Abstract
Introduction
Given the continuing COVID-19 pandemic and much of the U.S. implementing social distancing owing to the lack of alternatives, there has been a push to develop a vaccine to eliminate the need for social distancing.
Methods
In 2020, the team developed a computational model of the U.S. simulating the spread of COVID-19 coronavirus and vaccination.
Results
Simulation experiments revealed that to prevent an epidemic (reduce the peak by >99%), the vaccine efficacy has to be at least 60% when vaccination coverage is 100% (reproduction number=2.5–3.5). This vaccine efficacy threshold rises to 70% when coverage drops to 75% and up to 80% when coverage drops to 60% when reproduction number is 2.5, rising to 80% when coverage drops to 75% when the reproduction number is 3.5. To extinguish an ongoing epidemic, the vaccine efficacy has to be at least 60% when coverage is 100% and at least 80% when coverage drops to 75% to reduce the peak by 85%–86%, 61%–62%, and 32% when vaccination occurs after 5%, 15%, and 30% of the population, respectively, have already been exposed to COVID-19 coronavirus. A vaccine with an efficacy between 60% and 80% could still obviate the need for other measures under certain circumstances such as much higher, and in some cases, potentially unachievable, vaccination coverages.
Conclusions
This study found that the vaccine has to have an efficacy of at least 70% to prevent an epidemic and of at least 80% to largely extinguish an epidemic without any other measures (e.g., social distancing).
INTRODUCTION
With the continuing coronavirus disease 2019 (COVID-19) pandemic and much of the U.S. having implemented social distancing measures and other nonpharmaceutical interventions (e.g., wearing masks) owing to the lack of alternatives, there has been a push for efforts to develop a vaccine. However, as these vaccine development efforts progress, it is not yet clear what vaccine efficacies are necessary to achieve this goal with a vaccine alone, thereby eliminating the need for measures such as social distancing. As described previously,1 , 2 it is important to determine the efficacy thresholds to aim for early on and during a vaccine's development. This can help identify the best candidates for various roles (e.g., vaccines that can prevent or extinguish an epidemic versus reduce the impact of an epidemic) to help guide resource allocation and also properly manage expectations once each vaccine reaches the market. Currently, there are 16 vaccine candidates under clinical Phase I–II evaluation, and more than 100 preclinical candidate vaccines are in the immunization pipeline.3, 4, 5, 6 To help establish the ideal goals for the vaccine efficacies needed to prevent and extinguish a COVID-19 coronavirus epidemic, the team developed a computational simulation model representing the U.S. population, the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the impact of a vaccine under various conditions.
METHODS
Model Structure
Using Microsoft Excel, version 16, with the Crystal Ball add-in, the team developed a computational transmission, clinical, and economics outcomes model (in 2020) representing the U.S. population (357,157,434 people) and their various interactions with each other as well as the spread of SARS-CoV-2 and the potential health and economic outcomes.7 The model (Appendix Figure 1, available online) advances in discrete, 1-day time steps for 2.5 years (which is the longest epidemic duration in the scenarios evaluated in this study). On any given day, each individual in the model is in 1 of 5 mutually exclusive SARS-CoV-2 states: (1) susceptible (S, not infected and able to become infected), (2) exposed (E, infected but not able to transmit to others), (3) infectious and asymptomatic (Ia, infected but without symptoms and able to transmit to others), (4) infectious and symptomatic (Is, infected, showing symptoms, and able to transmit to others), or (5) recovered/immune (R, not infected and unable to become infected). On Day 1, a set number of individuals start in the Ia state and Is state (i.e., coronavirus seed), with the remainder starting in the S state. Each day, individuals interact with each other, and a person who is infectious can potentially transmit the virus to a person who is susceptible. If a susceptible person comes in contact with an infectious person and is exposed, they move from the S state to the E state. The following equation determines the number of susceptible individuals who became exposed each day: β × S × Is + (β × 0.5) × S × Ia. Beta (β) equals to the basic reproduction number (R0, the average number of secondary cases generated by 1 infectious case) divided by the infectious period duration and the number of individuals in the population; S and I represent the number of susceptible and infectious individuals, respectively, on any given day. Exposed individuals remain in the E state for the latent period duration (i.e., the time between exposure and ability to transmit) before becoming infectious and moving to the I state (at a rate of 1/latent period duration). Because individuals can transmit the virus before disease onset,8 they could transmit 1 day before the start of symptoms. Each individual moving to the I state has a probability of being symptomatic, which governs whether they are in the Is or Ia state. Each person in the model draws an infectious period duration from a distribution (range=4–15 days, including the day before symptom onset). Infectious individuals remain in the I state until they recover and are no longer infectious, moving from the I state to the R state (at the rate of 1/infectious period duration).
Vaccination occurs when different percentages of the population have been exposed to SARS-CoV-2 during the epidemic (varying with scenario) and protects in 2 different ways: (1) by preventing infection and (2) by preventing symptomatic disease (i.e., reducing viral shedding). Individuals who are vaccinated move into the V state. Each day, vaccinated individuals mix with others, and if they come in contact with an infectious person, they move to the E state. When the vaccine prevents infection, individuals in the E state will move to the R state on the basis of the vaccine efficacy. Individuals for whom the vaccine is not effective move to either the Ia state or Is state. When the vaccine reduces viral shedding, it reduces the individuals’ probability of moving to the Is state on the basis of vaccine efficacy. Effectively vaccinated individuals in the E state move to the Ia state, where they transmit at a lower rate (e.g., β × 0.5). Vaccination is defined as the timepoint at which the onset of protection occurs (e.g., if onset is 2 weeks after vaccination, vaccination start date occurs 2 weeks before the simulated day), and the vaccine has no impact on individuals who had already been infected with or exposed to SARS-CoV-2.
