir ( Chen et al., 2020a; Liu et al., 2020a). Nonetheless, our results are in line with recent observations by Choy and colleagues (Choy et al., 2020). Interestingly, umifenovir, berberine and cyclosporine A, three compounds previously reported to have broad antiviral activity, displayed dose-dependent antiviral responses against SARS-CoV-2. Umifenovir (IC50: 3.5 μM and SI: 21) was reported to inhibit fusion between the viral envelope and the membrane of the target cell, although the exact mechanism of this inhibition is still not well described. Besides its anti-influenza properties, umifenovir has been shown to inhibit some arthropod-borne flaviviruses, including Zika, West Nile and tick-borne encephalitis virus (Haviernik et al., 2018). Umifenovir is currently being evaluated in clinical trials against COVID-19 ( Chen et al., 2020a; Liu et al., 2020a). Berberine (IC50: 10.6 μM and SI: >37) has demonstrated in vitro and in vivo (mice) antiviral activity against influenza, Chikungunya and enterovirus 71, possibly by downregulating autophagy and the MEK/ERK signaling pathway (Varghese et al., 2016b, 2016b). Cyclosporine A (IC50: 3.0 μM and SI: >29) was shown to be very effective in inhibiting two different coronaviruses in vitro, human coronavirus 229E (HCoV-229E) and mouse hepatitis virus (MHV) but not SARS-CoV (de Wilde et al., 2011). Although the exact mechanism by which cyclosporine A inhibits coronavirus replication remains to be established, an early blockage of viral RNA and protein synthesis has been suggested (de Wilde et al., 2011).
Table 1.
Antiviral activity of post-infection treatment with candidate molecules against SARS-CoV-2 in vitro.
| Drug class | Drug indication | CAS Number | IC50 (M) | CC50 (M) | SI | |
|---|---|---|---|---|---|---|
| Remdesivir | Antimetabolites Antiviral agents |
Treatment of Ebola virus disease | 1809249-37-3 | 0.987 | 275 | 278.62 |
| Lopinavir | Antiviral agents | Treatment of HIV-infection | 192725-17-0 | 5.246 | 45 | 8.57 |
| Chloroquine | Antimalarial agents | Treatment of malaria, rheumatoid arthritis, glioma | 54-05-7 | 1.38 | 238 | 172.46 |
| Berberine | Alkaloid | Treatment of parasitic and fungal infections | 2086-83-1 | 10.577 | >400 | >37.84 |
| Cyclosporine A | Enzyme inhibitors Immunosuppressive agents |
Prophylaxis of organ rejection, treatment of rheumatoid arthritis & dermatologic diseases | 59865-13-3 | 3.048 | >90 | >29.53 |
| Arbidol (umifenovir) | Antiviral agents | Treatment of Influenza infection | 131707-25-0 | 3.537 | 75 | 21.20 |
| Diltiazem | antihypertensive & vasodilating agents |
Management of hypertension | 42399-41-7 | >45 | 424 | <9.42 |
| Ribavirin | Hepatitis C & antiviral agents | Treatment of HCV infection | 36791-04-5 | >10 | >100 | nd |
| Favipiravir | Antiviral agents | Treatment of Influenza infection | 259793-96-9 | >100 | 631 | <6.31 |
Fig. 1.
Evaluation of antiviral activity of six drug candidates against SARS-CoV-2 in vitro. Vero E6 cells were infected by SARS-CoV-2 at a MOI of 0.01 and treated 1 h post infection (hpi) with serial dilutions of remdesivir, lopinavir, chloroquine, berberine, cyclosporine A and arbidol (umifenovir). Viral production was measured 48 hpi and expressed in relative values compared to the vehicle-treated control. The impact of treatment on cell viability was assessed by MTS assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega), and expressed in relative values compared to control. Cell viability measurements were performed to ensure that molecular viral quantification was performed within a non-cytotoxic concentration range that could bias the measurement.
