Abstract
Introduction
In the current time where we face a COVID-19 pandemic, there is no vaccine or effective treatment at this time. Therefore, the prevention of COVID-19 and the rapid diagnosis of infected patients is crucial.
Method
We searched all relevant literature published up to February 28, 2020. We used Random-effect models to analyze the appropriateness of the pooled results.
Result
Eighty studies were included in the meta-analysis, including 61,742 patients with confirmed COVID-19 infection. 62.5% (95% CI 54.5–79, p < 0.001) of patients had a history of recent travel endemic area or contact with them. The most common symptoms among COVID-19 infected patients were fever 87% (95% CI 73–93, p < 0.001), and cough 68% (95% CI 55.5–74, p < 0.001)), respectively. The laboratory analysis showed that thrombocytosis was present in 61% (95% CI 41–78, p < 0.001) CRP was elevated in 79% (95% CI 65–91, p < 0.001), and lymphopenia in 57.5% (95% CI 42–79, p < 0.001).
The most common radiographic signs were bilateral involvement in 81% (95% CI 62.5–87, p < 0.001), consolidation in 73.5% (95% CI 50.5–91, p < 0.001), and ground-glass opacity 73.5% (95% CI 40–90, p < 0.001) of patients. Case fatality rate (CFR) in <15 years old was 0.6%, in >50 years old was 39.5%, and in all range group was 6%.
Conclusions
Fever and cough are the most common symptoms of COVID-19 infection in the literature published to date. Thombocytosis, lymphopenia, and increased CRP were common lab findings although most patients included in the overall analysis did not have laboratory values reported. Among Chinese patients with COVID-19, rates of hospitalization, critical condition, and hospitalization were high in this study, but these findings may be biased by reporting only confirmed cases.
Keywords: COVID-19, SARS-CoV-2, Coronavirus, Severe acute respiratory syndrome coronavirus, meta-Analysis
Highlights
-
•
Eighty studies (61,742 patients) with confirmed COVID-19 infection included in this study.
-
•
Bilateral involvement (81%), consolidation (73.5%), and ground-glass opacity (73.5%) was most common radiographic signs.
-
•
Case fatality rate (CFR) in <15 years old age group was 0.6%, in >50 years old was 39.5%, and in all range group was 6%.
1. Introduction
In December 2019, the new COVID-19 coronavirus was recognized as a cause of respiratory illness. The first reports of pneumonia were from people who worked or lived in the Huanan seafood wholesale market in Wuhan, China raising concerns about a zoonotic viral infection [1,2]. Phylogenetic analysis showed that the COVID-19 belong to the beta-coronavirus [1]. Epidemiological studies have shown that the virus is spread relatively easily and can be transmitted by aerosol, droplets, and through infected surfaces [3]. The COVID-19 has now spread to more than 50 countries from December 2019 to February 2020 [4]. Most symptoms are non-specific in patients with respiratory disease. According to the latest WHO report, out of 83,652 confirmed cases of COVID-19 worldwide, 2791 deaths occurred in China and 67 deaths is recorded in other countries [4].
Thus far, 6 coronaviruses that are able to infect humans have been identified, coronavirus infections are typically asymptomatic or associated with mild respiratory symptoms [1]. The first coronavirus to cause severe disease in humans was the Severe Acute Respiratory Syndrome virus (SARS), which was appeared in the Guangdong province of southern China in 2002, there were 8098 reported case and 774 deaths [5]. In Saudi Arabia in 2012, the Middle East respiratory syndrome coronavirus (MERS-CoV), which was transmitted from the camels to humans, caused 2458 infections with 848 deaths [6].
Clinical studies have shown that COVID-19 can rapidly cause pulmonary damage and severe respiratory symptoms [3]. There is no vaccine or targeted treatment currently available for COVID-19 infection. Treatment is largely supportive although multiple experimental antiviral medications are being evaluated [7,8]. Thus, prevention and rapid diagnosis of infected patients is crucial. To date, the published clinical studies are quite small and give variable findings. With this in mind, here we evaluate the clinical features and laboratory findings using a large sample size of COVID-19 infected patients in order to assist in its understanding, prevention and treatment.
2. Methods
2.1. Search strategy
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) guidelines [9]. We searched all studies published up to February 28, 2020 from the following databases: Embase, Scopus, PubMed, Web of Science and the Cochrane library. Search medical subject headings (MeSH) terms used were: “COVID-19”, “Coronavirus”, “severe acute respiratory syndrome coronavirus”, and all their synonyms like “Wuhan Coronavirus”, “SARS-CoV-2”, and “COVID-19”. Moreover, we searched for unpublished and grey literature with Google scholar, Center for Disease Controls (CDC) and WHO databases. We also examined references of included articles to find additional relevant studies. There was no language restriction and all included studies are written in English or Chinese languages, the latter were translated by https://translate.google.com/. Additional search strategy details are provided in Table S1 (supplementary material) [10].
2.2. Study selection
Duplicate studies were removed using EndNote X7 (Thomson Reuters, New York, NY, USA). Records were initially screened by title and abstract by independently two authors (AP, SG). The full-text of potentially eligible records was retrieved and examined. Any discrepancies were resolved by consensus.
2.3. Inclusion criteria
Studies had to fulfil the following pre-determined criteria to be eligible for inclusion in our meta-analysis. Studies were included if they reported the number of confirmed cases of patients with demographic data, [AND] [OR] clinical data, [AND] [OR] laboratory data, [AND] [OR] risk factor data. Confirmed patients were defined as any patient with positive nucleic acid testing (most of the studies with Real-Time PCR) or those meeting CDC and WHO criteria at the time of their publication.
2.4. Exclusion criteria
Studies were excluded if they did not report number of confirmed cases, were letters to the editor or individual case reports or reviews. News reports were also excluded.
2.5. Data extraction
All included publications were published in 2020 and all patients are from China. The following items were extracted from each article: first author, Center and study location in China, sample collection time period, patient follow-up time, reference standard for infection confirmation, number of confirmed cases, and all demographic, clinical, laboratory data, and risk factor data. Two of our authors (AP and SG) independently extracted data and differences were resolved by consensus.
2.6. Quality assessment
Quality assessments of studies were performed by two reviewers independently according to the Critical Appraisal Checklist recommended by the Joanna Briggs Institute [11], and disagreements were resolved by consensus. The checklist is composed of nine questions that reviewers addressed for each study. The ‘Yes’ answer for each question received one point. Thus, final scores for each study could range from zero to nine (Table S2 in Supplementary Material).
2.7. Analysis
Data cleaning and preparation was done in Microsoft Excel 2010 (Microsoft©, Redmond, WA, USA) and further analyses were carried out via Comprehensive Meta-Analysis Software Version 2.0 (Biostat, Englewood, NJ). Determination of heterogeneity among the studies was undertaken using the chi-squared test (Cochran's Q) to assess the appropriateness of pooling data. We used Random effect model (M − H heterogeneity) for pooled results [12]. P values reflect study heterogeneity with <0.05 being significant. We also used the Begg's and Egger's tests based on the symmetry assumption to detect publication bias.