Each symptomatically infected person (i.e., COVID-19 case) travels through a probability tree to determine their clinical outcomes, with all the probabilities and outcomes being age-specific (Appendix Figure 1B, available online).7 An infected person showing symptoms starts with mild infection and has a probability of seeking ambulatory care or calling their physician (i.e., telephone consult). This person then has a probability of progressing to severe disease requiring hospitalization. If this person has only mild illness and is not hospitalized, they self-treat with over-the-counter medications and miss school or work for the duration of symptoms. If this person is hospitalized, they have a probability of developing severe pneumonia or severe nonpneumonia symptoms and a probability of intensive care unit admission. This patient then has a probability of having either sepsis or acute respiratory distress syndrome, with or without sepsis. If this patient has acute respiratory distress syndrome, they require the use of a ventilator. If the person is hospitalized, they have a probability of dying from COVID-19 complications. The person accrues relevant costs and health effects as they travel through the model.
The third-party payer perspective includes direct medical costs (e.g., ambulatory care, hospitalization), and the societal perspective includes direct and indirect (i.e., productivity losses due to absenteeism and mortality) costs. Hourly wage across all occupations9 serves as a proxy for productivity losses. Absenteeism results in productivity losses for the duration of symptoms. All COVID-19 cases accrue productivity losses, regardless of age or employment status, as everyone is assumed to contribute to society. Death results in the net present value of productivity losses for missed lifetime earnings based on annual wage9 for the years of life lost based on an individual's life expectancy.10 The Appendix and Appendix Table 1 (available online) describe the model inputs, along with their values, distributions, and data sources.
Modeled Scenarios and Sensitivity Analyses
This study evaluated the impact of introducing a vaccine with varying efficacies in the absence of other measures from the third-party payer and societal perspectives. The overall goal of this study was to identify the vaccine efficacy thresholds above which vaccination could prevent a wave of the epidemic or extinguish an ongoing wave of the epidemic across a range of possible scenarios rather than predict exactly what will happen with the current pandemic. Predicting the specific course of the pandemic can be challenging with such variable social distancing measures being applied and limited data on the actual spread of the virus. Therefore, the team ran ranges of possible epidemic scenarios that varied R0 and the proportion of the population that has already been exposed to SARS-CoV-2 before vaccination onset. In doing so, the aim was to represent the spectrum of possibilities if social distancing measures were relaxed completely. The initial scenario assumes