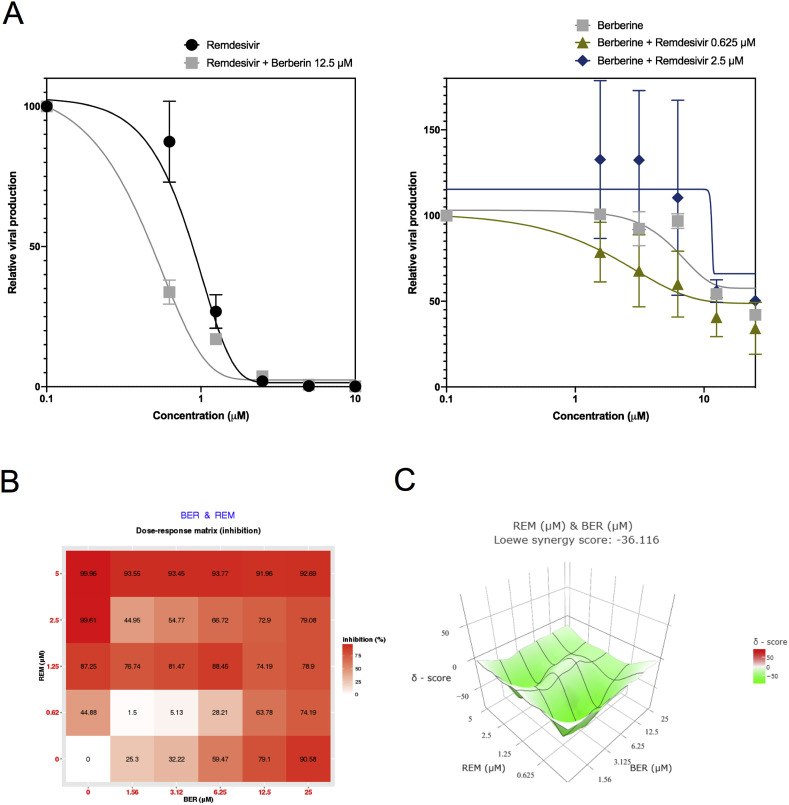
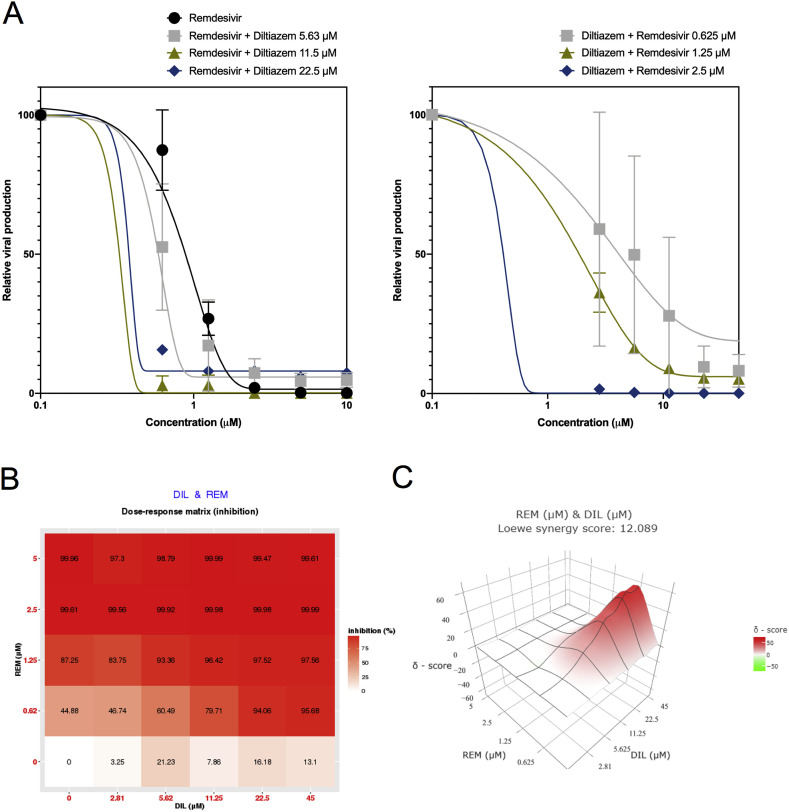
There is arguably a consensus among virologists that combining two compounds with antiviral activity and different viral or cellular targets could result in better virological and physiological responses than those of antiviral monotherapy, for which we decided to explore this hypothesis in the case of SARS-CoV-2. Provided recent results of clinical trials failed to show a therapeutic benefit of the use of either the lopinavir-ritonavir association or chloroquine (or its derivative hydroxychloroquine) in COVID-19 patients (Cao et al., 2020; Chen et al., 2020b; Magagnoli et al., 2020), we then selected remdesivir as the main antiviral agent for our drug combination studies. Following the same infection protocol described above, remdesivir dose-response experiments were repeated in the presence of berberine or diltiazem, given their expected host-directed antiviral properties. Alternatively, berberine and diltiazem dose-response curves were performed in the presence of different fixed concentrations of remdesivir. Our results show that the presence of 12.5 μM berberine induces a shift of the remdesivir dose-response towards lower drug concentration values (Fig. 2 A, left panel), as evidenced by a >2-fold reduction of remdesivir IC50 (0.41 μM versus 0.99 μM), with the associated increase on the SI (Table 2 ). A similar trend was observed when we analyzed the berberine dose-response profile in the presence of 0.625 μM remdesivir but not at higher remdesivir concentrations (Fig. 2A, right panel and Table 2). To further explore this combination, we used SynergyFinder (Ianevski et al., 2017) to calculate and visualize synergy scores, using a wider range of remdesivir/berberine concentrations spanning 24 different pairwise combinations. Interestingly, although the dose-response matrix shows a large range of pairwise combination with significant inhibitory effect (Fig. 2B), calculated Loewe synergy scores (Fig. 2C) account for a strong antagonism between remdesivir and berberine, with negative values distributed all throughout the synergy map regardless of drug concentrations (Fig. 2B and C). Following an analogous approach, the presence of diltiazem induced a stronger shift in the dose-response curve of remdesivir (Fig. 3 A, left panel), with an up to 8.5-fold reduction of remdesivir IC50 values (0.12 μM versus 0.99 μM in presence of 11.5 μM diltiazem) and the consequent SI increase (Table 3 ). A similar trend was observed for diltiazem dose-response curves in the presence of remdesivir (Fig. 3A, right panel). SynergyFinder analysis revealed a clear synergistic inhibitory