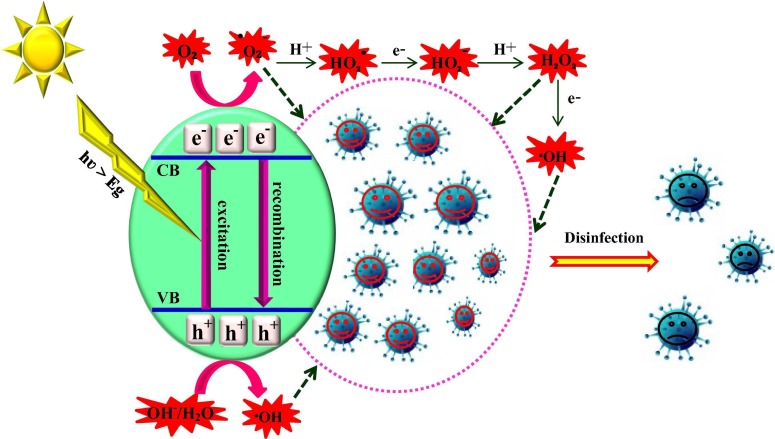
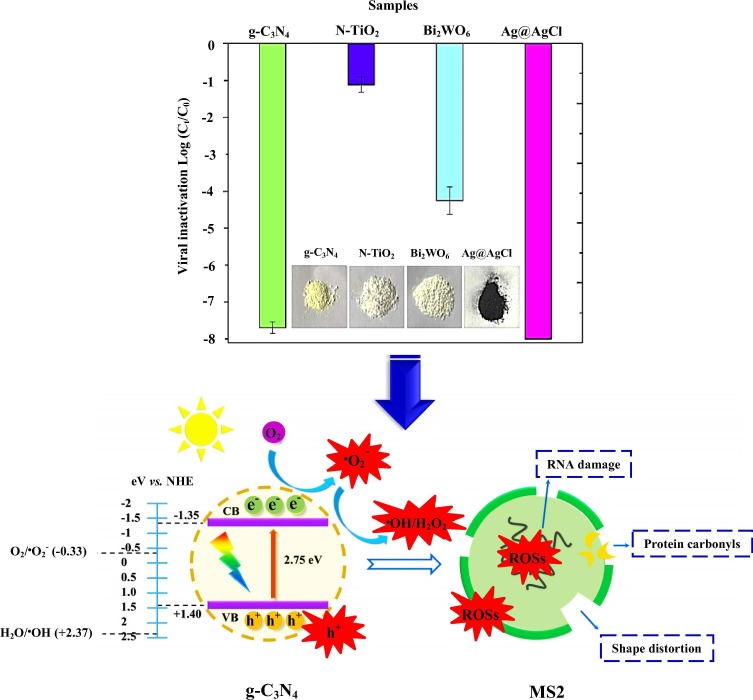
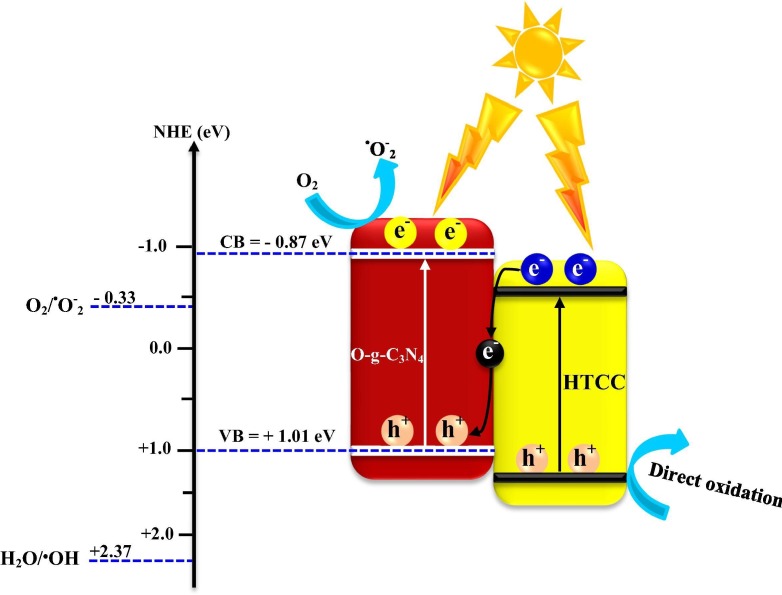
Graphical abstract
Keywords: Viral pathogenic contaminations, Semiconductor-assisted photocatalysis, Disinfection of viruses
Abstract
Microbial pathogenic contaminations have world widely represented a serious health hazard to humans. Viruses, as a member of microbial contaminants, seriously threaten human health due to their high environmental resistance, having small sizes, and causing an extensive range of diseases. Therefore, selecting an appropriate technology to remove viral contaminants from the air, water, and foods is of prominent significance. Traditional methods for viral disinfection have not proven to be highly practical and effective because they need high energy resources and operational expenses. In recent years, semiconductor-based photocatalysis has attracted more attention in the field of microorganism inactivation due to its outstanding performance and mild reaction conditions. Therefore, this review primarily concentrates on the recent development in viral inactivation/disinfection by heterogeneous photocatalysts. Moreover, the photocatalytic viral inactivation of waterborne, airborne, and foodborne viruses is discussed. Given the appealing merits of heterogeneous photocatalytic disinfection of viruses, there is no doubt that this technology will be an impressively active research field and a source of comfort and confidence to humans in battling against viruses.
1. Introduction
Nowadays, microbial pathogenic contaminations with viruses, bacteria, and protozoa have become a growing environmental concern, because these pathogens threaten human health and cause dangerous infectious illnesses [1], [2], [3]. Among different kinds of microbial contaminations, viruses have proven as a global challenge due to their small-sized particles, difficult inactivation, high environmental endurance, and also because of an extensive range of illnesses and diseases caused by them [4], [5]. Viruses are extremely ubiquitous microorganisms failing to survive outside the host organisms. Moreover, their survival/disinfection is primarily subject to the host-virus dynamics [6]. Viral contaminants, associated with drinking water, breathing air, and food, have become a notable threat due to their adverse effects on the environment and human health. Foodborne, waterborne, and airborne pathogens inter the body through different modes of infection and cause over 15 million deaths around the world annually. Water-borne and foodborne outbreaks correlate with climatic changes and disturbances in the ecosystem. In addition to airborne transmission, these pathogens are also transmitted by direct surface contact [7], [8]. In particular, the risk of disease through exposure to the airborne and waterborne viruses is higher than those of other microbial pathogenic contaminants [9], [10], [11], [12], [13], [14], [15], [16]. Infectious diseases caused by these viruses are the most widespread and common health risks. Spiraling worries about epidemic and pandemic viral diseases, such as severe acute respiratory syndrome coronavirus (SARS-CoV), swine influenza virus (H1N1), middle-east respiratory syndrome coronavirus (MERS-CoV), and novel coronavirus disease-2019 (COVID-19), have drawn the attention of many scientists for viral treatments [17], [18], [19], [20], [21], [22], [23]. Accordingly, to treat the diseases caused by viruses, different methods have been utilized and many attempts have been allocated for disinfection of various viruses presented in the environment [24], [25]. For waterborne viruses, there is a diversity of disinfection processes like ultraviolet (UV) disinfection, membrane filtration, and chemical disinfectants (ozone, chlorine dioxide, and chlorine) [26]. In practice, however, these processes suffer from some limitations. The production of noxious byproducts has restricted the use of chemical disinfectants [27]. Membrane methods like nanofiltration and ultrafiltration, due to the small membrane pore sizes relative to the size of viruses, can eliminate viruses efficiently, although these methods are unaffordable and energy consuming [13]. In addition to the high expense of the UV method, some viruses show a high resistance to UV illumination [28], [29]. Further, conventional methods for airborne viral disinfection like UV light, thermal treatment, and non-thermal plasma are not highly practical and effective, because they require high energy resources and high operational expenses [30]. Therefore, an effective technology with eco-friendly features, high proficiency, low energy consumption, and affordable expense is required for inactivation of viruses.