3. Results
3.1. Characteristics of included studies
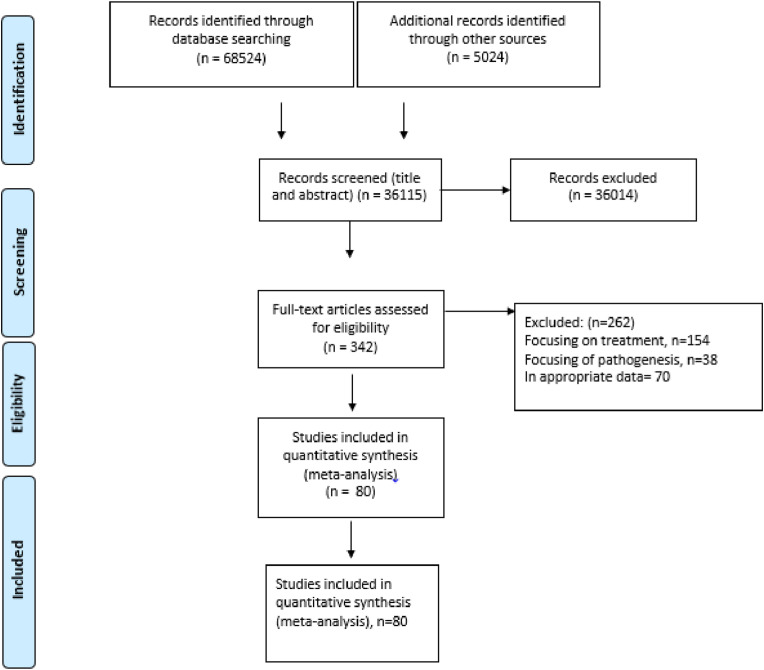
The process of study selection is displayed in Fig. 1 . A total of 36,115 reports were screened for the analysis of patients with COVID-19, 36,014 were excluded after title and abstract screening and the full text of 342 reports were reviewed in full text. We excluded studies that did not report sufficient data and finally 80 studies met the inclusion criteria (Fig. 1). Characteristics of the selected articles are summarized in Table 1 . Of the 80 studies that were included in the analysis, 79 studies were in English and the one of them was in the language of Chinese [13]. All studies were retrospective, published in 2020, and all patients were from China.
Fig. 1.
Flow Diagram of Literature Search and Study Selection (PRISMA flow chart).
Table 1.
Characterization of Included Studies with total 61, 742 COVID-19 Confirmed Patients. All Studies are Retrospective, from China, and Published in 2020.
| First Author | Sampling Center | Sample collection time | Patient follow up (days) | N Confirmed Patients | Mean age in years (IQR) | N sex (male) | Reference standard |
|---|---|---|---|---|---|---|---|
| Nanshan Chen [14] | Wuhan Jinyintan Hospital | Jan 1 to Jan 20, 2020 | 5–24 | 99 | 55·5 | 67 | RT-PCR |
| (21–82) | |||||||
| Kaiyuan Sun [30] | Multicenter | Jan 20- Jan 29, 2020 | 42 | 288 | 49 | 62.3 | CDC guideline |
| (2–89) | |||||||
| Jie Li [31] | Dazhou Central Hospital | 22 January- February 10, 2020 | 1–21 | 17 | 45.1 | 9 | RT-PCR |
| (32–65) | |||||||
| Dawei Wang [15] | Zhongnan Hospital of Wuhan | January 1-January 28, 2020 | 6–34 | 138 | 56 | 75 | RT-PCR |
| (42–68) | |||||||
| Chaolin Huang [16] | Jin Yintan Hospital (Wuhan) | Dec 31, 2019-UN | NA | 41 | 49 | 30 | RT-PCR |
| (41–58) | |||||||
| Weijie Guan [17] | Multicenter | NA | NA | 1099 | 47 | 640 | RT-PCR |
| (35–58) | |||||||
| Yang Yang [32] | NA | NA | 51 days | 4021 | 49 | 2211 | NA |
| Lei Chen (Chinese) [13] | Tongji hospital in Wuhan | January 14–29, 2020 | 15 day | 29 | 56 | 21 | RT-PCR |
| (26–79) | |||||||
| Adam Bernheim [3] | Multicenter | January 18-February 2, 2020 | 12 days | 121 | 45 | 61 | RT-PCR & CT scan |
| (18–80) | |||||||
| Feng Pan [33] | Union Hospital | 12 Jan-6 Fen 2020 | NA | 21 | 40 | 15 | RT-PCR |
| (25–63) | |||||||
| jin Zhang [18] | No.7 hospital of Wuhan | Jan 16th to Feb 3rd, 2020 | NA | 140 | 57 | 71 | RT-PCR |
| (25–87) | |||||||
| Yichun Cheng [19] | Tongji hospital in Wuhan | January 28-February 11, 2020 | 10 (7–13) | 710 | 63 | 374 | RT-PCR |
| (51–71) | |||||||
| Ming-Yen [34] | Hong Kong-Shenzhen Hospital | NA | NA | 21 | 56 | 13 | RT-PCR |
| (37–65) | |||||||
| Sijia Tian [35] | Beijing Emergency Medical Service | Jan 20 to Feb 10, 2020 | Feb.10 20 | 262 | 47.5 | 127 | RT-PCR |
| (1–94) | |||||||
| Qun Li [20] | NA | NA | NA | 425 | 15–89 | 240 | WHO guideline |
| (26–82) | |||||||
| De Chang [36] | 3 hospitals in Beijing | January 16- January 29, 2020 | Feb.4 2020 |
13 | 34 | 10 | NA |
| (34–48) | |||||||
| Xiao-Wei Xu [21] | Zhejiang province | 10 January −26 January 2020 | 10 days | 62 | 41 | 36 | WHO guideline |
| (32–52) | |||||||
| Fengxiang Song [22] | Center for Disease Control, Shanghai | January 20- January 27, 2020 | NA | 51 | 49 | 25 | CT scan & nucleic acid test |
| (16–76) | |||||||
| Michael Chung [37] | Multicenter | January 18–27, 2020 | NA | 21 | 51 | 13 | CT scan, NA |
| (29–77) | |||||||
| Zunyou Wu (CDC) [38] | Multicenter | through February 11, 2020 | 15 days | 44,672 | 30–79 | 22,981 | nucleic acid test result |
| Bicheng Zhang [39] | hospitalized death | January 11, 2020 to February 10 | 30 day | 82 | 72.5 | 54 | rt-pcr |
| Bing-Liang Lin [40], | Multicenter | January 20 to February 19, | 29 day | 91 | 50 | 52 | rt-pcr |
|---|---|---|---|---|---|---|---|
| Bo Hu [41] | Multicenter | January 8 to February 9 | 20 day | 50 | 62 | 34 | rt-pcr |
| Chuansheng Zheng [42] | Union Hospital, Wuhan | 16 Jan 2020 to 15 Feb, | 30 day | 64 | 35 | 23 | rtpcr |
| Lin Fu [43] | Union Hospital | January 1 to January 30 | 30 day | 200 | 99 | rtpcr | |
| Fei Zhou [44] | Multicenter | 191 | 56 | 119 | rtpcr | ||
| Guo-Qing Qian [45] | Multicenter | as of 11 February | NA | 91 | 50 | 37 | rt-pcr and clinical |
| Guqin Zhang [46] | Zhongnan Hospital | anuary 2 to February 10, | NA | 221 | 55 | 108 | rtpcr |
| Qiannan Guo [47] | Tongji Hospital | UN | UN | 11 | 57.55 | 9 | rtpcr |