effect of the combination, driven mainly by remdesivir but also by diltiazem, as shown by both the dose-response matrix (Fig. 3B) and the three-dimensional synergy map (Fig. 3C), with mean Loewe synergy scores of 12 and peak values above 50. Similar results were obtained using the Zero Interaction Potency (ZIP), Highest Single Agent (HAS), and Bliss independence models. Interestingly, the Loewe synergy scores obtained were quite high in comparison to the previously published synergistic combination of remdesivir and emetine (Choy et al., 2020).
Fig. 2.
Evaluation of remdesivir-berberine combined treatment against SARS-CoV-2 in vitro. A. Vero E6 cells were infected by SARS-CoV-2 at a MOI of 0.01 and treated 1 hpi with serial dilutions of remdesivir in the presence of different fixed concentrations of berberine (A, left panel) or, alternatively, serial dilutions of berberine in the presence of different fixed concentrations of remdesivir (A, right panel). Viral production was measured 48 hpi and expressed in relative values compared to the vehicle-treated control. B. Dose-response percent inhibition matrix of single and combined remdesivir-berberine treatments C. Interaction landscape between remdesivir and berberine as calculated using the Loewe additive model. Areas with synergy scores of −10 or lower (green) represent zones of drug antagonism.
Table 2.
Inhibitory effect of remdesivir-berberine combined treatment against SARS-CoV-2 in vitro. For each experimental condition, IC50, CC50 and the corresponding SI values are listed.
| Combination treatment | IC50 (M) | CC50 (M) | SI |
|---|---|---|---|
| Remdesivir | 0.987 | 275 | 278.62 |
| Remdesivir + Berberine 12.5 M |
0.409 | 264 | 645.47 |
| Berberine | 10.577 | > 400 | > 37.84 |
| Berberine + Remdesivir 0.625 M |
6.95 | > 57.55 | |
| Berberine + Remdesivir 2.5 M |
8.237 | > 48.56 |
Fig. 3.
Synergistic effect of remdesivir-diltiazem combined treatment against SARS-CoV-2 in vitro. A. Vero E6 cells were infected by SARS-CoV-2 at a MOI of 0.01 and treated 1 hpi with serial dilutions of remdesivir in the presence of different fixed concentrations of diltiazem (A, left panel) or, alternatively, serial dilutions of diltiazem in the presence of different fixed concentrations of remdesivir (A, right panel). Viral production was measured 48 hpi and expressed in relative values compared to the vehicle-treated control. B. Dose-response percent inhibition matrix of single and combined remdesivir-diltiazem treatments C. Interaction landscape between remdesivir and diltiazem as calculated using the Loewe additive model. Areas with synergy scores of 10 or higher (red) represent zones of drug synergy.
Table 3.