Over the past decades, we have witnessed extensive and unprecedented research in the field of advanced oxidation processes (AOPs), which are judged to be the most encouraging method for the removal of pollutants, including organic, inorganic, and microbial contaminants, compared with traditional purification procedures [31], [32]. In the AOPs, highly reactive oxygen species (ROS) like •OH, •O2 −, h+, and HO2 • are produced, which have large oxidizing ability. These species can oxidize organic contaminants to CO2 and inorganic ions, reduce inorganic contaminants to nontoxic ions, and inactivate microorganisms producing no noxious compounds [33], [34]. Of these AOPs, semiconductor-assisted photocatalysis is introduced to be the most desirable method due to its proven ability in the purification of an immense range of contaminants [35], [36]. Photocatalysis is an extremely effective and promising technology, because it requires only solar light (or artificial light) and a photoactive material. The heterogeneous photocatalytic procedure begins with the absorption of the utilized light with a higher amount of energy than the photocatalyst band gap, which transfers the valence band (VB) electrons into the conduction band (CB), leading to production of charge carriers (e−/h+ pairs). The photoexcited charge carriers migrate to the surface of the photoactive material and take part in the reduction and oxidation (redox) reactions. The holes in VB and the electrons in CB can thermodynamically oxidize water molecules and reduce O2 to produce ROS, respectively. Subsequently, the reactions of harmful microorganisms and environmental pollutants with these radicals result in disinfection and elimination of contaminants found in the environment, respectively [37], [38], [39], [40].
2. Inactivation of viruses by heterogeneous photocatalysis
After viral inactivation on TiO2 photocatalyst discovered by Sierka and Sjogren in 1994, heterogeneous photocatalysis has been given widespread recognition as an outstanding technology in the field of viral disinfection [41]. The ROS created by photocatalytic processes destroy the shell and/or capsid of viruses, resulting in release of genetic materials, minerals, and proteins inside viruses. Additionally, organic compounds presented in the structure of viruses could be completely mineralized, which causes their inactivation/disinfection. As shown in Fig. 1 , Zhang et al. suggested three mechanisms for viral disinfection in photocatalytic processes, which include physical damage of viruses, metal ion toxicity obtained from metal-including photocatalysts, and chemical oxidation by ROS generated over the photocatalysts [5]. These mechanisms are affected when the photocatalyst surface and the virus come into contact, which leads to an increase of the photocatalytic efficiency. The contact built up between the viral cells and the photocatalyst particles in suspension may supply an extended surface area for production of more ROS [42], [43].
Fig. 1.
Three main mechanisms of viral inactivation induced by heterogeneous photocatalysts (Reproduced with permission from reference [5] Copyright 2019 Elsevier).
It is witnessed that relevant publications in the field of viral inactivation through heterogeneous photocatalysis are increasing with time, confirming the rising popularity of the field.
The photocatalytic disinfection of viruses mainly takes place by chemical oxidation initiated by ROS over heterogeneous photocatalysts. The disinfection mechanism includes decomposition of the cell wall and the cytoplasmic membrane as a result of the generation of ROS like •OH and H2O2 [44], through several steps as follows:
-
1)
Electron/hole (e−/h+) pairs are produced after excitation of the selected photocatalyst and transfer of charges to its surface.
-
2)
The holes in VB react with surface adsorbed OH− or H2O species to generate hydroxyl radical (•OH), which subsequently oxidize the chemicals presented in the virus (especially the shell and capsid) adsorbed on the photocatalyst surface.
-
3)
The electrons in CB, after reaction with O2, produce highly efficient radicals (•O2 −, •OH, and •OOH), and then, the generated ROS initiates reactions, leading to destruction of the virus adsorbed on the photocatalyst surface.
As stated, the diverse highly efficient species, produced on the photocatalyst surface, can oxidize viruses adsorbed on the photocatalyst surface, leading to their disinfection [45]. Fig. 2 demonstrates the formation of e−/h+ pairs, recombination step, generation of ROS, and viral disinfection. Finally, the photocatalytic disinfection efficiency of viruses is obtained using Eq. (1):
| (1) |
where Nt and N0 stand for concentrations at the time t and the initial time, respectively, and Q is the microorganism removal efficiency [46].
Fig. 2.
Disinfection mechanism of viruses by photocatalysts.
The heterogeneous photocatalytic performance of viral disinfection principally pertains to the capability for retardation of e−/h+ pairs from recombination and the ability to fully utilize sunlight. Regrettably, the photocatalytic ability of most semiconductors is trivial because of several disadvantages including poor capability for e−/h+ separation, fast recombination of charges, and low utilization efficiency of solar light [47], [48]. Up to now, diverse photocatalysts such as g-C3N4, CuO, ZnO, TiO2, and Ag3PO4 prepared with different morphologies, structures, and procedures have been used for the inactivation of viruses due to their nonhazardous nature and high stability [49], [50], [51]. Moreover, they are eco-friendly and are used in biomedical fields such as drug delivery systems, biological sensors, cell imaging, and the photodynamic therapy of cancer [52]. However, the practical and large scale utilization of the mentioned semiconductors in photocatalysis is normally hindered by the rapid recombination of charges, inadequate visible-light absorption, and a low specific surface area. In the past years, considerable efforts have been made to overcome the mentioned shortfalls and increase the photoactivity of sole semiconductors through extrinsic and intrinsic doping [53], [54], surface modification [55], sensitization [56], and coupling with other semiconductors to form heterojunctions [57]. Therefore, by considering the actual state of the review, this study focuses on a survey of the photocatalytic inactivation of waterborne, airborne, and foodborne viruses using semiconductor-assisted photocatalysis and the perspective of this important research field to tackle issues related to the spread of different viruses worldwide. Thus, in the following sections, we will review the literature on photocatalytic viral inactivation for waterborne, airborne, and foodborne viruses.
2.1. Waterborne pathogenic viruses
Waterborne pathogenic viruses (like enteroviruses, adenoviruses, noroviruses, and rotaviruses) are usually found in groundwater, surface water, and even treated drinking water, which could pose serious hazards to human health [58], [59], [60]. For this reason, most studies have been focused on the antiviral ability of various photocatalysts against waterborne viruses [61], [62], [63]. In 1994, Sierka and Sjogren as pioneers in managing the photocatalytic inactivation of viruses explored MS2 disinfection, as a waterborne virus, with TiO2 [41]. Near-UV photocatalytic inactivation was performed in TiO2 suspensions. The phage MS2 disinfection level reached to 99.9% after adding 2 µM of FeSO4. The increased MS2 disinfection level might be described by impressive targeting of OH oxidation directed by FeSO4 adsorbed to MS2. In 1998, in another research, Lee et al. used Bacteriophage Qβ, as a model waterborne virus [64]. They concluded that UV illumination on TiO2 suspension was more effective in virus disinfection than only UV illumination. Accordingly, after 2 min of irradiation, 3.5-log10 Qβ disinfection was obtained by UV illumination on TiO2, while UV disinfected only 2-log10 Qβ. In another research, Koizumi and Taya investigated the photocatalytic disinfection of phage MS2 over TiO2, in different situations [65]. It was observed that the considerable disinfection of MS2 occurred only with the presence of light and TiO2. Concerning the efficacy of pH on MS2 disinfection, the rate constant was determined to show an outstanding property with the maximal amount at pH 6.0 (9.1 × 10−2 s−1).
Noroviruses (NVs) are identified as major waterborne viruses, which cause outbreaks of gastroenteritis. Hence, in 2005, Kato et al. explicated methods to quantitatively specify NVs in wastewater and determine the probability of the inactivation procedure [66]. It was found that the UV-assisted TiO2 system was effective in disinfection of viruses. In this study, the UV-assisted TiO2 system was a developed inactivation system wherein •OH species, created through the oxidative decomposition of H2O/−OH species, led to disinfection of viruses. After that, in 2006, the photocatalytic disinfection of MS2 was carried out with the mixture of rutile and anatase-type TiO2 upon black-light illumination [67]. As illustrated in Fig. 3 , due to the coexistence of two types of TiO2, they can be in close contact, which may facilitate the exchange of h+ and e- among these phases with the balanced acceleration of reduction and oxidation rates. As a consequence, the quantum yield can increase production of ROS.