an epidemic with no vaccination, whereas experimental scenarios consisted of vaccinating individuals to either prevent infection or prevent symptomatic disease. Sensitivity analyses varied the vaccine efficacy (20%–100%), population coverage (those vaccinated and those otherwise immune, 50%–100%), percentage of the population exposed before vaccination onset (0%–30%), and R0 (2.5–3.5).11, 12, 13 Additional scenarios varied the probability of an infected individual being asymptomatic (from the distribution in Appendix Table 1, available online, to 35%) and the infectiousness of asymptomatic individuals (0.5–1) compared with that of the symptomatic individuals.14 Experiments consisted of 1,000 trial Monte Carlo simulations, varying each parameter throughout its distribution (Appendix Table 1, available online).
RESULTS
No Vaccine
Table 1 presents the results of the initial scenario (i.e., no vaccination), showing the number of cases, clinical outcomes, resources used, and costs incurred over the epidemic duration. When R0 is 2.5, an epidemic results in 282.5 million total SARS-CoV-2 cases, and a higher R0 of 3.5 results in 312.9 million total cases.
Table 1.
Clinical Outcomes, Resource Use, and Costs During the Course of a COVID-19 Epidemic
| Scenario | Total SARS-CoV-2 cases (in millions)a |
Symptomatic cases (in millions) |
Hospitalized cases (in millions) |
Number of patients ventilated (in millions) |
Deaths (in thousands) |
Total beds days (in millions) | Ventilated days (in millions) |
Direct medical costs (in billions) |
Productivity losses (in billions) |
|---|---|---|---|---|---|---|---|---|---|
|
Median (95% CI) |
Median (95% CI) |
Median (95% CI) |
Median (95% CI) |
Median (95% CI) |
Median (95% CI) |
Median (95% CI) |
Median (95% CI) |
Median (95% CI) |
|
| R0 of 2.5 | |||||||||
| No vaccination | 282.5 (280.7, 284.3) |
232.1 (223.9, 240.0) |
48.1 (46.4, 49.8) |
8.5 (6.5, 9.7) |
4,160.9 (4,014.2, 4,302.0) |
267.2 (251.7, 282.6) |
68.0 (51.8, 77.9) |
883.5 (808.5, 980.8) |
2,796.4 (1,661.8, 4,515.5) |
| Vaccination occurs when 5% of the population has been exposed to SARS-CoV-2 | |||||||||
| 60% vaccine efficacy, 75% coverage | 86.0 (51.9, 277.3) |
76.5 (44.7, 228.2) |
15.9 (9.3, 47.3) |
2.78 (1.46, 8.82) |
1,371.4 (801.5, 4,090.4) |
88.1 (51.2, 262.9) |
22.1 (11.7, 70.2) |
295.6 (165.7, 878.5) |
934.4 (402.2, 3,373.4) |
| 70% vaccine efficacy, 60% coverage | 103.4 (76.5, 278.5) |
89.3 (62.3, 229.2) |
18.5 (12.9, 47.5) |
3.2 (2.1, 8.7) |
1,600.7 (1,116.8, 4,109.1) |
101.8 (71.1, 264.3) |
25.7 (16.5, 70.1) |
339.6 (231.3, 891.4) |
1,124.1 (519.7, 3,427.5) |
| 80% vaccine efficacy, 75% coverage | 45.2 (5.9, 275.2) |
38.8 (4.9, 225.7) |
8.0 (1.0, 46.8) |
1.39 (0.17, 8.78) |
694.9 (88.5, 4,045.8) |
44.5 (5.7, 262.6) |
11.2 (1.4, 70.7) |
146.0 (18.4, 870.0) |
441.6 (52.6, 3,462.1) |
| Vaccination occurs when 15% of the population has been exposed to SARS-CoV-2 | |||||||||
| 70% vaccine efficacy, 75% coverage | 104.0 (27.8, 282.5) |
89.8 (23.7, 235.6) |
18.6 (4.9, 48.8) |
3.24 (0.81, 9.22) |
1,609.4 (424.3, 4,223.7) |