Inhibitory effect of remdesivir-diltiazem combined treatment against SARS-CoV-2 in vitro. For each experimental condition, IC50, CC50 and the corresponding SI values are listed.
| Combination treatment | IC50 (M) | CC50 (M) | SI |
|---|---|---|---|
| Remdesivir | 0.987 | 275 | 278.62 |
| Remdesivir + Diltiazem 5.62 M |
0.627 | 267 | 425.83 |
| Remdesivir + Diltiazem 11.5 M |
0.116 | 2301.72 | |
| Remdesivir + Diltiazem 22.5 M |
0.228 | 1171.05 | |
| Diltiazem | >45 | 424 | <9.42 |
| Diltiazem + Remdesivir 0.625 M |
5.268 | 414 | 78.59 |
| Diltiazem + Remdesivir 1.25 M |
1.892 | 218.81 | |
| Diltiazem + Remdesivir 2.5 M |
0.554 | 747.29 |
In conclusion, our study demonstrated the in vitro efficacy against SARS-CoV-2 of broad-spectrum antivirals such as Remdesivir, Lopinavir, chloroquine, Arbidol and Ribavirin and repurposable host-targeted drug candidates such as Berberine or cyclosporine A in monotherapy. As already reported by our group and others for the treatment of severe forms of influenza infection (Dunning et al., 2014; Pizzorno et al., 2019), drug combination highlighted the added value of combining drugs with different modes of action as a strategy to improve antiviral therapy. From this perspective, the remdesivir/diltiazem combination seems particularly interesting for the treatment of COVID-19, with a synergistic antiviral affect against SARS-CoV-2 in a wide dose range that remains below reported remdesivir therapeutic plasma concentrations. Although berberine, cyclosporine A and the remdesivir-diltiazem combination showed interesting potential as SARS-CoV-2 inhibitors, we are nonetheless aware of the inherent limits of the Vero E6 in vitro model, which may not accurately reflect the potential efficacy of antiviral molecules in more physiological models. In addition, the a priori counter-intuitive antagonistic effect observed for the remdesivir/berberine combination further illustrates the challenge of proposing effective drug combinations based on individual drug-efficacy data. We therefore expect our data constitute a starting point for further validation of selected candidates in more complex and biologically relevant pre-clinical models of SARS-CoV-2 infection before consideration for evaluation in the clinical setting.
Acknowledgments
This work was funded by INSERM REACTing (REsearch & ACtion emergING infectious diseases), CNRS, and Mérieux research grants. AP, BP, TJ, AT, OT and MRC are co-inventors of a patent application filed by INSERM, CNRS, Université Claude Bernard Lyon 1 and Signia Therapeutics for the repurposing of diltiazem for the treatment of SARS-CoV-2 infections (FR 20/02351).
References
- Al-Bari MdA.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017;5 doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaising J., Polyak S.J., Pécheur E.-I. Arbidol as a broad-spectrum antiviral: an update. Antivir. Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang Jingli, Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li Huadong, Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li Hui, Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang Juan, Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir–ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhang Yi, Huang J., Yin P., Cheng Z., Wu J., Chen S., Zhang Yongxi, Chen B., Lu M., Luo Y., Ju L., Zhang J., Wang X. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020;2020 doi: 10.1101/2020.03.17.20037432. 03.17.20037432. [DOI] [Google Scholar]
- Chen Z., Hu J., Zhang Zongwei, Jiang S., Han S., Yan D., Zhuang R., Hu B., Zhang Zhan. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020;2020 doi: 10.1101/2020.03.22.20040758. 03.22.20040758. [DOI] [Google Scholar]
- Choy K.-T., Wong A.Y.-L., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.-H., Huang X., Peiris M., Yen H.-L. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.M. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covid-19 living Data. 2020. https://covid-nma.com/ [WWW Document] (accessed 5.4.20) [Google Scholar]