Fig. 3.
Possible mechanism for enhancement of photocatalytic disinfection of viruses caused by contacting between anatase and rutile phases of TiO2 (The modified illustration with permission from reference [67] 2006 Elsevier).
In another research, Sang et al. inactivated rotavirus, astrovirus, and feline calicivirus using a TiO2 film under visible light [68]. They displayed that under the activation of TiO2 with visible light through a fluorescent white lamp, •OH and O2 − species are generated in a significant amount. The authors inferred that virus disinfection over this photocatalyst may be related to the production of these ROS. They also underscored the potential and importance of the fabricated TiO2 film in the future specific treatment of enteropathogenic viral infection. In another attempt, Li et al. fabricated palladium-modified/nitrogen-doped TiO (denoted as TiON/PdO) nanocomposite via a sol–gel method [69]. In the fabrication of this nanocomposite, the palladium additive appeared as PdO. For the first time, a great amount of coliphage MS2, as a nonpathogenic virus, was inactivated upon visible-light irradiation. In this study, highly reactive •OH was generated by TiON/PdO under visible light to disinfect MS2 phage. In 2010, a specific procedure was presented for identical viral and bacterial adhesion on the selected photocatalysts. Bacillus thuringiensis, Mycobacterium smegmatis, and vaccinia viruses were disinfected after adhering to the TiO2 and Pt/TiO2 photocatalysts [70]. An enhanced disinfection rate was observed on the Pt/TiO2 upon UVA illumination. A disinfection rate of 99.8% and 90% was obtained in 30 min of the light illumination on the Pt/TiO2 and TiO2 photocatalysts, respectively. The outcomes illustrated that TiO2 or Pt/TiO2 photocatalytic filters are able to disinfect viral microorganisms and successfully decompose them into inorganic products.
Another research group in 2010, illustrated that boosted photocatalytic efficiency over TiO2 for bacteriophage MS2 virus disinfection was obtained through adding SiO2 [71]. They reported that by adding SiO2, the virus deposition increased on the photocatalyst. The increased photoactivity for the TiO2-SiO2 composite could be related to the reduced recombination of the charge carriers, production of a higher concentration of •OH, and higher adsorption ability of MS2 to the photocatalyst surface. Moreover, as can be viewed in Fig. 4 , the TiO2-SiO2 photocatalysts were better than TiO2 and the rate constant for the TiO2-SiO2 photocatalyst fabricated with refluxing method was 3.1 min−1, which was almost 3-folds premier than that of TiO2 (0.95 min−1). Hence, the enhanced ability of this photocatalyst for MS2 disinfection makes it a more noteworthy alternative for using as an antiseptic.
Fig. 4.
Photocatalytic inactivation of MS2 by TiO2 (P25) (green, R2 = 0.935), and TiO2 (P25)-SiO2 (blue, R2 = 0.970) photocatalysts (Reproduced with permission from reference [71] Copyright 2011 Elsevier). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In plasmonic photocatalysts, metal noble particles are dispersed over photocatalysts, which possess outstanding properties due to the surface plasmonic resonance (SPR). A further combination of light with metal particles at special photon energies is because of the excitation of the electron in noble particles under the light illumination, introduced as SPR. The individual capability of noble metal particles for light absorption and scattering depends on the particle geometry and metal type. Recently, it has been illustrated that plasmon-excited nanoparticles can be an effective resource of hot electrons. Under optical excitation, any plasmon quantum can decay either nonradiatively into an electron-hole pair or radiatively into a photon (scattering). Hence, this process can be completely impressive and if a nearby semiconductor as an electron acceptor exists, hot electrons can move into its electronic states [72], [73]. Photocatalysis can benefit from the SPR in different ways. First, the resonance wavelengths for Ag and Au nanoparticles can be manipulated to be in the range of visible or near-UV lights, based on the shape, size, and surrounding media. Second, the SPR can remarkably improve the visible-light harvesting ability of the wide-band gap photocatalysts [74], [75].
Liga et al. studied silver-doped TiO2 nanoparticles (denoted as nAg/TiO2) to disinfect MS2 [76]. The disinfection rate of MS2 increased with the increase of the Ag amount. These photocatalysts were excited by visible light through SPR generated by the silver nanoparticles. They showed that the nAg/TiO2 photocatalyst remarkably increased the photocatalytic disinfection of the virus through enhancing •OH generation in addition to slightly enhancing virus adsorption. Liga et al. also synthesized SiO2-TiO2 photocatalysts through modifying the TiO2 surface with SiO2 [77]. Although the SiO2-TiO2 photocatalysts slightly decreased •OH generation, they presented excellent photocatalytic disinfection rates for MS2, due to the increased MS2 adsorption. These outcomes suggested that modifying TiO2 surface to enhance adsorption of viruses is a major method in developing photocatalytic disinfection performance.
In another research, Zuo et al. used Phi X174 and MS2 viruses as viral disinfection models [78]. They applied three disinfection procedures (UV, chlorine, and UV/TiO2) to eliminate the abovementioned viruses. Based on the obtained results, the successful disinfection in the presence of the UV-assisted TiO2 system was observed for the mentioned viruses from water sources. Li et al. for the first time evaluated the photocatalytic disinfection of MS2 virus on g-C3N4, as a metal-free semiconductor, upon visible-light excitation [79]. Briefly, the inactivation process was as follows: By preparing 1 × 108 PFU/mL of the primary virus, the MS2 stock suspension was diluted into 100 mL of PBS. Then, the obtained g-C3N4 photocatalyst was appended for inactivation of the virus. The suspension was stirred at 25 ˚C. Before illumination, to reach an adsorption–desorption balance, the reaction was left in the dark upon stirring for 30 min. Viruses were sampled at different irradiation times using a 300 W Xenon lamp, and then were enumerated immediately to stop any further inactivation. A reaction solution containing the sample and viruses, without the light irradiation, was utilized as a dark control. Moreover, as a light control experiment, a suspension of viruses without the photocatalyst was illuminated under the same circumstance. Upon the light illumination for 5 h, the ability of g-C3N4 was compared with those of other visible-light-induced systems, like NTiO2, Bi2WO6, and Ag@AgCl photocatalysts in the viral disinfection. Accordingly, g-C3N4 disinfected >7 log of the virus, while the Bi2WO6 and N-TiO2 photocatalysts reduced the amount of the virus only ~4 log and ~1 log, respectively (Fig. 5 a). The g-C3N4 photocatalyst demonstrated long-term and durable anti-virus efficacy against MS2. As demonstrated in Fig. 5b, the disinfection of this virus by this photocatalyst was explained as nonselective reactions initiated by e−, •OH, and •O2 − species, which finally led to removal of virus without regrowth. Thus, it was inferred that g-C3N4 and g-C3N4–based photocatalysts are excellent selections for inactivation of different viruses.
Fig. 5.
(a) Comparison between the photocatalytic performance of g-C3N4, N-TiO2, Bi2WO6, and Ag@AgCl photocatalysts for MS2 inactivation under 300 min visible-light irradiation. (b) Schematic illustration of proposed mechanism for the viral inactivation by g-C3N4 (Reproduced with permission from reference [77] Copyright 2016 Elsevier).