| Hang Fu [48] | Chengdu, hospital | Jan 1 to Feb 20, | NA | 52 | 44.5 | rtpcr | |
| Heshui Shi [49] | Union Hospital | Dec 20, 2019, and Jan 23 | NA | 81 | 49·5 | 42 | rtpcr |
| Huijun Chen [50] | Multicenter | 20-Jan | NA | 9 | 26–40 | rtpcr | |
| Jian Wu [51] | Multicenter | 22-Jan | NA | 80 | 46.1 | 39 | rtpcr |
| Jianlei Cao [52] | Multicenter | 3-Jan | NA | 102 | rtpcr | ||
| Jie Liu [53] | Union Hospital | 16 Jan 2020 to 15 Feb | NA | 64 | 35 | 23 | rtpcr |
| Jing Yuan [54] | Shenzhen hospital | Jan 23 23rd 2020 to Feb 21 21st | NA | 25 | 28 | 8 | rtpcr |
| Jinjun Zhang [55] | Multicenter | Jan 20 to Feb 20, | 30 DAY | 478 | 46.9 | 238 | rtpcr |
| Jin-Wei Ai [56] | Hubei | UN | UN | 102 | 50.38 | 52 | rtpcr |
| Jiong Wu [57] | Yancheng City | 22-Jan | NA | 80 | 44 | 42 | rtpcr |
| Jun Chen [58] | Shanghai | Jan 20 to Feb 6, | 14 DAY | 249 | 51 | 126 | rtpcr |
| Kaiyuan Sun [59] | Multicenter | Jan 13 and Jan 31 | NA | 507 | 46 | 281 | rtpcr |
| Kaiyue Diao [60] | Wuhan | January 17th to February 5th | 30 DAY | 6 | 47.5 | 3 | rtpcr |
| Kenneth W. Tsang [61] | Hong Kong | February 22, 2003, and March 22 | 30 DAY | 10 | 52.5 | 5 | rtpcr |
| Kui Liu [62] | Multicenter | December 30, 2019 to January 24 | 24 DAY | 137 | 57 | 61 | rtpcr |
| L. Zhang [63] | Multicenter | Jan 13, 2020, to Feb 26 | 40 DAY | 28 | 65 | 17 | rtpcr |
| Lei Liu [64] | Hospital in Chongqing | January 20 to February 3, | 14 DAY | 51 | 45 | 32 | rtpcr |
| lei shu [65] | Wuhan Stadium Cabin Hospital | Feb 13 to Feb 29, | 16 DAY | 545 | 50 | 264 | rtpcr |
| Lei Wang [66] | Zhengzhou University | Jan 21 to Feb 05, 2020, | 14 DAY | 18 | 39 | 10 | rtpcr |
| Li Yan [67] | Tongji Hospital | January 10th to February 18th | 18 DAY | 375 | 58.83 | 220 | rtpcr |
| Li-Li Ren [68] | wuhan | December 18 to December 29, 2019 | 12 DAY | 5 | UN | 3 | rtpcr |
| Lin Fu [69] | Union Hospital | January 1 to January 30 | 30 DAY | 200 | UN | 99 | rtpcr |
| Xiang Li [70] | Multicenter | 24-Feb-20 | NA | 292 | 47·83 | 134 | rtpcr |
| Matt Arentz [71] | Evergreen hospital | February 20, 2020, and March 5 | 15 DAY | 21 | 70 | 11 | rtpcr |
| Naibin Yang [72] | Zhejiang | 25th January to 28th February | NA | 10 | 33 | 3 | rtpcr |
| Ping Wu [73] | Yichang Central People's Hospital | February 9 to 15 | NA | 38 | 65.8 | 25 | rtpcr |
| Qifang Bi [74] | Shenzhen, | January 14 to February 12 | 25 DAY | 391 | 45 | 187 | rtpcr |
| Qiurong Ruan [75] | Multicenter | 150 | rtpcr | ||||
| Tao Yao [76] | Renmin hospital | NA | 55 | 70.7 | 37 | rtpcr | |
| Wen Zhao [77] | Beijing YouAn Hospital | 21st Jan and 8th February | 14 DAY | 77 | 52 | 34 | rtpcr |
| Yani Kuang [78] | Zhejiang | January 17, | NA | 143 | 47 | 77 | rtpcr |
| Yani Kuang [79] | Zhejiang, | 1-Jan | NA | 944 | 47.4 | 476 | rtpcr |
| Wan Chen [80] | Hospital of Guangxi Zhuang | 15-Jan | NA | 85 | 41 | 34 | rtpcr |
| Xiaomin Luo [81] | Renmin hospital | Jan 30 to Feb 25 | 25 DAY | 403 | 56 | 193 | rtpcr |
| Xiaoyu Han [82] | Union Hospital, | December 20 th and February 2 | 12 DAY | 17 | 40 | 5 | rtpcr |
| Xun Li [83] | wuhan | As of February 13 | NA | 25 | 71.48 | 10 | rtpcr |
| Yan Deng [84] | wuhan | January 1, | NA | 225 | 54 | 124 | rtpcr |
| Yang Wu [85] | wuhan | 13-Jan | NA | 14 | 59 | 5 | ct and rtpcr |
| Yangli Liu [86] | Guangdong, | December 8, 2019, | NA | 13 | rtpcr | ||
| Yanli Liu [87] | Hospital of Wuhan | January 2 to February | NA | 109 | 55 | 59 | rtpcr |
| Ying Huang [88] | wuhan | Jan 21 and Feb 10 | 20 DAY | 36 | 69.22 | 25 | rtpcr |
| Ying Wen [89] | Multicenter | NA | 417 | 45.4 | 197 | rtpcr | |
| Yingjie Wu [90] | wuhan | 12-Jan | NA | 402 | 198 | rtpcr | |
| Yuhui Wang [91] | wuhan | January 16 to February 17 | 30 DAY | 90 | 45 | 33 | rtpcr |
| Zhibing Lu [92] | Multicenter | January 1 to February15 | 15 DAY | 123 | 57.78 | 61 | rtpcr |
| Zhiliang Hu [93] | Multicenter | from Jan 28 to Feb 9, 2020 | 19 DAY | 24 | rtpcr | ||
| Ping Yu [94] | Shanghai | 7-Jan-20 | NA | 4 | 74.25 | ct scan | |
| Ali Aminian [95] | tehran | 9-Feb | NA | 4 | 63.5 | ct scan | |
| Hui Yu [96] | wuhan | Feb. 1 to Mar. 3, | NA | 105 | 1–16 year | 64 | ct scan |
| Matthieu Million [97] | France, multi center | March 3rd to March 31s | NA | 1061 | 43.6 14–95 |
492 | Ct scan/rt pcr |
| Bai shaoli | Gansu Prov center | 22-january | NA | 8 | 53.71 | 4 | Rt pcr |
NA = not known, RT-PCR= Real Time Polymerase Chain Reaction, CDC= Centers for Disease Control and Prevention, WHO= World Health Organization, CT scan = CT scan of chest, N = number, IQR = interquartile range.