- Crotty S., Maag D., Arnold J.J., Zhong W., Lau J.Y.N., Hong Z., Andino R., Cameron C.E. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 2000;6:1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- de Wilde A.H., Zevenhoven-Dobbe J.C., van der Meer Y., Thiel V., Narayanan K., Makino S., Snijder E.J., van Hemert M.J. Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 2011;92:2542–2548. doi: 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning J., Baillie J.K., Cao B., Hayden F.G. Antiviral combinations for severe influenza. Lancet Infect. Dis. 2014;14:1259–1270. doi: 10.1016/S1473-3099(14)70821-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir. Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Takahashi K., Fukuda Y., Kuno M., Kamiyama T., Kozaki K., Nomura N., Egawa H., Minami S., Watanabe Y., Narita H., Shiraki K. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 2002;46:977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haviernik J., Štefánik M., Fojtíková M., Kali S., Tordo N., Rudolf I., Hubálek Z., Eyer L., Ruzek D. Arbidol (umifenovir): a broad-spectrum antiviral drug that inhibits medically important arthropod-borne flaviviruses. Viruses. 2018;10:184. doi: 10.3390/v10040184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond. Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianevski A., He L., Aittokallio T., Tang J. SynergyFinder: a web application for analyzing drug combination dose–response matrix data. Bioinformatics. 2017;33:2413–2415. doi: 10.1093/bioinformatics/btx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Fang X., Tian L., Chen X., Chung U., Wang K., Li D., Dai X., Zhu Q., Xu F., Shen L., Wang B., Yao L., Peng P. The effect of Arbidol Hydrochloride on reducing mortality of Covid-19 patients: a retrospective study of real world date from three hospitals in Wuhan. medRxiv. 2020;2020 doi: 10.1101/2020.04.11.20056523. 04.11.20056523. [DOI] [Google Scholar]
- Liu Y., Yan L.-M., Wan L., Xiang T.-X., Le A., Liu J.-M., Peiris M., Poon L.L.M., Zhang W. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect. Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magagnoli J., Narendran S., Pereira F., Cummings T., Hardin J.W., Sutton S.S., Ambati J. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. 2020;2020 doi: 10.1101/2020.04.16.20065920. 04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangu S., Dodd L.E., Davey R.T., Tshiani Mbaya O., Proschan M., Mukadi D., Lusakibanza Manzo M., Nzolo D., Tshomba Oloma A., Ibanda A., Ali R., Coulibaly S., Levine A.C., Grais R., Diaz J., Lane H.C., Muyembe-Tamfum J.-J., the Palm Writing Group A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorno A., Padey B., Julien T., Trouillet-Assant S., Traversier A., Errazuriz-Cerda E., Fouret J., Dubois J., Gaymard A., Lescure F.-X., Dulière V., Brun P., Constant S., Poissy J., Lina B., Yazdanpanah Y., Terrier O., Rosa-Calatrava M. Characterization and treatment of SARS-CoV-2 in nasal and bronchial human airway epithelia. bioRxiv. 2020;2020 doi: 10.1101/2020.03.31.017889. 03.31.017889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzorno A., Terrier O., Nicolas de Lamballerie C., Julien T., Padey B., Traversier A., Roche M., Hamelin M.-E., Rhéaume C., Croze S., Escuret V., Poissy J., Lina B., Legras-Lachuer C., Textoris J., Boivin G., Rosa-Calatrava M. Repurposing of drugs as novel influenza inhibitors from clinical gene expression infection signatures. Front. Immunol. 2019;10:60. doi: 10.3389/fimmu.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J., Hall P., Acuna-Retamar M., Ye C., Wathelet M.G., Ebihara H., Feldmann H., Hjelle B. New world hantaviruses activate IFNλ production in type I IFN-deficient Vero E6 cells. PloS One. 2010;5 doi: 10.1371/journal.pone.0011159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell R.W., Huffman J.H., Khare Lois G.P., Allen B., Witkowski Roland J.T., Robins K. Broad-spectrum antiviral activity of virazole: 1-f8- D-ribofuranosyl- 1,2,4-triazole- 3-carboxamide. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- Solidarity Clinical trial for COVID-19 treatments. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments [WWW Document]. accessed 4.27.20.
- Varghese F.S., Kaukinen P., Gläsker S., Bespalov M., Hanski L., Wennerberg K., Kümmerer B.M., Ahola T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir. Res. 2016;126:117–124. doi: 10.1016/j.antiviral.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Varghese F.S., Thaa B., Amrun S.N., Simarmata D., Rausalu K., Nyman T.A., Merits A., McInerney G.M., Ng L.F.P., Ahola T. The antiviral alkaloid berberine reduces chikungunya virus-induced mitogen-activated protein kinase signaling. J. Virol. 2016;90:9743–9757. doi: 10.1128/JVI.01382-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.-Q., Fu Y.-J., Wu S., Qin H.-Q., Zhen X., Song B.-M., Weng Y.-S., Wang P.-C., Chen X.-Y., Jiang Z.-Y. Anti-influenza activity of berberine improves prognosis by reducing viral replication in mice. Phytother. Res. PTR. 2018;32:2560–2567. doi: 10.1002/ptr.6196. [DOI] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., Chen W., Wang Q., Zhang D., Liu Y., Gong R., Ma Z., Lu S., Xiao Y., Gu Y., Zhang J., Yao H., Xu K., Lu X., Wei G., Zhou J., Fang Q., Cai H., Qiu Y., Sheng J., Chen Y., Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]