In 2018, Cheng et al. prepared Ag3PO4/g-C3N4 (denoted as AgCN) nanocomposites through a hydrothermal procedure [49]. The photocatalytic inactivation was studied with bacteriophage f2, as a viral model. Meanwhile, the mechanism of boosted inactivation by the mentioned binary photocatalyst was evaluated through radical quenching tests, denoting that •OH and h+ played major roles in the inactivation process. The photocatalytic disinfection efficiency of f2 virus through the mentioned binary photocatalysts reached 6.5 log in 80 min upon visible-light excitation. This impressive enhancement was related to the synergistic effects of Ag3PO4 and g-C3N4 for the supreme segregation of the charge carriers and broad visible-light absorption. To describe the improved photocatalytic performance of the mentioned binary photocatalysts, a photocatalytic Z-scheme mechanism was suggested, as represented in Fig. 6 . The selected virus was decomposed by the ROS produced after efficiently separation of the charge carriers of g-C3N4 and Ag3PO4 components. This work completely created a type of promising nanocomposite for disinfection of different viruses present in contaminated water sources.
Fig. 6.
The possible mechanism for photocatalytic inactivation of bacteriophage f2 by AgCN photocatalysts under visible light (Reproduced with permission from reference [49] Copyright 2018 Elsevier).
In another attempt, some novel metal-free nanocomposites of oxygen-doped g-C3N4/hydrothermal carbonation carbon (denoted as O-g-C3N4/HTCC) microspheres were successfully constructed through a simple two-step method [13]. The optimum nanocomposite had strong visible-light absorption and showed outstanding virucidal efficacy for HAdV-2, which disinfected 5-log in 2 h of the light illumination. The boosted viral disinfection ability of the optimum nanocomposite was related to the Z-scheme mechanism for separation of charges, which significantly developed charge segregation (Fig. 7 ). Furthermore, the Z-scheme mechanism simplified the generation of •OH, leading to the lethal rupture of the rigid capsid of HAdV-2.
Fig. 7.
The possible Z-scheme mechanism proposed for the O-g-C3N4/HTCC photocatalyst (The modified form with permission from reference [13] 2019 Elsevier).
2.2. Airborne pathogenic viruses
The emission of airborne microorganisms through animal feeding operations has prompted growing worries in the field of epidemiology and health [80]. Airborne microorganisms may create negative immune reactivity in animals and trigger inflammatory respiratory dysfunction in humans. Moreover, several pathogenic species are suspected to be airborne transmittable species among farms [81], [82]. Several infectious viruses have been discovered at the outlet of animal houses, and other extremely infectious illnesses are spread via aerial transmission. Hence, impressive procedures are needed to hinder pathogen transmission and decrease microbial emissions, and thus safeguard human and animal health.
Although many studies about the photocatalytic disinfection of bacteria have been carried out, few investigations have been reported about disinfection of airborne viruses. Hence, in a research conducted in 2012, Nakano et al. observed that TiO2 thin film significantly disinfected the influenza virus through degradation of viral proteins. They realized that the degradation relied on the illumination time of UV and its intensity [83]. Despite the partial intensity of UVA, a viral decrease of almost 4 log10 was seen during the illumination time. These outcomes demonstrated that the TiO2 thin film could be applied to disinfect the influenza virus in the air. As a result, TiO2 might be an efficient photocatalyst for disinfection of other airborne viruses and it can be applied to hinder viral transition through air. In addition, Daikoku et al. constructed a porous ceramic substrate coated with nanosized-TiO2 and used it for disinfection of airborne influenza infection [84]. The airborne infectivity of the influenza virus was attenuated within 5 min through this photocatalytic system under black light. The photocatalytic air cleaner impressively inactivated airborne influenza virus infection. Moreover, as known, air contamination created by fine particulate matters (PM2.5), bioaerosols, and volatile organic compounds, is a major risk to health. In 2017, Shiraki et al. constructed a photocatalytic air cleaner to decrease the contamination levels of indoor air. They studied the photocatalytic activity using UV-LED [85]. For this purpose, they fabricated a TiO2-coated aluminum plate system as a photocatalyst. This photocatalytic air cleaner was utilized for inactivation of the aerosol-associated influenza virus. This photocatalytic system effectively disinfected aerosol-associated influenza virus, showing that it could detoxify and clean the air in a closed place.
Doss et al. introduced the first LED photocatalytic system constructed by TiO2/β-SiC solid alveolar foams for air purification from airborne viruses such as T2 bacteriophage [12]. They demonstrated that the purification efficiency associated with the elimination of this virus resulted from the passive filtration effect and the photocatalytic ability of the TiO2/β-SiC solid alveolar foams. Moreover, it was reported that utilizing 56 LEDs versus 40 LEDs significantly improved the logarithmic reduction. These outcomes demonstrated the potential application of LEDs and solid alveolar foam as an impressive and energy-saving method to disinfect viruses via a photocatalytic procedure.
Although some UV-based inactivation procedures have been introduced for viral disinfections, these techniques often need long illumination periods or use of the recirculating mode due to their low photon energy. Vacuum UV (V-UV) was lately discovered to be a highly effective light source. In a research conducted by Kim and Jang [18], the photocatalytic processes were investigated by V-UV with short illumination times to simultaneously disinfect MS2 as an airborne virus (nearly 90% disinfection efficiency at a VUV illumination time of 0.009 s) and remove the produced ozone toward an air inactivation system. In this study, the Pd-TiO2/VUV system demonstrated the ability for simultaneous MS2 disinfection and ozone elimination and the photocatalytic ability was efficient regardless of relative humidity.
In another study carried out by Choi and Cho, novel visible-light-driven nanocomposites based on some metals (Mg, Fe, and Mn) deposited on TiO2 were investigated for their antiviral ability against airborne viruses such as influenza H1N1 [86]. Under wavelengths higher than 410 nm supplied with fluorescent lamps, >99% of the virus was inactivated within half an hour. Thus, they concluded that the mentioned nanocomposites could be satisfactorily utilized to decrease viral transmission.
2.3. Foodborne pathogenic viruses
Human norovirus (denoted as HuNoV) is the main cause of viral gastroenteritis [87], [88]. Hence, it is necessary to determine intervention procedures to decrease the danger of foodborne diseases. The inactivation yield of disinfection procedures is hard to investigate for vegetables and fruits, due to their irregular surface characteristics and inconsistent degree of pollution [89], [90]. Park et al. investigated the disinfection efficiency of murine norovirus 1 (MNV-1), as a HuNoV, by a constructed solidified agar matrix (SAM) as a simulated blueberries under UVA, UVB, and UVC irradiations in the absence and presence of TiO2 [91]. Among them, UVC with TiO2 obtained a good level of virus decrease for both externally inoculated and internalized MNV-1. Furthermore, the Weibull model was used to explain the disinfection mechanism of MNV-1, which proved an excellent fit to the data. There are many studies which agree with the Weibull model, as a better fit than first-order models for kinetic analysis of disinfection processes. More importantly, the Weibull model has been extensively utilized because of its simplicity and flexibility [92], [93]. A good correlation was quantified between the viral disinfection and the steady-state concentration of •OH through a probe compound, indicating that the generated •OH was the main species for MNV-1 disinfection. It was found that UVC in the absence and presence of TiO2 was more efficient for MNV-1 disinfection than UVA and UVB irradiations. Hence, the UVC/TiO2 inactivation system can disinfect the virus found inside and on the surface of blueberries, which is an impressive alternative technique to common chlorine inactivation for decreasing the danger of HuNoV infection in various foods. Moreover, the viral disinfection procedure presented by this research group indicated that the use of SAM as an efficient simulated food for evaluation of the yield of disinfection procedures indicated favorable outcomes.
As mentioned, HuNoV is the main cause of foodborne diseases, which can also associate with shellfish consumption [94]. Kim et al. injected MNV-1 inside SAM for virus internalization and specified the effect of high hydrostatic pressure (HHP) and UV-assisted TiO2 [95]. The internalized MNV-1 decreased by 5.5-log10, when HHP was followed by the UV-assisted TiO2 system, suggesting a synergistic disinfection effect. Moreover, as can be viewed in Fig. 8 a, untreated MNV-1 particles were round with a 35–40 nm diameter, while the TEM image of the treated MNV-1 virus demonstrated considerable virus deformation due to a synergistic efficacy of the inactivation procedure. This suggested that the outer surface was degraded (Fig. 8b). As a result, a hurdle strategy of the HHP-UV-assisted TiO2 combined method proved efficient for internalized MNV-1 via a simulated food.