103.9 (27.3, 273.3) |
25.9 (6.5, 73.4) |
341.2 (90.5, 922.9) |
1,119.1 (236.1, 3,775.6) |
| 80% vaccine efficacy, 50% coverage | 144.3 (96.9, 282.6) |
120.3 (77.1, 234.6) |
24.9 (16.0, 48.6) |
4.3 (2.6, 9.1) |
2,156.8 (1,382.5, 4,206.3) |
138.8 (88.3, 273.3) |
34.6 (20.9, 73.4) |
460.1 (285.8, 920.8) |
1,486.5 (686.1, 3,879.1) |
| Vaccination occurs when 30% of the population has been exposed to SARS-CoV-2 | |||||||||
| 40% vaccine efficacy, 50% coverage | 224.5 (179.4, 283.4) |
190.7 (152.6, 236.9) |
39.5 (31.6, 49.1) |
6.8 (4.8, 9.4) |
3,418.0 (2,736.2, 4,247.6) |
220.6 (173.5, 276.6) |
54.5 (38.1, 75.8) |
732.9 (557.6, 935.0) |
2,269.4 (1,213.9, 4,170.8) |
| 70% vaccine efficacy, 75% coverage | 156.5 (39.2, 283.4) |
131.9 (33.3, 236.0) |
27.4 (6.9, 48.9) |
4.71 (1.20, 9.35) |
2,365.2 (596.2, 4,231.2) |
152.3 (38.7, 274.0) |
37.5 (9.5, 75.0) |
502.2 (128.1, 937.7) |
1,605.2 (342.4, 4,072.7) |
| R0 of 3.5 | |||||||||
| No vaccination | 312.9 (311.9, 314.0) |
257.1 (248.7, 264.8) |
53.3 (51.6, 54.9) |
9.5 (7.2, 10.7) |
4,608.3 (4,458.0, 4,747.6) |
296.0 (281.4, 312.1) |
76.2 (57.6, 85.7) |
978.9 (896.4, 1,082.3) |
3,141.3 (1,871.3, 5,059.2) |
| Vaccination occurs when 5% of the population has been exposed to SARS-CoV-2 | |||||||||
| 60% vaccine efficacy, 75% coverage | 125.8 (110.0, 310.7) |
113.5 (97.5, 256.6) |
23.5 (20.2, 53.2) |
4.2 (3.0, 10.0) |
2,034.5 (1,748.2, 4,600.4) |
130.8 (110.4, 297.2) |
33.6 (24.1, 79.7) |
438.0 (353.8, 996.9) |
1,593.9 (794.5, 4,087.0) |
| 80% vaccine efficacy, 60% coverage | 125.7 (108.4, 311.5) |
107.1 (89.8, 257.2) |
22.2 (18.6, 53.3) |
4.0 (2.7, 9.9) |
1,920.4 (1,609.0, 4,611.8) |
123.3 (101.9, 298.2) |
31.8 (21.8, 79.0) |
406.7 (328.6, 1,003.9) |
1,459.1 (724.9, 4,077.8) |
| 80% vaccine efficacy, 75% coverage | 82.3 (46.1, 311.4) |
71.2 (38.4, 256.8) |
14.8 (8.0, 53.2) |
2.6 (1.3, 10.0) |
1,276.8 (687.8, 4,603.1) |
81.8 (44.0, 297.9) |
20.6 (10.1, 80.0) |
270.5 (141.8, 1,001.8) |
901.9 (338.5, 4,051.8) |
| Vaccination occurs when 15% of the population has been exposed to SARS-CoV-2 | |||||||||
| 80% vaccine efficacy, 75% coverage | 120.1 (50.1, 313.3) |
102.1 (42.0, 260.3) |
21.2 (8.7, 54.0) |
3.7 (1.4, 10.2) |
1,831.3 (753.3, 4,666.6) |
117.0 (48.5, 302.1) |
29.8 (11.3, 82.1) |
389.0 (155.3, 1,029.4) |
1,224.6 (394.3, 4,540.0) |
| 80% vaccine efficacy, 50% coverage | 180.0 (147.1, 313.4) |
150.9 (121.8, 260.6) |
31.3 (25.3, 54.0) |
5.5 (3.9, 10.2) |
2,704.8 (2,184.4, 4,672.6) |
174.0 (139.1, 302.8) |
44.2 (31.0, 81.7) |
576.8 (451.0, 1,026.9) |
1,974.4 (1,027.7, 4,339.1) |
| Vaccination occurs when 30% of the population has been exposed to SARS-CoV-2 | |||||||||
| 40% vaccine efficacy, 50% coverage | 254.2 (217.1, 313.9) |
217.0 (188.1, 262.3) |
45.0 (39.0, 54.4) |
7.9 (5.9, 10.3) |
3,890.6 (3,372.5, 4,702.9) |
250.2 (214.1, 305.3) |
63.1 (47.3, 82.4) |
836.9 (691.0, 1,035.3) |
2,672.2 (1,551.1, 4,697.4) |
| 80% vaccine efficacy, 50% coverage | 210.3 (148.7, 313.7) |
176.3 (123.7, 261.1) |
36.6 (25.6, 54.1) |
6.5 (3.9, 10.4) |
3,161.3 (2,217.4, 4,680.5) |
201.5 (141.1, 305.2) |
51.6 (31.2, 82.9) |