3.2. Quality assessment
Quality assessment of included studies were performed based on the Critical Appraisal Checklist and the final scores for quality of included studies are represented in Table S2 (in supplementary material). In brief, studies by Chen [14], Wang [15], Huang [16], Guan [17], Zhang [18], Cheng [19], Li [20], Wei Xu [21], and Song [22] had the highest quality of the studies available in the purpose of this study.
3.3. Demographics, baseline characteristics, and clinical characterization
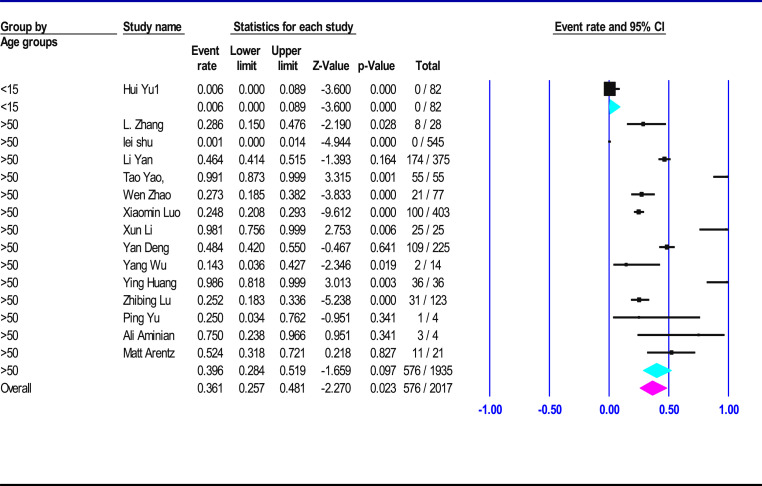
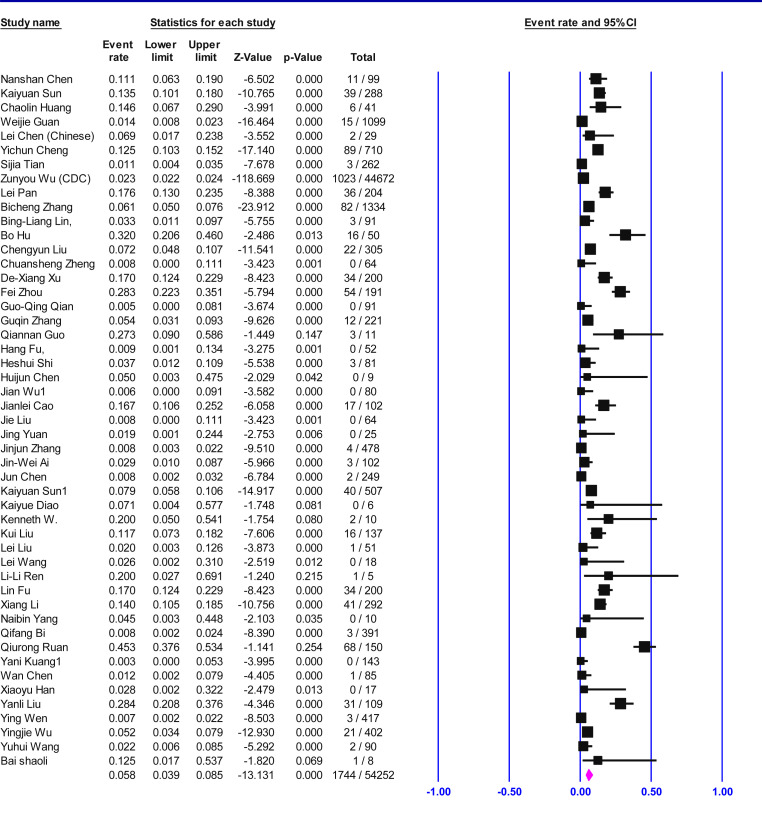
Table 2 shows that 61, 742 confirmed patients with COVID-19 infection were included in the Meta-analysis, of which 55% (95% CI 50–57.5, p < 0.001) were male. The most of the patients had fever 87% (95% CI 73–93, p < 0.001) and cough 68% (95% CI 55.5–74, p < 0.001). A much smaller proportion of patients had sore throat 14% (95% CI 7.8–17, p 0.06), headache 14% (95% CI 8.3–18, p < 0.001), diarrhea 8% (95% CI 4.6–11.4, p < 0.001), rhinorrhea 7% (95% CI 3–12, p 0.43) or nausea and vomiting 6.5% (95% CI 2.7–13, p < 0.001). Most patients required hospitalization 81% (95% CI 68–94, p < 0.001), 25.6% (95% CI 6.7–48, p < 0.001) were deemed to be in critical condition and the mortality rate was 6% (95% CI 4–8.5, p < 0.001) between all infected patients. Table 3 shows that case fatality rate (CFR) in <15 years old age groups was 0.6% (95% CI 0–0.9, p 1), >50 years old was 39.5% (95% CI 28.5–52, p < 0.001) (Fig. 2 ), all range group was 6% (95% CI 4–8.5, p < 0.001) (Fig. 3 ).
Table 2.
Demographics, baseline characteristics, and clinical outcomes of patients with confirmed COVID-19.
| Clinical presentation* | Confidence interval 95% | Heterogeneity test, I2 (%)** | Heterogeneity test, P Value** | Number of Studies | |
|---|---|---|---|---|---|
| Age, years | 48 (mean) | 43–50 | 98 | <0.001 | 23 |
| Sex (Male) | 55 (%) | 50–57.5 | 88.4 | <0.001 | 24 |
| Fever | 87 (%) | 73–93 | 98 | <0.001 | 18 |
| Cough | 68 (%) | 55.5–74 | 86 | <0.001 | 18 |
| Fatigue | 39 (%) | 29–52.5 | 93 | <0.001 | 14 |
| Sputum production/Expectoration | 31 (%) | 19–39 | 92 | <0.001 | 9 |
| Myalgia | 24 (%) | 14–43 | 92 | <0.001 | 9 |
| Dyspnea | 24 (%) | 12.6–32 | 92 | <0.001 | 11 |
| Sore throat | 14 (%) | 7.8–17 | 52 | 0.06 | 9 |
| Headache | 14 (%) | 8.3–18 | 77 | <0.001 | 16 |
| Diarrhea | 8 (%) | 4.6–11.4 | 70 | <0.001 | 18 |
| Rhinorrhea | 7 (%) | 3–12 | 0 | 0.43 | 6 |
| Nausea and vomiting | 6.5 (%) | 2.7–13 | 84 | <0.001 | 6 |
| Outcome | |||||
| Hospitalized | 81 (%) | 68–94 | 95 | <0.001 | 7 |
| Critical condition/ICU | 25.6 (%) | 6.7–48 | 99 | <0.001 | 8 |
| CFR (all age group) | 6 (%) | 4–8.5 | 89.6 | <0.001 | 49 |
*Age is an exception, presented in mean age in years. ** Greater than 50% is considered high heterogeneity, less than 50% is considered low heterogeneity. A low p value (<0.05) is consistent with high heterogeneity. Case fatality rate (CFR).