Fig. 8.
(a) TEM analysis of purified MNV-1 (untreated) and (b) HHP-UV-assisted TiO2 treatment (Reproduced with permission from reference [93] Copyright 2017 Elsevier).
A summary of details about photocatalysts and operational parameters applied for viral disinfection is presented in Table 1 . Furthermore, we know that heterogeneous photocatalytic processes are non-selective technology for degradation of pollutants and inactivation of microorganisms. Up to now, a wide range of organic contaminants and microorganisms have been removed/inactivated over photoactive materials. Despite non-selectivity in viral disinfections, the most important issue is the lack of any research about heterogeneous photocatalytic disinfection of novel viruses. As a consequence, more scientific activities in the field of heterogeneous photocatalytic disinfection of new viruses should be stimulated to obtain more insights about the exact potential of this technology to tackle the diseases caused by these viruses. After designing and fabricating more efficient photocatalysts, these photoactive materials could be used for inactivation of waterborne viruses in water decontamination plants and fabrication of more effective filters to disinfect airborne viruses. Additionally, walls of infectious parts of hospitals can be covered with photoactive materials to sustainably disinfect/inactivate viruses adhered over them using the irradiations applied for lightning of these parts. Furthermore, on streets of cities, photoactive materials may be used to sustainably inactivate/disinfect viruses using solar energy, without using any compounds currently used for inactivation of viruses.
Table 1.
Summary of utilized photocatalysts for various viral disinfection.
| Photocatalyst | Virus | Operational condition |
Light source | Disinfection efficiency | Type of virus | Ref. | |
|---|---|---|---|---|---|---|---|
| Catalystloading(mg/L) | Virus level(PFU*/mL) | ||||||
| TiO2 | Phage MS2 | 1000 | 6 × 104 | UV | 2.8-log in 65 min | waterborne | [41] |
| TiO2 | Phage MS2 | 1000 | 6 × 105 | 18 W BLB* lamp | 1.8-log in 180 min | waterborne | [61] |
| TiO2 | Bacteriophage Qβ | 1000 | 1 × 106 | UV lamp | 3.5-log in 2 min | waterborne | [64] |
| TiO2 | Phage f2 | 1000 | 1010–1011 | 6 W black light lamp | 6-log in 15 min | waterborne | [78] |
| TiO2 | Influenza virus | No data | 4.0 × 108 | 1 mW black light | Eliminated in 5 min | airborne | [83] |
| TiO2 | Influenza virus | No data | 0.0 or 0.1 mg ml−1 | 20 W black light | 4-log in a short irradiation time | airborne | [84] |
| TiO2 | H1N1 | No data | No data | UV-LED lamp | Eliminated in 7 min | airborne | [85] |
| TiO2 | MNV-1 | No data | No data | UV lamp | 3.2-log in 10 min | foodborne | [91] |
| TiO2 | MNV-1 | No data | No data | UV lamp | >5.5-log in 15 min | foodborne | [95] |
| TiO2 | MS-2 bacteriophage | No data | 2 × 105 | 4 W BLB lamp | 2-log in 109 min | waterborne | [96] |
| TiO2 | Phage f2 | 100 | >20 | 4 W UV-Clamp | 5–6-log in 160 min | waterborne | [97] |
| TiO2 | Murine norovirus | 10 | 1 × 108 | UV lamp | 3.3-log in 24 h | waterborne | [98] |
| Pd-TiO2 | MS2 | No data | 2.30 (±1.27) × 108 | VUV | 90% in 0.009 s | airborne | [18] |
| Cu-TiO2 nanofibers | Bacteriophage f2 | 50 | 1 × 104 | Xe lamp | 4.0-log in 120 min | waterborne | [46] |
| Fe, Mg, and Mn-loaded TiO2 | H1N1 | No data | 100 μl | Fluorescent lamp | 99% in 30 min | airborne | [86] |
| Cu-TiO2 nanofibers | Bacteriophage f2 | 10 | 1 × 108 | Xe lamp | > 5-log in 240 min | waterborne | [99] |
| Mn-TiO2 | Phage MS2 | 100 | 1 × 105 | 150 W Xe ozone-free lamp | 4-log in 60 min | waterborne | [100] |
| TiO2/β-SiC | T2 Bacteriophage | No data | 6 × 106 | 56 LEDs | 3-log in 60 min | airborne | |
| TiO2/CuO films | Phage T4 | No data | 8 × 109 | 40 W UVA lamp | 9.9-log in 80 min | waterborne | [50] |
| SiO2-TiO2 | Phage MS2 | 100 | 3 × 107 | 8 W UVA lamp | 5-log in 1.8 min | waterborne | [71] |
| nAg/TiO2 | Phage MS2 | 100 | 3 × 107 | 8 W UVA lamp | 9.9-log in 80 min | waterborne | [76] |
| g-C3N4 | Phage MS2 | 150 | 1 × 108 | 300 W Xe lamp | 8-log in 300 min | waterborne | [79] |
| g-C3N4 | Phage MS2 | 135.4 | 1 × 108 | Xe lamp | 8-log in 240 min | waterborne | [101] |
| Ag3PO4/g-C3N4 | bacteriophage f2 | 100 | 3 × 107 | 8 W UVA lamp | 6.5-log in 80 | waterborne | [49] |
| g-C3N4/EP* | MS2 | 0.06 | 1 × 108 | 300 W Xe lamp | 8-log in 240 min | waterborne | [102] |
| O-g-C3N4/HTCC* | HAdV-2 | 3 | No data | 7 W white LED* lamp | 5-log in 120 min | waterborne | [13] |
| TiON/PdO | Phage MS2 | 100 | 3 × 108 | 1000 W Xe arc lamp | 1.5-log in 60 min | waterborne | [69] |
| G-WO3 films | Phage MS2 | No data | 2 × 106 | Mercury lamp | 5.9-log in 180 min | waterborne | [103] |
| FeO | Phage MS2 | 5 | 1 × 106 | Simulated solar | 5-log in 30 min | waterborne | [104] |
| C60/SiO2 | Phage MS2 | 300 | 5 × 106 | UVA, FL*, BLB | [UV]: 3.55-log in 75 min[FL]: 2.8-log in 75 min[BLB]: 2.3-log in 75 min | waterborne | [105] |
| C70/SiO2 | Phage MS2 | 300 | 3 × 108 | Vis, Sunlight | [Vis]: 4.35-log in 90 min[Sunlight]: 4.4-log in 90 min | waterborne | [106] |
| Rh-SrTiO3 | Phage Qβ | 3000 | 5 × 107 | Vis | 5-log in 120 min | waterborne | [107] |
[*]: PFU = plaque forming unit; HRV-Wa = human rotavirus type 2 Wa; Xe = Xenon; BLB = black-light-blue; EP = expanded perlite; FL = Fluorescent light; HTCC = Hydrothermal carbonation carbon; LED = Light emitting diode.
3. Conclusions and future perspectives
Given the fact that viruses are potential dangers to human health, their removal from water, air, and foods is of prominent concern for researchers worldwide. Luckily, semiconductor-based photocatalysis has indicated a potential alternative technology with valuable merits of the facility, high efficiency, and energy-saving procedure to inactivate various viruses present in water, air, and foods. This is the first review on the development of heterogeneous photocatalytic disinfection of waterborne, airborne, and foodborne viruses. The research domains recommended below can open up new opportunities for exploring the potential application of the photocatalytic viral inactivation technology in addressing new challenges provoked by viral epidemic and pandemic cases.