671.6 (452.0, 1,039.0) |
2,162.2 (1,054.8, 4,396.9) |
| 70% vaccine efficacy, 75% coverage | 182.9 (85.1, 313.9) |
155.9 (74.1, 262.1) |
32.3 (15.4, 54.3) |
5.74 (2.44, 10.24) |
2,794.9 (1,328.3, 4,698.6) |
180.4 (84.3, 304.6) |
45.5 (19.6, 81.7) |
599.2 (277.5, 1,029.7) |
1,825.9 (655.5, 4,468.1) |
Note: Number of clinical outcomes, resource use, and costs during the course of a COVID-19 epidemic when vaccination occurs when varying percentages of the population had been exposed to SARS-CoV-2 with a vaccine that prevents infection, varying with vaccine efficacy. Costs are in 2020 $US.
Total SARS-CoV-2 cases include those infected before vaccination onset.
R0, reproduction number.
Vaccine Efficacy Needed to Prevent an Epidemic
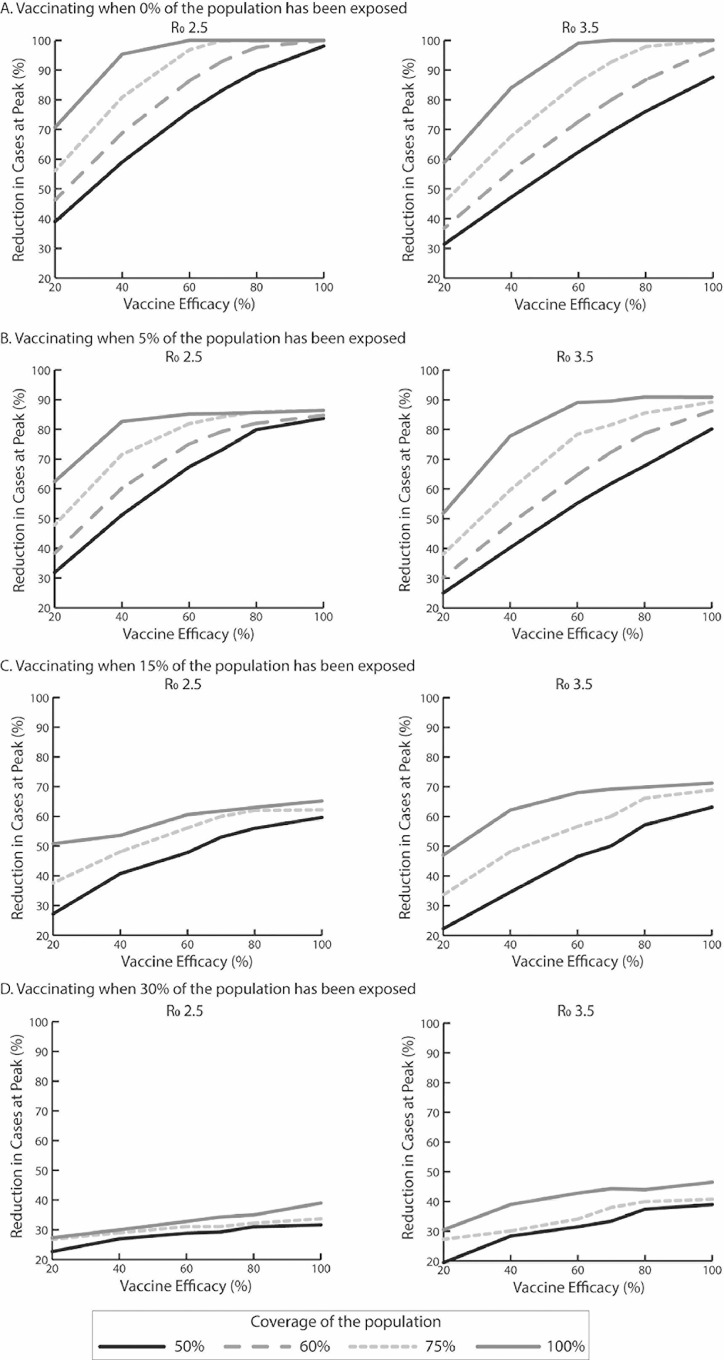
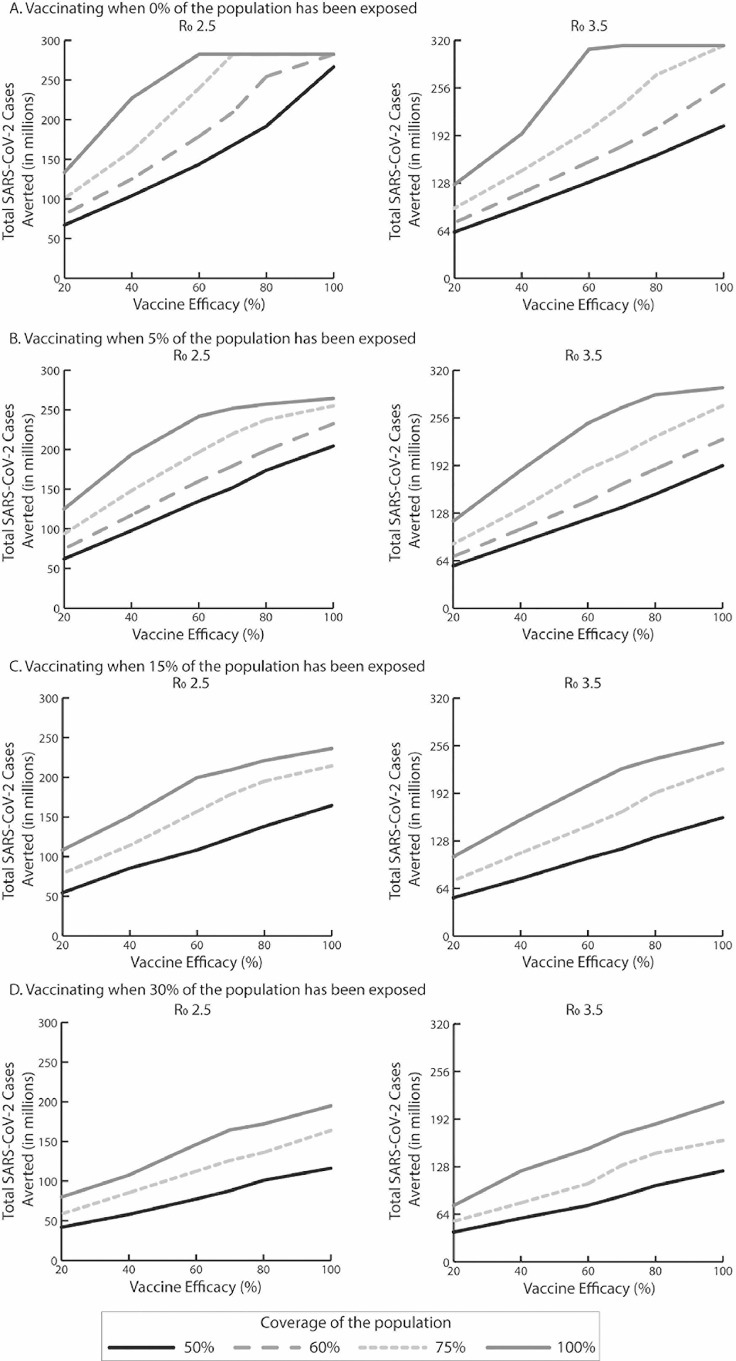
These scenarios determine what would happen if vaccination took place at the very early stage of the epidemic or before a second or subsequent wave. The question then is whether vaccination can prevent a peak from occurring. Figure 1 shows how vaccine efficacy (x-axis) and coverage (those vaccinated and those otherwise immune) can reduce the size of the peak (i.e., the maximum number of daily SARS-CoV-2 infections; y-axis) in terms of percentage reduction of what the peak would be without vaccination. As Figure 1A shows, vaccine efficacy has to be at least 60% (when it prevents infection) to reduce the peak by >99% (i.e., number of new cases per day never exceeds the initial number of cases on Day 1) when R0 is 2.5 and vaccination coverage is 100%. This vaccine efficacy threshold rises to 70% when coverage drops to 75% and up to 80% when coverage drops to 60%. When coverage drops to 50%, it is no longer possible to eliminate the peak, even when vaccine efficacy is 100%. Of course, eliminating the peak does not necessarily mean that there will be no cases, because the infection can continue to occur at a stable level. Figure 2 shows how the vaccine efficacy (x-axis) impacts the total number of SARS-CoV-2 cases averted (y-axis) compared with no vaccination over the simulated period. As Figure 2 shows, when vaccination coverage is 100%, the number of cases averted plateaus after the vaccine efficacy reaches and exceeds 60%, which is consistent with the threshold identified to eliminate the peak. Similarly, when vaccination coverage is 75%, cases averted plateau after vaccine efficacy reaches and exceeds 70%, resulting in 90,760 symptomatic cases (Appendix Table 2, available online).
Figure 1.
Percentage reduction in SARS-CoV-2 cases at the epidemic peak for a vaccine that prevents infection compared with no vaccination, varying with vaccine efficacy, vaccination coverage, R0, and vaccination onset (percentage of population exposed to SARS-CoV-2) occurring when (A) 0% of the population has been exposed, (B) 5% of the population has been exposed, (C) 15% of the population has been exposed, and (D) 30% of population has been exposed.
Note: Epidemic peak is the maximum number of daily SARS-CoV-2 cases. R0, reproduction number.
Figure 2.