Table 3.
Meta-analysis on clinical presentation of case fatality rate (CFR) in different age groups of confirmed COVID-19 cases.
| Age groups (year) | CFR (%) | Confidence Interval |
patients |
Heterogeneity test* |
|||
|---|---|---|---|---|---|---|---|
| Lower limit (%) | Upper limit (%) | Number Studies | Included patients | I-squared | P-value | ||
| All Range | 6 | 4 | 8.5 | 49 | 54,252 | 89.6 | <0.001 |
| >50 | 39.5 | 28.5 | 52 | 14 | 1935 | 97 | <0.001 |
| <15 | 0.6 | 0 | 0.9 | 1 | 82 | 0 | 1 |
Case fatality rate (CFR), * Greater than 50% is considered high heterogeneity, less than 50% is considered low heterogeneity. A low p value.
Fig. 2.
Forest plot of the meta-analysis on clinical presentation of case fatality rate (CFR) in different age groups of confirmed COVID-19 cases.
Fig. 3.
Forest plot of the meta-analysis on clinical presentation of case fatality rate (CFR) in all age groups of confirmed COVID-19 cases.
3.4. Clinical characteristics, and Comorbid conditions of patients infected with COVID-19
The majority of patients, 62.5% (95% CI 54.5–79, p < 0.001), had a history of recent travel endemic area or contact with them. A significant minority of patients (39.5%, 95% CI 20–56, p < 0.001) had a history of chronic diseases and 26.5% (95% CI 9.6–49, p < 0.001) had exposure at the seafood market(s) (Table 4 ).
Table 4.
Clinical Characteristics and Comorbid Conditions of patients with confirmed COVID-19.
| Risk Factor | Patients with risk factor (%) | Confidence interval 95% | Heterogeneity test, I2 (%)* | Heterogeneity test, P Value* | Number of Studies reporting |
|---|---|---|---|---|---|
| History of recent travel endemic area or contact with them | 62.5 | 54.5–79 | 96 | <0.001 | 11 |
| Chronic diseases | 39.5 | 20–56 | 95 | <0.001 | 6 |
| Exposure to seafood market | 26.5 | 9.6–49 | 95 | <0.001 | 8 |
| Sick contacts with respiratory illness | 18 | 4.5–39.6 | 97 | <0.001 | 7 |
| Hypertension | 18 | 8.5–24.6 | 97.5 | <0.001 | 17 |
| ARDS | 17.5 | 4–26.7 | 95.7 | <0.001 | 8 |
| Diabetes | 9 | 4–15 | 96 | <0.001 | 11 |
| Current smoker | 8.2 | 3.7–15 | 69 | 0.01 | 8 |
| Chronic liver disease | 7 | 3.8–8.4 | 6 | 0.38 | 12 |
| Digestive system disease | 4.5 | 2.5–4.9 | 95 | <0.001 | 8 |
| Health care worker | 16 | 2–4.6 | 79 | 0.008 | 12 |
| Past smoker | 4 | 1.1–7.5 | 80 | 0.02 | 6 |
| Cardiovascular and cerebrovascular diseases | 3.3 | 2.2–2.5 | 98 | <0.001 | 14 |
| Chronic respiratory disease | 3.2 | 0.6–8 | 93 | <0.001 | 7 |
| Cancer | 2.7 | 0.4–7.4 | 96.3 | <0.001 | 9 |
ARDS = acute respiratory distress syndrome * Greater than 50% is considered high heterogeneity, less than 50% is considered low heterogeneity. A low p value (<0.05) is consistent with high heterogeneity.
3.5. Laboratory findings of patients infected with COVID-19
The laboratory analysis and features showed that the most infected patients had increased platelets 61% (95% CI 41–78, p < 0.001), and CRP 79% (95% CI 65–91, p < 0.001), while others showed decreased lymphocytes, 57.5% (95% CI 42–79, p < 0.001) (Table 5 ).
Table 5.
Laboratory features for confirmed patients with COVID-19.
| Confidence interval 95% | normal range | Total Patient Number | Number of Studies | ||
|---|---|---|---|---|---|
| Leucocytes (WBCs) (mean) | 6.2 ( × 10⁹ per L) | 5.3–6.9 | 3.5–9.5 | 2961 | 17 |
| Increaseda | 18.3 (%) | 6.4–25.6 | |||
| Decreased | 28 (%) | 21–33 | |||
| Neutrophils (mean) | 4.6 ( × 10⁹ per L) | 3.1–5.1 | 1.8–6.3 | 1212 | 12 |
| Lymphocytes (mean) | 0.94 ( × 10⁹ per L) | 0.9–1.06 | 1.1–3.2 | 3161 | 18 |
| Decreased | 57.5 (%) | 42–79 | |||
| Platelets (mean) | 196.5 ( × 10⁹ per L) | 167–205 | 125–350 | 2900 | 15 |
| Decreased | 13 (%) | 5–30 | |||
| Increased | 61 (%) | 41–78 | |||
| CRPa(mean) | 32 (mg/L) | 19.7–46.5 | 0–0.5 | 880 | 10 |
| Increased | 79 (%) | 65–91 | |||
| Hemoglobin (mean) | 113 (g/L) | 106–132 | 130–175 | 2862 | 12 |
| ESR**(mean) | 44 (mm/h) | 46–57 | 0–15 | 320 | 4 |
| Albumin (mean) | 36.8 (g/L) | 24.5–46 | 40–55 | 420 | 5 |
| Decreased | 81% | 72–87 | |||
| Interleukin-6 (mean) | 8.1 (pg/mL) | 6.8–8.6 | 0.0–7 | 509 | 6 |
| Increased | 56% | 42–61 | |||
| LDH*** (mean) | 286 | 268–294 | 120–250 | 2383 | 12 |
| Increased | 69.3 (%) | 58–83 |
CRP= C Reaction Protein, ESR = Erythrocyte sedimentation rate. WBCs= White blood cells.
Increased or Decreased refers to values above or below the normal range.
3.6. Chest X-ray and CT scan findings in patients infected with COVID-19
Analysis showed that the most abnormality which finding with Chest X-ray and CT are bilateral involvement of chest radiography 81% (95% CI 62.5–87, p < 0.001), consolidation 73.5% (95% CI 50.5–91, p < 0.001), and ground-glass opacity 73.5% (95% CI 40–90, p < 0.001) (Table 6 ).