Although utilized photocatalysts have presented promising results for disinfection of different viruses, more efficient photocatalysts are still needed to be applied for viral disinfections. Therefore, it is urgently necessary to explore ideal materials for photocatalytic disinfection processes with relatively high redox potential, low price, and long-term durability. To use sustainable resources in the photocatalytic viral inactivation processes, it is highly desired to develop more efficient visible/solar-light driven photocatalysts to tackle viral issues. In our opinion, recently developed photocatalysts based on g-C3N4, metal–organic frameworks, layered double hydroxides, and MXenes will be hot research topics as viral disinfection materials in the next decade. Furthermore, magnetically separable materials to simplify the separation of utilized photocatalysts from the aquatic systems are another bottleneck for the semiconductor-based photocatalytic viral disinfection procedures. To maximize the light/oxygen utilization to produce more ROS, floating photocatalysts are promising choices. In addition, contrary to waterborne viruses, the research on photocatalytic viral disinfections for airborne and foodborne viruses is in its infancy. Hence, more studies should be conducted to illustrate the potential application of the heterogeneous photocatalysis technology to disinfect these viruses. In spite of the non-selectivity of photocatalytic disinfection of viruses, the most important issue is the lack of any research about heterogeneous photocatalytic disinfection of novel viruses such as SARS-CoV and COVID-19. Therefore, future scientific studies should be undertaken on the field of heterogeneous photocatalysis processes to gain more insights into the exact potential of this technology to tackle new viral diseases. Consequently, there is no doubt that the heterogeneous photocatalysis viral disinfection technology, with many appealing merits, will be an impressively active research field in the future to provide comfort and confidence to humans worldwide.
CRediT authorship contribution statement
Aziz Habibi-Yangjeh: Conceptualization, Writing - review & editing, Supervision. Soheila Asadzadeh-Khaneghah: Writing - original draft, Methodology. Solmaz Feizpoor: Writing - original draft, Methodology. Afsar Rouhi: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are honestly thankful to the University of Mohaghegh Ardabili-Iran for the financial support of this study. We would also like to kindly appreciate Miss Hesane Habibi for her insightful comments on viruses.
References
- 1.Pang L. Microbial removal rates in subsurface media estimated from published studies of field experiments and large intact soil cores. J. Environ. Qual. 2009;38:1531–1559. doi: 10.2134/jeq2008.0379. [DOI] [PubMed] [Google Scholar]
- 2.Bradford S.A., Morales V.L., Zhang W., Harvey R.W., Packman A.I., Mohanram A., Welty C. Transport and fate of microbial pathogens in agricultural settings. Critic. Rev. Environ. Sci. Technol. 2013;43:775–893. [Google Scholar]
- 3.Arnone R.D., Walling J.P. Waterborne pathogens in urban watersheds. J. Water Health. 2007;5:149–162. doi: 10.2166/wh.2006.001. [DOI] [PubMed] [Google Scholar]
- 4.Gall A.M., Mariñas B.J., Yi L.u., Shisler J.L. Waterborne viruses: a barrier to safe drinking water. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C., Li Y., Shuai D., Shen Y., Wang D. Progress and challenges in photocatalytic disinfection of waterborne viruses: a review to fill current knowledge gaps. Chem. Eng. J. 2019;355:399–415. [Google Scholar]
- 6.Moelling K. Viruses More Friends than Foes. Electroanalysis. 2020;32:669–673. [Google Scholar]
- 7.Cesewski E., Johnson B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020;159 doi: 10.1016/j.bios.2020.112214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Sayed A., Kamel M. Climatic changes and their role in emergence and re-emergence of diseases. Environ. Sci. Pollut. Res. Int. 2020 doi: 10.1007/s11356-020-08896-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson K.E. Viral pathogens in water: occurrence, public health impact, and available control strategies. Curr. Opin. Virol. 2014;4:50–57. doi: 10.1016/j.coviro.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbaszadegan M., Alum A. Waterborne enteric viruses: diversity, distribution, and detection. Manual Environ. Microbiol. 2016:1–13. Fourth Edition 3.1.5. [Google Scholar]
- 11.Prevost B., Goulet M., Lucas F.S., Joyeux M., Moulin L., Wurtzer S. Viral persistence in surface and drinking water: Suitability of PCR pre-treatment with intercalating dyes. Water Res. 2016;91:68–76. doi: 10.1016/j.watres.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 12.Doss N., Carré G., Keller V., André P., Keller N. Photocatalytic decontamination of airborne T2 bacteriophage viruses in a small-size TiO2/β-SiC alveolar foam LED reactor. Water Air Soil Pollut. 2018;229:29. [Google Scholar]
- 13.Zhang C., Zhang M., Li Y., Shuai D. Visible-light-driven photocatalytic disinfection of human adenovirus by a novel heterostructure of oxygen-doped graphitic carbon nitride and hydrothermal carbonation carbon. Appl. Catal. B. 2019;248:11–21. [Google Scholar]
- 14.Lupia T., Scabini S., Pinna S.M., Di Perri G., De Rosa F.G., Corcione S. 2019-novel coronavirus outbreak: A new challenge. J. Global Antimicrob. Resist. 2020;21:22–27. doi: 10.1016/j.jgar.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease (COVID-19): a systematic review of imaging findings in 919 patients. Am. J. Roentgenol. 2019;2020:1–7. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Xie M., Chen Q. Insight into 2019 novel coronavirus—an updated intrim review and lessons from SARS-CoV and MERS-CoV. Int. J. Infect. Diseases. 2020 doi: 10.1016/j.ijid.2020.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Z., Wu Y., Shen F., Chen Q., Tan M., Yao M. Bioaerosol science, technology, and engineering: past, present, and future. Aerosol Sci. Technol. 2011;45:1337–1349. [Google Scholar]
- 18.Kim J., Jang J. Inactivation of airborne viruses using vacuum ultraviolet photocatalysis for a flow-through indoor air purifier with short irradiation time. Aerosol Sci. Technol. 2018;52:557–566. [Google Scholar]
- 19.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleibtreu A., Bertine M., Bertin C., Houhou-Fidouh N., Visseaux B. Focus on Middle East respiratory syndrome coronavirus (MERS-CoV) Medecine et Maladies Infectieuses. 2019 doi: 10.1016/j.medmal.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020;27:1–4. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Liu S.-M., Yu X.-H., Tang S.-L., Tang C.-K. Coronavirus disease (COVID-19): current status and future perspective. Int. J. Antimicrob. Agents. 2019;2020 doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ren H., Koshy P., Chen W.-F., Qi S., Sorrell C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017;325:340–366. doi: 10.1016/j.jhazmat.2016.08.072. [DOI] [PubMed] [Google Scholar]
- 25.Ateia M., Alalm M.G., Awfa D., Johnson M.S., Yoshimura C. Modeling the degradation and disinfection of water pollutants by photocatalysts and composites: A critical review. Sci. Total Environ. 2020;698 doi: 10.1016/j.scitotenv.2019.134197. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C., Li Y., Shuai D., Shen Y., Xiong W., Wang L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere. 2019;214:462–479. doi: 10.1016/j.chemosphere.2018.09.137. [DOI] [PubMed] [Google Scholar]