The total number of SARS-CoV-2 cases averted by vaccination during the course of the epidemic for a vaccine that prevents infection compared with no vaccination, varying with vaccine efficacy, vaccination coverage, R0, and vaccination onset (percentage of population exposed to SARS-CoV-2) occurring when A) 0% of the population has been exposed, B) 5% of the population has been exposed, C) 15% of the population has been exposed, and D) 30% of the population has been exposed.
Note: Total number of SARS−CoV-2 cases averted is equal to total SARS-CoV-2 cases without vaccination minus total SARS-CoV-2 cases with vaccination. R0, reproduction number.
When R0 is 3.5 (Figures 1A and 2A and Appendix Table 2, available online), vaccine efficacy has to be at least 60% to reduce the peak by >99% when vaccination coverage is 100%. This vaccine efficacy threshold increases to 80% when coverage drops to 75%. These vaccine efficacy and vaccination coverage thresholds are also the same thresholds at which the number of cases averted plateaus (Figure 2A). When coverage drops to ≤60%, it is no longer possible to eliminate the peak, even with 100% vaccine efficacy (Figure 1A).
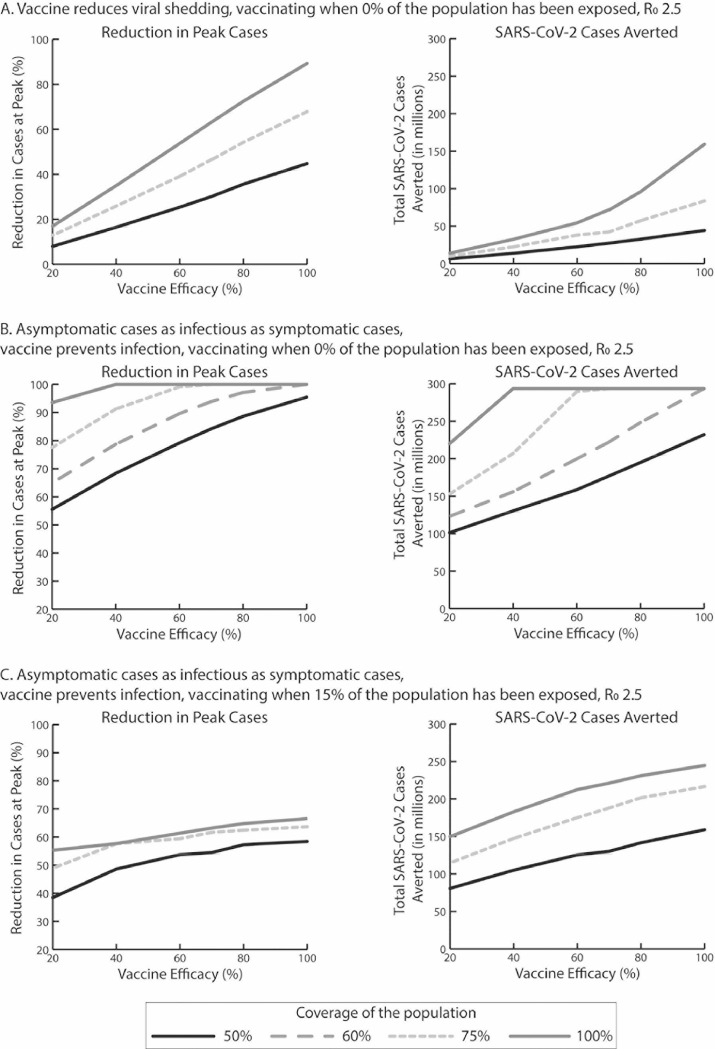
If the vaccine only reduced viral shedding (Figure 3A ), even a 100% vaccine efficacy with 100% coverage of the population is not enough to eliminate the peak and still results in a total of 122.9–239.8 million SARS-CoV-2 cases during the epidemic when R0 is 2.5–3.5.
Figure 3.
Percentage reduction in SARS-CoV-2 cases at the epidemic peak and the total number of SARS-CoV-2 cases averted by vaccination during the course of the epidemic compared with no vaccination for (A) a vaccine that reduces viral shedding when vaccination onset occurs when 0% of the population has been exposed to SARS-CoV-2, (B) for a vaccine that prevents infection when asymptomatic cases are as infectious as symptomatic cases when vaccination onset occurs when 0% of the population has been exposed, and (C) for a vaccine that prevents infection when asymptomatic cases are as infectious as symptomatic cases when vaccination onset occurs when 15% of the population has been exposed.
R0, reproduction number.
As Figure 3B shows, when individuals have a 35% probability of being asymptomatic and are equally infectious as symptomatic cases, vaccine efficacy has to be at least 40% to reduce the peak by 100% when R0 is 2.5 and vaccination coverage is 100%. This efficacy threshold rises to 60% when coverage drops to 75% and 100% when coverage drops to 60%. The number of cases averted also plateaus after reaching and exceeding these vaccine efficacy and coverage thresholds (Figure 3B).
Vaccine Efficacy Needed to Extinguish an Ongoing Epidemic
The next set of scenarios evaluated what would occur if vaccination took place during the course of an epidemic and to what degree the vaccination could extinguish the epidemic. This would correspond to rolling out a vaccine at various points during an epidemic. Again, Figures 1B–D and 2B–D show how vaccine efficacy and coverage can reduce the size of the peak and impact the total number of SARS-CoV-2 cases averted compared with no vaccination when different percentages of the population have already been exposed to SARS-CoV-2 (assuming that natural infection confers immunity). Table 1 shows the clinical and economic outcomes. As shown in Figure 1B, when vaccination prevents infection and occurs after 5% of the population has already been exposed, the peak can only be reduced by, at the most, 86% when R0 is 2.5. Vaccine efficacy has to be at least 60% to reduce the peak by 85% when coverage is 100%. This vaccine efficacy threshold increases to 80% when coverage drops to 75% (86% reduction) and 100% when coverage drops to 60% (85% reduction). However, while reducing the peak, the number of cases averted continues to increase, not reaching the maximum until both vaccine efficacy and coverage are 100% (Figure 2B).