Table 6.
Chest X-ray and CT scan Findings in Patients with Confirmed COVID-19.
| Abnormality (%) | Confidence interval 95% | Heterogeneity test, I2 (%)a | Heterogeneity test, P Valuea | Number of Studies | |
|---|---|---|---|---|---|
| Bilateral involvement of chest radiography | 81 | 62.5–87 | 93 | <0.001 | 18 |
| Consolidation | 73.5 | 50.5–91 | 89 | <0.001 | 9 |
| Ground-glass opacity | 73.5 | 40–90 | 97 | <0.001 | 16 |
| Unilateral involvement of chest radiography | 18.5 | 8.5–29.5 | 94 | <0.001 | 9 |
Greater than 50% is considered high heterogeneity, less than 50% is considered low heterogeneity. A low p value (<.05) is consistent with high heterogeneity. CT scan = CT scan.
4. Discussion
COVID-19 belongs to the Coronaviridae family and is the newest serious zoonotic virus after the related viruses SARS and MERS [23,24]. Prior to 2002, coronaviruses were associated with mild respiratory illness, but with the emergence of SARS in 2002, MERS in 2012, and now in late 2019, COVID-19, establishes that coronaviruses can be associated with severe respiratory disease. Genetic variation and phylogenetic analysis of these viruses show that the COVID-19 virus has 84% homology to other beta-coronaviruses, 96% sequence similarity at the whole genome level to a bat coronavirus and 79.5% similarity to the SARS virus [8,25]. These results suggest that bats are important coronavirus reservoirs.
A study by Adam Bernheim et al. showed that among 121 COVID-19 patients, fever, cough and sputum production were the most common clinical symptoms [3]. Our study found utilizing data from 52,251 patients with COVID-19 infection, that in additional to these, fatigue and myalgia (muscle soreness) were also common.
The large data set here finds that 81% of patients required hospitalization, 25.6% were found to be in critical condition and the mortality rate was 6% between all infected patients. The mortality rate is lower than some studies (for example, 11% in Nanshan et al. [14]), but still higher than many viral infections. It should be recognized that these numbers are bias due to the data set including publications related to screening practices (e.g. only those with symptoms being screened) increased the % value. The true mortality rate from COVID-19 is almost certainly much lower than that found in this study. As more data emerges from screening asymptomatic or mildly symptomatic individuals in China and around the world, the true mortality rate will be better understood. Additionally, at the time of submission of this manuscript only ~50% of reported infected patients had recovered (gisanddata.maps.arcgis.com). Lymphopenia, age, multilobular infiltration, smoking history, hypertension, and bacterial co-infection have been reported as mortality risk factors. Underlying cardiovascular disease (40%) and bilateral pneumonia (81%) were common among those who have died. Recent travel endemic area or contact with them, exposure to persons with respiratory symptoms, and seafood market exposures were common amongst those contracting COVID-19. Among 2361 COVID-19 patients with laboratory data available, leukocytosis was found in 18.3% and leukopenia in 28% with lymphocytopenia in 57.5%. Among 2200 patients, thrombocytosis occurred in 61% and in a smaller sample (n = 290) CRP was increased in 79%.
A study by Yu Zhao et al. showed that ACE2 is a COVID-19 virus receptor and that it is normally expressed on pulmonary alveolar epithelial cells [26]. ACE2 activates the RAS cascade, which can lead to hypertension. The pathology in this pathway can also stimulate fibrogenesis, inflammation, cell hypertrophy, and cell proliferation [27,28]. ACE2 expression is increased in people with pulmonary ARDS and acute respiratory injury [29]. The data collected here shows that ARDS occurred in 17.5% of reported patients with COVID-19 infection.
4.1. Limitations
Several limitations of this study exist. Publication bias and study heterogeneity are unavoidable in this type of study, therefore it should be considered when interpreting the outcomes of the reports and our final data set. Further, this study likely overestimates disease severity due to lack of screening of asymptomatic or mildly symptomatic individuals and subsequent publication bias related to these factors. It is very likely that many infected persons have not been detected, thus falsely elevating the rates of hospitalization, critical condition, and mortality.
5. Conclusions
Fever and cough are the most common symptoms of COVID-19 infection in the literature published to date. Thombocytosis, lymphopenia, and increased CRP were common lab findings although most patients included in the overall analysis did not have laboratory values reported. The most common radiographic sign was bilateral involvement in and consolidation. Among Chinese patients with COVID-19, rates of hospitalization, critical condition, and hospitalization were high in this study, but these findings may be biased by reporting of only confirmed cases.
Declaration of competing interest
The authors have declared that no competing interests exist.
Acknowledgments
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.micpath.2020.104390.
Funding
Dr. Bahr receives funding from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health, K23 NS110470.
Author contributions
Conceived and designed the study: AP, SG, Comprehensive research: SG, AK, AP, Analyzed the data: A P, MAM, Wrote and revised the paper: AP, SG, BB, AK, RT, MAM, NB, DK, JPI, Participated in data analysis and manuscript editing: AP, SG, BB, AK, RT, MAM, NB, DK, JPI.
Ethical statement
The manuscript is a systematic review, so the ethical approval was not required for the study.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019 doi: 10.1056/NEJMoa2001017. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020 doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Who. Rational Use of Personal Protective Equipment for Coronavirus Disease 2019 (COVID-19) 2020, Feb 27.
- 5.Lam W., Zhong N., Tan W. Overview on SARS in Asia and the world. Respirology. 2003;8:S2–S5. doi: 10.1046/j.1440-1843.2003.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng J., Hassan S., Alagaili A.N., Alshukairi A.N., Amor N.M., Mukhtar N. Middle East respiratory syndrome coronavirus seropositivity in camel handlers and their families, Pakistan. Emerg. Infect. Dis. 2019;25:2307. doi: 10.3201/eid2512.191169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contini A. 2020. Virtual Screening of an FDA Approved Drugs Database on Two COVID-19 Coronavirus Proteins. [Google Scholar]
- 8.Zhavoronkov A., Aladinskiy V., Zhebrak A., Zagribelnyy B., Terentiev V., Bezrukov D.S. 2020. Potential COVID-2019 3C-like Protease Inhibitors Designed Using Generative Deep Learning Approaches. [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 10.Pormohammad A., Ghorbani S., Khatami A., Farzi R., Baradaran B., Turner D.L. Comparison of confirmed COVID‐19 with SARS and MERS cases‐Clinical characteristics, laboratory findings, radiographic signs and outcomes: a systematic review and meta‐analysis. Rev. Med. Virol. 2020 Jun 5 doi: 10.1002/rmv.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munn Z., Moola S., Riitano D., Lisy K. The development of a critical appraisal tool for use in systematic reviews: addressing questions of prevalence. Int. J. Health Pol. Manag. 2014;3:123. doi: 10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies. J. Natl. Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 13.Chen L., Liu H., Liu W., Liu J., Liu K., Shang J. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Chin. J. Tuberc. Respir. Dis. (Zhonghua Jiehe He Huxi Zazhi) 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 14.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan W-j, Ni Z-y, Hu Y., Liang W-h, Ou C-q, He J-x. MedRxiv; 2020. Clinical Characteristics of 2019 Novel Coronavirus Infection in China. [Google Scholar]
- 18.Zhang Jj, Dong X., Cao Y.Y., Yuan Yd, Yang Yb, Yan Yq. Allergy; 2020. Clinical Characteristics of 140 Patients Infected by SARS‐CoV‐2 in Wuhan, China. [DOI] [PubMed] [Google Scholar]
- 19.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L. medRxiv; 2020. Kidney Impairment Is Associated with In-Hospital Death of COVID-19 Patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X.-W., Wu X.-X., Jiang X.-G., Xu K.-J., Ying L.-J., Ma C.-L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. bmj. 2020:368. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. Emerging coronavirus 2019-nCoV pneumonia. Radiology. 2020:200274.