- 27.Cedergren M.I., Selbing A.J., Löfman O., Källen B.A.J. Chlorination byproducts and nitrate in drinking water and risk for congenital cardiac defects. Environ. Res. 2002;89:124–130. doi: 10.1006/enrs.2001.4362. [DOI] [PubMed] [Google Scholar]
- 28.Hijnen W.A.M., Beerendonk E.F., Medema G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo) cysts in water: A review. Water Res. 2006;40:3–22. doi: 10.1016/j.watres.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 29.Li D., Gu A.Z., He M., Shi H.-C., Yang W. UV inactivation and resistance of rotavirus evaluated by integrated cell culture and real-time RT-PCR assay. Water Res. 2009;43:3261–3269. doi: 10.1016/j.watres.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 30.Yu B.F., Hu Z.B., Liu M., Yang H.L., Kong Q.X., Liu Y.H. Review of research on air-conditioning systems and indoor air quality control for human health. Int. J. Refrig. 2009;32:3–20. [Google Scholar]
- 31.Miklos D.B., Remy C., Jekel M., Linden K.G., Drewes J.E., Hübner U. Evaluation of advanced oxidation processes for water and wastewater treatment–A critical review. Water Res. 2018;139:118–131. doi: 10.1016/j.watres.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 32.Sillanpää M., Ncibi M.C., Matilainen A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manage. 2018;208:56–76. doi: 10.1016/j.jenvman.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Yang L., Zhou H., Fan T., Zhang D. Semiconductor photocatalysts for water oxidation: current status and challenges. PCCP. 2014;16:6810–6826. doi: 10.1039/c4cp00246f. [DOI] [PubMed] [Google Scholar]
- 34.Kanakaraju D., Glass B.D., Oelgemöller M. Advanced oxidation process-mediated removal of pharmaceuticals from water: a review. J. Environ. Manage. 2018;219:189–207. doi: 10.1016/j.jenvman.2018.04.103. [DOI] [PubMed] [Google Scholar]
- 35.Friedmann D., Hakki A., Kim H., Choi W., Bahnemann D. Heterogeneous photocatalytic organic synthesis: state-of-the-art and future perspectives. Green Chem. 2016;18:5391–5411. [Google Scholar]
- 36.Kou J., Lu C., Wang J., Chen Y., Xu Z., Varma R.S. Selectivity enhancement in heterogeneous photocatalytic transformations. Chem. Rev. 2016;117:1445–1514. doi: 10.1021/acs.chemrev.6b00396. [DOI] [PubMed] [Google Scholar]
- 37.Robertson P.K.J., Robertson J.M.C., Bahnemann D.W. Removal of microorganisms and their chemical metabolites from water using semiconductor photocatalysis. J. Hazard. Mater. 2012;211:161–171. doi: 10.1016/j.jhazmat.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 38.Chen D., Cheng Y., Zhou N., Chen P., Wang Y., Li K., Huo S., Cheng P., Peng P., Zhang R., Wang L., Liu H., Liu Y., Ruan R. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Cleaner Prod. 2020;121725 [Google Scholar]
- 39.Rueda-Marquez J.J., Levchuk I., Ibañez P.F., Sillanpää M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Cleaner Prod. 2020;120694 [Google Scholar]
- 40.Dalrymple O.K., Stefanakos E., Trotz M.A., Goswami D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B. 2010;98:27–38. [Google Scholar]
- 41.Sjogren J.C., Sierka R.A. Inactivation of phage MS2 by iron-aided titanium dioxide photocatalysis. Appl. Environ. Microbiol. 1994;60:344–347. doi: 10.1128/aem.60.1.344-347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foster H.A., Ditta I.B., Varghese S., Steele A. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011;90:1847–1868. doi: 10.1007/s00253-011-3213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao P., Li A., Sun D.D., Ng W.J. Effects of various TiO2 nanostructures and graphene oxide on photocatalytic activity of TiO2. J. Hazard. Mater. 2014;279:96–104. doi: 10.1016/j.jhazmat.2014.06.061. [DOI] [PubMed] [Google Scholar]
- 44.Linsebigler A.L., Lu G., Yates J.T., Jr Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 1995;95:735–758. [Google Scholar]
- 45.Khezerlou A., Alizadeh-Sani M., Azizi-Lalabadi M., Ehsani A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018;123:505–526. doi: 10.1016/j.micpath.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Zheng X., Shen Z.-P., Cheng C., Shi L., Cheng R., Yuan D.-H. Photocatalytic disinfection performance in virus and virus/bacteria system by Cu-TiO2 nanofibers under visible light. Environ. Pollut. 2018;237:452–459. doi: 10.1016/j.envpol.2018.02.074. [DOI] [PubMed] [Google Scholar]
- 47.Dong S., Feng J., Fan M., Pi Y., Hu L., Han X., Liu M., Sun J., Sun J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: a review. RSC Adv. 2015;5:14610–14630. [Google Scholar]
- 48.Hitam C.N.C., Jalil A.A. A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J. Environ. Manage. 2020;258 doi: 10.1016/j.jenvman.2019.110050. [DOI] [PubMed] [Google Scholar]
- 49.Cheng R., Shen L.-J., Yu J.-H., Xiang S.-Y., Zheng X. Photocatalytic inactivation of bacteriophage f2 with Ag3PO4/g-C3N4 composite under visible light irradiation: Performance and mechanism. Catalysts. 2018;8:406. [Google Scholar]
- 50.Ditta I.B., Steele A., Liptrot C., Tobin J., Tyler H., Yates H.M., Sheel D.W., Foster H.A. Photocatalytic antimicrobial activity of thin surface films of TiO2, CuO and TiO2/CuO dual layers on Escherichia coli and bacteriophage T4. Appl. Microbiol. Biotechnol. 2008;79:127. doi: 10.1007/s00253-008-1411-8. [DOI] [PubMed] [Google Scholar]
- 51.Ishwarya R., Vaseeharan B., Kalyani S., Banumathi B., Govindarajan M., Alharbi N.S., Kadaikunnan S., Al-Anbr M.N., Khaled J.M., Benelli G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol., B. 2018;178:249–258. doi: 10.1016/j.jphotobiol.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 52.Khan I., Saeed K., Khan I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019;12:908–931. [Google Scholar]
- 53.Mittal A., Mari B., Sharma S., Kumari V., Maken S., Kumari K., Kumar N. Non-metal modified TiO2: a step towards visible light photocatalysis. J. Mater. Sci.: Mater. Electron. 2019;30:3186–3207. [Google Scholar]
- 54.Kumari V., Mittal A., Jindal J., Yadav S., Kumar N. S-, N-and C-doped ZnO as semiconductor photocatalysts: A review. Front. Mater. Sci. 2019;13:1–22. [Google Scholar]
- 55.Kumar S.G., Rao K.K. Comparison of modification strategies towards enhanced charge carrier separation and photocatalytic degradation activity of metal oxide semiconductors (TiO2, WO3 and ZnO) Appl. Surf. Sci. 2017;391:124–148. [Google Scholar]
- 56.Singh P., Shandilya P., Raizada P., Sudhaik A., Rahmani-Sani A., Hosseini-Bandegharaei A. Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arab. J. Chem. 2020;13:3498–3520. [Google Scholar]
- 57.Meng S., Zhang J., Chen S., Zhang S., Huang W. Perspective on construction of heterojunction photocatalysts and the complete utilization of photogenerated charge carriers. Appl. Surf. Sci. 2019;476:982–992. [Google Scholar]
- 58.Watts R.J., Kong S., Orr M.P., Miller G.C., Henry B.E. Photocatalytic inactivation of coliform bacteria and viruses in secondary wastewater effluent. Water Res. 1995;29:95–100. [Google Scholar]
- 59.Reddy P.V.L., Kavitha B., Reddy P.A.K., Kim K.-H. TiO2-based photocatalytic disinfection of microbes in aqueous media: a review. Environ. Res. 2017;154:296–303. doi: 10.1016/j.envres.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 60.Kakita Y., Kashige N., Miake F., Watanabe K. Photocatalysis-dependent inactivation of Lactobacillus phage PL-1 by a ceramics preparation. Biosci. Biotechnol. Biochem. 1997;61:1947–1948. doi: 10.1271/bbb.61.1947. [DOI] [PubMed] [Google Scholar]