When vaccination occurs after 15% of the population has already been exposed, the resulting reduction in the peak is, at most, 65%. As Figure 1C shows, the reduction in the peak size plateaus after vaccine efficacy reaches and exceeds 60% with a 100% coverage and 80% with a 75% coverage. However, the number of cases averted continues to accrue with increases in efficacy, until reaching the maximum when both vaccine efficacy and coverage are 100% (Figure 2C).
As shown in Figure 2D, the lines for various coverage levels move closer together and are lower on the y-axis (at most, the peak can be reduced by 39%) when vaccination occurs after 30% of the population has already been exposed. Whereas increases in efficacy result in similar reductions in the peak size (e.g., with a 50% coverage, a 40% vaccine efficacy reduces the peak by 27%, and an 80% efficacy reduces it by 31%), reductions in the number of cases averted continue to accrue (Figure 2D).
When R0 is 3.5, vaccination cannot reduce the peak by more than 91%, 71%, and 46% when vaccination occurs after 5%, 15%, and 30% of the population has already been exposed, respectively. As Figure 1B–D shows, the reduction in the peak size starts to plateau as vaccine efficacy reaches and exceeds 60% when vaccination coverage is 100% and 80% when coverage drops to 75%. When coverage drops to 50% and ≥5% of the population has been exposed, it is no longer possible to extinguish an ongoing epidemic, even when vaccine efficacy is 100%. Again, increasing efficacy continues to avert cases, except when vaccination occurs after 5% of the population has already been exposed when vaccine efficacy reaches and exceeds 80% when coverage is 100% (Figure 2B–D).
As shown in Figure 3C, when individuals have a 35% probability of being asymptomatic and are as infectious as symptomatic cases, vaccination when 15% of the population has already been exposed can reduce the peak, at most, by 67%. Vaccine efficacy has to be at least 60% to reduce the peak by 61% when R0 is 2.5 and vaccination coverage is 100% and 70% when vaccination coverage drops to 75% (61% reduction).
DISCUSSION
This study found that in the absence of other interventions to prevent an epidemic, the vaccine has to have an efficacy (i.e., probability of preventing infection) of at least 70% when vaccination covers at least 75% of the population. To extinguish an ongoing epidemic and obviate the need for any other measures (e.g., social distancing), the vaccine has to have an efficacy of at least 80% with a 75% vaccine coverage. These efficacy and coverage thresholds hold as the proportion of the population exposed increases from 5% to 30%, at which point the peak of the epidemic is rapidly approached as more and more people become exposed and immune. Achieving a 75% coverage is not trivial, although recent poll by Reuters/Ipsos of 1,215 American adults found that 75% of respondents would get a SARS-CoV-2 vaccine if assured it was safe.15 Even if enough people were willing to get the vaccine, there would have to be sufficient production capacity, vaccination supplies, and staffing as well as an adequate supply chain to reach everyone.
There are circumstances in which a vaccine with an efficacy between 40% and 70% could prevent an epidemic and between 60% and 80% could extinguish an ongoing epidemic, but these would require a potentially unachievable 100% coverage of the population. All of this suggests that a vaccine alone may not allow everything to return to normal immediately (i.e., stop social distancing) unless both vaccine efficacy and vaccination coverage are fairly high. Therefore, it will be important to manage the expectations of the public who may believe that no more social distancing will be needed as soon as a vaccine becomes available,16, 17, 18, 19, 20 especially if that vaccine has an efficacy below 70%–80% range. Of course, this does not mean that a vaccine with a lower efficacy may not be useful. For example, a vaccine could also help reduce the burden on the healthcare system so that it is not overwhelmed (e.g., a vaccine efficacy of 40% could prevent ≥2.8 million patients from requiring a ventilator and ≥89.5 million hospital bed days). In addition, vaccination could be combined with other control measures (e.g., test–trace–isolate, mandatory masking). A vaccine with a lower efficacy could also be used for targeted vaccination (e.g., response to a local outbreak or to protect certain groups). As this study shows, even if a vaccine does not prevent or extinguish an epidemic, it can still save a considerable number of lives, hospitalizations, and costs. The focus of the study, however, was not on measuring the cost effectiveness of the vaccine, which may be addressed in future studies.
Limitations
All models, by definition, are simplifications of real life and cannot account for every possible outcome.21 Model inputs drew from various sources, and new data on SARS-CoV-2 continue to emerge. For example, estimates of R0 vary widely and in many studies, are calculated considering only symptomatic infections.11, 12, 13 Because the course of an actual SARS-CoV-2 epidemic may not be very predictable, this study explored a range of possible scenarios and parameter values in sensitivity analyses. In addition, individuals mixed equally with each other, whereas in actuality, transmission and thus vaccination's impact may be higher among closer contacts, raising the possibility that targeted vaccination approaches may have a higher impact. Simulation experiments assumed an optimistic situation in which the population was vaccinated in 1 day. In reality, vaccination would take place over an extended period, which would reduce the impact of vaccination. Moreover, assumptions about levels of herd immunity required may depend on differing individual susceptibility to SARS-CoV-2. The model also assumes that there are sufficient healthcare resources (e.g., intensive care unit beds and ventilators) for all patients. However, if the healthcare system is overburdened, patients with COVID-19 may not receive proper care, leading to higher mortality.