- 23.Ji W., Wang W., Zhao X., Zai J., Li X. Homologous recombination within the spike glycoprotein of the newly identified coronavirus may boost cross‐species transmission from snake to human. J. Med. Virol. 2020 doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun P., Qie S., Liu Z., Ren J., Xi J. 2020. Clinical Characteristics of 5732 Patients with 2019-nCoV Infection. Available at: SSRN 3539664. [Google Scholar]
- 25.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. BioRxiv. 2020 [Google Scholar]
- 26.Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. BioRxiv; 2020. Single-cell RNA Expression Profiling of ACE2, the Putative Receptor of Wuhan 2019-nCov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole-Jeffrey C.T., Liu M., Katovich M.J., Raizada M.K., Shenoy V. ACE2 and microbiota: emerging targets for cardiopulmonary disease therapy. J. Cardiovasc. Pharmacol. 2015;66:540. doi: 10.1097/FJC.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y.C. Molecular mechanism of vitamin D in the cardiovascular system. J. Invest. Med. 2011;59:868–871. doi: 10.231/JIM.0b013e31820ee448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai Y., Kuba K., Penninger J.M. Angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Cell. Mol. Life Sci. 2007;64:2006–2012. doi: 10.1007/s00018-007-6228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun K., Chen J., Viboud C. medRxiv; 2020. Early Epidemiological Analysis of the 2019-nCoV Outbreak Based on a Crowdsourced Data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J., Li S., Cai Y., Liu Q., Li X., Zeng Z. medRxiv; 2020. Epidemiological and Clinical Characteristics of 17 Hospitalized Patients with 2019 Novel Coronavirus Infections outside Wuhan, China. [Google Scholar]
- 32.Yang Y., Lu Q., Liu M., Wang Y., Zhang A., Jalali N. medRxiv; 2020. Epidemiological and Clinical Features of the 2019 Novel Coronavirus Outbreak in China. [Google Scholar]
- 33.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020:200370. [DOI] [PMC free article] [PubMed]
- 34.Ng M.-Y., Lee E.Y., Yang J., Yang F., Li X., Wang H. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiology: Cardiothorac. Imag. 2020;2 doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian S., Hu N., Lou J., Chen K., Kang X., Xiang Z. 2020. Characteristics of COVID-19 Infection in Beijing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang D., Lin M., Wei L., Xie L., Zhu G., Cruz C.S.D. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. Jama. 2020 doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 2020:200230. [DOI] [PMC free article] [PubMed]
- 38.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease Control and prevention. J. Am. Med. Assoc.. [DOI] [PubMed]
- 39.Zhang B., Zhou X., Qiu Y., Feng F., Feng J., Jia Y. medRxiv; 2020. Clinical Characteristics of 82 Death Cases with COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu B., Lei Z.-Y., Wu K.-L., He J.-R., Cao H.-J., Fu J. 2020. Epidemiological and Clinical Features of Imported and Local Patients with Coronavirus Disease 2019 (COVID-19) in Hainan. China. [Google Scholar]
- 41.Peng Z., Hu B., Wang D., Hu C., Hu M., Zhu F. 2020. Clinical Features of Critically Ill Patients with COVID-19 Infection in China. [Google Scholar]
- 42.Liu J., Ouyang L., Fu P., Cao Y., Yang D., Han X. 2020. Epidemiological, Clinical, Radiological Characteristics and Outcomes of Medical Staff with COVID-19 in Wuhan, China: A Single-Centered, Retrospective Case Series Analysis. [Google Scholar]
- 43.Fu L., Fei J., Xiang H.-X., Xiang Y., Tan Z.-X., Li M.-D. Analysis of death risk factors among 200 COVID-19 patients in Wuhan, China: a hospital-based case-cohort study. In: Fang-Fang, Hong-Yan Liu, Ling Zheng, Ying Li, Hui Zhao, De-Xiang Xu., editors. vol. 200. 2020. (Analysis of Death Risk Factors Among). [Google Scholar]
- 44.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian G.-Q., Yang N.-B., Ding F., Ma A.H.Y., Wang Z.-Y., Shen Y.-F. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM: Int. J. Med. 2020 doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J. MedRxiv; 2020. Clinical Features and Outcomes of 221 Patients with COVID-19 in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo Q., Nampoukime K-pB., Ma K., Wang H. 2020. Clinical Features of 11 COVID-19 Patients with History of Thoracotomy: A Descriptive Study in Wuhan, China. China (3/5/2020) [Google Scholar]
- 48.Fu H., Xu H., Zhang N., Xu H., Li Z., Chen H. medRxiv; 2020. Association between Clinical, Laboratory and CT Characteristics and RT-PCR Results in the Follow-Up of COVID-19 Patients. [Google Scholar]
- 49.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao J., Hu X., Cheng W., Yu L., Tu W.-J., Liu Q. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J., Ouyang L., Guo P., sheng Wu H., Fu P., liang Chen Y. medRxiv; 2020. Epidemiological, Clinical Characteristics and Outcome of Medical Staff Infected with COVID-19 in Wuhan, China: A Retrospective Case Series Analysis. [Google Scholar]
- 54.Yuan J., Kou S., Liang Y., Zeng J., Pan Y., Liu L. medRxiv; 2020. Clinical Characteristics on 25 Discharged Patients with COVID-19 Virus Returning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J., Yang S., Xu Y., Liu J., Guo J., Tian S. A Multicenter Study; China: 2020. Epidemiological and Clinical Characteristics of COVID-19 Infection outside Wuhan. [Google Scholar]
- 56.Ai J., Chen J., Wang Y., Liu X., Fan W., Qu G. 2020. The Cross-Sectional Study of Hospitalized Coronavirus Disease 2019 Patients in Xiangyang, Hubei Province. medRxiv. [Google Scholar]
- 57.Wu J., Wu X., Zeng W., Guo D., Fang Z., Chen L. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest. Radiol. 2020;55:257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun K., Chen J., Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population-level observational study. Lancet Digit. Health. 2020 doi: 10.1016/S2589-7500(20)30026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diao K., Han P., Pang T., Li Y., Yang Z. HRCT imaging features in representative imported cases of 2019 novel coronavirus pneumonia. Precis. Clin. Med. 2020;3:9–13. doi: 10.1093/pcmedi/pbaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 62.Liu K., Fang Y.-Y., Deng Y., Liu W., Wang M.-F., Ma J.-P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020 doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lei L., Jian-Ya G., Hu W., Zhang X., Gua L., Liu C. MedRxiv; China: 2020. Clinical Characteristics of 51 Patients Discharged from Hospital with COVID-19 in Chongqing. [Google Scholar]