CONCLUSIONS
This study describes the vaccine efficacy needed to prevent or extinguish a COVID-19 epidemic.
ACKNOWLEDGMENTS
Statements in the manuscript do not necessarily represent the official views of or imply the endorsement by NIH, Agency for Healthcare Research and Quality, or HHS.
This work was supported in part by the City University of New York's Graduate School of Public Health and Health Policy, Agency for Healthcare Research and Quality (through grant number R01HS023317), U.S. Agency for International Development (under agreement number AID-OAA-A-15-00064), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant numbers U01HD086861 and 5R01HD086013-02), and National Institute of General Medical Sciences through the Models of Infectious Disease Agent Study network under grant R01 GM127512.
The funders did not have any role in the study design; collection, analysis, or interpretation of data; writing the report; or the decision to submit the report for publication. The authors of this manuscript are responsible for its content, including data analysis. PJH and MEB develop vaccines against emerging and neglected diseases including coronavirus diseases such as COVID-19.
No financial disclosures were reported by the authors of this paper.
Footnotes
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2020.06.011.
Appendix. SUPPLEMENTAL MATERIAL
REFERENCES
- 1.Lee BY, Burke DS. Constructing target product profiles (TPPs) to help vaccines overcome post-approval obstacles. Vaccine. 2010;28(16):2806–2809. doi: 10.1016/j.vaccine.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee BY, Mueller LE, Tilchin CG. A systems approach to vaccine decision making. Vaccine. 2017;35(suppl 1):A36–A42. doi: 10.1016/j.vaccine.2016.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 treatment and vaccine tracker. Milken Institute, FasterCures. https://covid-19tracker.milkeninstitute.org. Accessed May 11, 2020.
- 4.Mapping COVID-19 research: the “map of hope” provides a geographical overview of planned, ongoing and completed clinical trials. Heidelberg Institute for Geoinformation Technology.https://covid-19.heigit.org/clinical_trials.html. Updated May 11, 2020. Accessed May 11, 2020.
- 5.Thanh Le T, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Draft landscape of COVID 19 candidate vaccines. Geneva, Switzerland: WHO.https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines. Published 2020. Accessed May 11, 2020.
- 7.Bartsch SM, Ferguson MC, McKinnell JA, et al. The potential health care costs and resource use associated with COVID-19 in the United States. Health Aff (Millwood) 2020;39(6):927–935. doi: 10.1377/hlthaff.2020.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Bureau of Labor Statistics; 2019. Occupational employment statistics, May 2018 national occupational employment and wage estimates United States.https://www.bls.gov/oes/2018/may/oes_nat.htm Published April 2, 2019. Accessed October 9, 2019. [Google Scholar]
- 10.The Human Morality Database. https://www.mortality.org/. Accessed January 7, 2020.
- 11.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao S, Lin Q, Ran J, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou T, Liu Q, Yang Z, et al. Preliminary prediction of the basic reproduction number of the Wuhan novel coronavirus 2019‐nCoV. J Evid Based Med. 2020;13(1):3–7. doi: 10.1111/jebm.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention; 2020. Coronavirus Disease 2019 (COVID-19), COVID–19 pandemic planning scenarios.https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html Updated May 20, 2020, Accessed June 1, 2020. [Google Scholar]
- 15.Bernstein S. Most Americans would take coronavirus vaccine if deemed safe: Reuters/Ipsos poll. Reuters. May 5, 2020 https://www.reuters.com/article/us-health-coronavirus-usa-poll/most-americans-would-take-coronavirus-vaccine-if-deemed-safe-reuters-ipsos-poll-idUSKBN22I019 [Google Scholar]
- 16.Lee BY. How long should social distancing last? When will COVID-19 coronavirus end? Forbes. May 26, 2020 https://www.forbes.com/sites/brucelee/2020/03/26/how-long-should-social-distancing-last-when-will-covid-19-coronavirus-end/#758080b6429a [Google Scholar]
- 17.Simmons-Duffin S, Aubrey A. What's it going to take to end the shutdown? 5 Keys to containing coronavirus. NPR. April 16, 2020 https://www.npr.org/sections/health-shots/2020/04/16/834555288/whats-it-going-to-take-to-end-the-shutdown-5-keys-to-containing-coronavirus [Google Scholar]
- 18.Kakaes K. Social distancing until 2022?! Hopefully not. MIT Technology Review. April 15, 2020 https://www.technologyreview.com/2020/04/15/999618/2022-social-distancing-science-lipsitch/ [Google Scholar]
- 19.Ewing P, Moore E. Testing could unlock a return to normal life, but obstacles persist. NPR. April 28, 2020 https://www.npr.org/2020/04/28/846816635/testing-could-unlock-a-return-to-normal-life-but-obstacles-persist [Google Scholar]
- 20.Gallagher J. Coronavirus: when will the outbreak end and life get back to normal? BBC NEWS. March 23, 2020 https://www.bbc.com/news/health-51963486 [Google Scholar]
- 21.Lee BY. Digital decision making: computer models and antibiotic prescribing in the twenty-first century. Clinical Infectious Diseases. 2008;46(8):1139-1141. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.