- 65.Shu L., Wang X., Li M., Chen X., Shi L., Wu M. 2020. Clinical Characteristics of 545 Cases Confirmed COVID-19 in Wuhan Stadium Cabin Hospital. Available at: SSRN 3552844. [Google Scholar]
- 66.Wang L., Gao Y.-H., Lou L.-L., Zhang G.-J. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan L., Zhang H.-T., Xiao Y., Wang M., Sun C., Liang J. medRxiv; 2020. Prediction of Criticality in Patients with Severe Covid-19 Infection Using Three Clinical Features: a Machine Learning-Based Prognostic Model with Clinical Data in Wuhan. [Google Scholar]
- 68.Ren L.-L., Wang Y.-M., Wu Z.-Q., Xiang Z.-C., Guo L., Xu T. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu L., Fei J., Xiang H.-X., Xiang Y., Tan Z.-X., Li M.-D. medRxiv; 2020. Influence Factors of Death Risk Among COVID-19 Patients in Wuhan, China: a Hospital-Based Case-Cohort Study. [Google Scholar]
- 70.Li X., Hu Y., Zhu S., Li Y., Huang L., Li Y. 2020. Epidemiological Feature and Outcome of 292 Hospitalized Patients with COVID-19 under Adequate Medical Resource Condition. Available at: SSRN 3550016. [Google Scholar]
- 71.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. Jama. 2020 doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang N., Shen Y., Shi C., Ma A.H.Y., Zhang X., Jian X. medRxiv; 2020. In-flight Transmission Cluster of COVID-19: A Retrospective Case Series. [DOI] [PubMed] [Google Scholar]
- 73.Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L. Characteristics of ocular findings of patients with coronavirus disease 2019 (covid-19) in Hubei Province, China. JAMA Ophthalmol. 2020 doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z. Epidemiology and Transmission of COVID-19 in Shenzhen China: analysis of 391 cases and 1,286 of their close contacts. MedRxiv. 2020 doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020:1–3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao T., Gao Y., Cui Q., Shen J., Peng B., Chen Y. Retrospective Case Series; 2020. Clinical Characteristics of 55 Cases of Deaths with COVID-19 Pneumonia in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao W., Yu S., Zha X., Wang N., Pang Q., Li T. medRxiv; 2020. Clinical Characteristics and Durations of Hospitalized Patients with COVID-19 in Beijing: a Retrospective Cohort Study. [Google Scholar]
- 78.Kuang Y., He S., Lin S., Zhu R., Zhou R., Wang J. 2020. Clinical Characteristics and CT Manifestations of 143 Hospitalized Patients with 2019 Novel Coronavirus Disease (COVID-19) outside Wuhan: A Multi-Center Study in Taizhou City. Zhejiang, China. Zhejiang, China (3/14/2020) [Google Scholar]
- 79.Kuang Y., Zhang H., Zhou R., Lin S., Lin M., Wang J. 2020. Epidemiological and Clinical Characteristics of 944 Cases of 2019 Novel Coronavirus Infection of Non-COVID-19 Exporting City. Zhejiang, China. Zhejiang, China (February 20, 2020) [Google Scholar]
- 80.Chen W., Chen C., Huang L., Ye K., Lv L., Qin Z. 2020. Clinical Characteristics of 85 Patients Infected by SARS-CoV-2 in Guangxi. China. [Google Scholar]
- 81.Luo X., Xia H., Yang W., Wang B., Guo T., Xiong J. MedRxiv; 2020. Characteristics of Patients with COVID-19 during Epidemic Ongoing Outbreak in Wuhan, China. [Google Scholar]
- 82.Han X., Cao Y., Jiang N., Chen Y., Alwalid O., Zhang X. Novel coronavirus pneumonia (COVID-19) progression course in 17 discharged patients: comparison of clinical and thin-section CT features during recovery. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li X., Wang L., Yan S., Yang F., Xiang L., Zhu J. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng Y., Liu W., Liu K., Fang Y.-Y., Shang J., Wang K. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hou J., Wan X., Shen Q., Leng Y., Xia Z., Zhao B. 2020. Epidemiologic and Clinical Characteristics of Surgical Patients Infected with COVID-19 in Wuhan. Available at: SSRN 3550044. [Google Scholar]
- 86.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J. Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y., Sun W., Li J., Chen L., Wang Y., Zhang L. MedRxiv; 2020. Clinical Features and Progression of Acute Respiratory Distress Syndrome in Coronavirus Disease 2019. [Google Scholar]
- 88.Huang Y., Zhou H., Yang R., Xu Y., Feng X., Gong P. medRxiv; 2020. Clinical Characteristics of 36 Non-survivors with COVID-19 in Wuhan, China. [Google Scholar]
- 89.Wen Y., Wei L., Li Y., Tang X., Feng S., Leung K. medRxiv; 2020. Epidemiological and Clinical Characteristics of COVID-19 in Shenzhen, the Largest Migrant City of China. [Google Scholar]
- 90.Wu Y., Guo W., Liu H., Qi B., Liang K., Xu B. medRxiv; 2020. Clinical Outcomes of 402 Patients with COVID-2019 from a Single Center in Wuhan, China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y., Dong C., Hu Y., Li C., Ren Q., Zhang X. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020:200843. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu Z., Chen M., Fan Y., Wu X., Zhang L., Guo T. 2020. Clinical Characteristics and Risk Factors for Fatal Outcome in Patients with 2019-Coronavirus Infected Disease (COVID-19) in Wuhan. Available at: SSRN 3546069. [Google Scholar]
- 93.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 2020:1–6. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu P., Zhu J., Zhang Z., Han Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aminian A., Safari S., Razeghian-Jahromi A., Ghorbani M., Delaney C.P. COVID-19 outbreak and surgical practice: unexpected fatality in perioperative period. Ann. Surg. 2020;10 doi: 10.1097/SLA.0000000000003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu H., Shao J., Guo Y., Xiang Y., Sun C., Zhang H.-T. Data-driven discovery of clinical routes for severity detection in COVID-19 pediatric cases. medRxiv. 2020 [Google Scholar]
- 97.Million M., Lagier J.-C., Gautret P., Colson P., Fournier P.-E., Amrane S. 2020. Full-length Title: Early Treatment of COVID-19 Patients with Hydroxychloroquine and Azithromycin: A Retrospective Analysis of 1061 Cases in Marseille, France. Travel Medicine and Infectious Disease; p. 101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.