- 61.Cho M., Chung H., Choi W., Yoon J. Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl. Environ. Microbiol. 2005;71:270–275. doi: 10.1128/AEM.71.1.270-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jang E.S., Won J.-H., Hwang S.-J., Choy J.-H. Fine tuning of the face orientation of ZnO crystals to optimize their photocatalytic activity. Adv. Mater. 2006;18:3309–3312. [Google Scholar]
- 63.Yang X., Wang Y. Photocatalytic effect on plasmid DNA damage under different UV irradiation time. Build. Environ. 2008;43:253–257. [Google Scholar]
- 64.Lee S., Nakamura M., Ohgaki S. Inactivation of phage Qß by 254nm UV light and titanium dioxide photocatalyst. J. Environ. Sci. Health Part A. 1998;33:1643–1655. [Google Scholar]
- 65.Koizumi Y., Taya M. Kinetic evaluation of biocidal activity of titanium dioxide against phage MS2 considering interaction between the phage and photocatalyst particles. Biochem. Eng. J. 2002;12:107–116. [Google Scholar]
- 66.Kato T., Tohma H., Miki O., Shibata T., Tamura M. Degradation of norovirus in sewage treatment water by photocatalytic ultraviolet disinfection. Nippon Steel Techn. Rep. 2005;92:41–44. [Google Scholar]
- 67.Sato T., Taya M. Enhancement of phage inactivation using photocatalytic titanium dioxide particles with different crystalline structures. Biochem. Eng. J. 2006;28:303–308. [Google Scholar]
- 68.Sang X., Xiaojie T.G., Phan S., Sugihara F., Yagyu S., Okitsu N., Maneekarn W.E.G., Muller H. Ushijima, Photocatalytic inactivation of diarrheal viruses by visible-light-catalytic titanium dioxide. Clin. Lab. 2007;53:413–422. [PubMed] [Google Scholar]
- 69.Li Q., Page M.A., Mariñas B.J., Shang J.K. Treatment of coliphage MS2 with palladium-modified nitrogen-doped titanium oxide photocatalyst illuminated by visible light. Environ. Sci. Technol. 2008;42:6148–6153. doi: 10.1021/es7026086. [DOI] [PubMed] [Google Scholar]
- 70.Kozlova E.A., Safatov A.S., Kiselev S.A., Marchenko V.Y., Sergeev A.A., Skarnovich M.O., Emelyanova E.K., Smetannikova M.A., Buryak G.A., Vorontsov A.V. Inactivation and mineralization of aerosol deposited model pathogenic microorganisms over TiO2 and Pt/TiO2. Environ. Sci. Technol. 2010;44:5121–5126. doi: 10.1021/es100156p. [DOI] [PubMed] [Google Scholar]
- 71.Jafry H.R., Liga M.V., Li Q., Barron A.R. Simple route to enhanced photocatalytic activity of P25 titanium dioxide nanoparticles by silica addition. Environ. Sci. Technol. 2011;45:1563–1568. doi: 10.1021/es102749e. [DOI] [PubMed] [Google Scholar]
- 72.Mukherjee S., Libisch F., Large N., Neumann O., Brown L.V., Cheng J., Lassiter J.B., Carter E.A., Nordlander P., Halas N.J. Hot electrons do the impossible: plasmon-induced dissociation of H2 on Au. Nano Lett. 2013;13:240–247. doi: 10.1021/nl303940z. [DOI] [PubMed] [Google Scholar]
- 73.Kuttner C. Plasmonics in sensing: from colorimetry to SERS analytics. Plasmonics. 2018:151–180. [Google Scholar]
- 74.Zhang X., Chen Y.L., Liu R.-S., Tsai D.P. Plasmonic photocatalysis. Rep. Prog. Phys. 2013;76 doi: 10.1088/0034-4885/76/4/046401. [DOI] [PubMed] [Google Scholar]
- 75.Li K., Chen P., Li J., Sun Y., Chu Y., Dong F. Enhanced plasmonic photocatalytic disinfection on noble-metal-free bismuth nanospheres/graphene nanocomposites. Catal. Sci. Technol. 2018;8:4600–4603. [Google Scholar]
- 76.Liga M.V., Bryant E.L., Colvin V.L., Li Q. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res. 2011;45:535–544. doi: 10.1016/j.watres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Liga M.V., Maguire-Boyle S.J., Jafry H.R., Barron A.R., Li Q. Silica decorated TiO2 for virus inactivation in drinking water–simple synthesis method and mechanisms of enhanced inactivation kinetics. Environ. Sci. Technol. 2013;47:6463–6470. doi: 10.1021/es400196p. [DOI] [PubMed] [Google Scholar]
- 78.Zuo X., Chu X., Hu J. Effects of water matrix on virus inactivation using common virucidal techniques for condensate urine disinfection. Chemosphere. 2015;136:118–124. doi: 10.1016/j.chemosphere.2015.04.083. [DOI] [PubMed] [Google Scholar]
- 79.Li Y., Zhang C., Shuai D., Naraginti S., Wang D., Zhang W. Visible-light-driven photocatalytic inactivation of MS2 by metal-free g-C3N4: Virucidal performance and mechanism. Water Res. 2016;106:249–258. doi: 10.1016/j.watres.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 80.Josset S., Taranto J., Keller N., Keller V., Lett M.-C. Photocatalytic treatment of bioaerosols: impact of the reactor design. Environ. Sci. Technol. 2010;44:2605–2611. doi: 10.1021/es902997v. [DOI] [PubMed] [Google Scholar]
- 81.Zhao Y., Aarnink A.J.A., Xin H. Inactivation of airborne Enterococcus faecalis and infectious bursal disease virus using a pilot-scale ultraviolet photocatalytic oxidation scrubber. J. Air Waste Manag. Assoc. 2014;64:38–46. doi: 10.1080/10962247.2013.831800. [DOI] [PubMed] [Google Scholar]
- 82.Gorvel L., Yver M., Robert E., Harmant M., Rosa-Calatrava M., Lina B., Gorvel J.P., Moulès V., Albalate R., Gaüzère C. Innovative germicidal UV and photocatalytic system dedicated to aircraft cabin eliminates volatile organic compounds and pathogenic micro-organisms. CLEAN–Soil Air Water. 2014;4:703–712. [Google Scholar]
- 83.Nakano R., Ishiguro H., Yao Y., Kajioka J., Fujishima A., Sunada K., Minoshima M., Hashimoto K., Kubota Y. Photocatalytic inactivation of influenza virus by titanium dioxide thin film. Photochem. Photobiol. Sci. 2012;11:1293–1298. doi: 10.1039/c2pp05414k. [DOI] [PubMed] [Google Scholar]
- 84.Daikoku T., Takemoto M., Yoshida Y., Okuda T., Takahashi Y., Ota K., Tokuoka F., Kawaguchi A.T., Shiraki K. Decomposition of organic chemicals in the air and inactivation of aerosol-associated influenza infectivity by photocatalysis. Aerosol Air Qual. Res. 2015;15:1469–1484. [Google Scholar]
- 85.Shiraki K., Yamada H., Yoshida Y., Ohno A., Watanabe T., Watanabe T., Watanabe H., Watanabe H., Watanabe H., Yamaguchi M., Tokuoka F., Hashimoto S., Kawamura M., Adachi N. Improved photocatalytic air cleaner with decomposition of aldehyde and aerosol-associated influenza virus infectivity in indoor air. Aerosol Air Qual. Res. 2017;17:2901–2912. [Google Scholar]
- 86.Choi S.-Y., Cho B. Extermination of influenza virus H1N1 by a new visible-light-induced photocatalyst under fluorescent light. Virus Res. 2018;248:71–73. doi: 10.1016/j.virusres.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 87.Ettayebi K., Crawford S.E., Murakami K., Broughman J.R., Karandikar U., Tenge V.R., Neill F.H., et al. Replication of human noroviruses in stem cell–derived human enteroids. Science. 2016;353:1387–1393. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bouwknegt M., Verhaelen K., Rzeżutka A., Kozyra I., Maunula L., von Bonsdorff C.-H., Vantarakis A., Kokkinos P., Petrovic T., Lazic S., Pavlik I., Vasickova P., Willems K.A., Havelaar A.H., Rutjes S.A., Husman A.M.R. Quantitative farm-to-fork risk assessment model for norovirus and hepatitis A virus in European leafy green vegetable and berry fruit supply chains. Int. J. Food Microbiol. 2015;198:50–58. doi: 10.1016/j.ijfoodmicro.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 89.Newman K.L., Leon J.S., Rebolledo P.A., Scallan E. The impact of socioeconomic status on foodborne illness in high-income countries: a systematic review. Epidemiol. Infect. 2015;143:2473–2485. doi: 10.1017/S0950268814003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yemmireddy V.K., Hung Y.-C. Using photocatalyst metal oxides as antimicrobial surface coatings to ensure food safety—Opportunities and challenges. Compr. Rev. Food Sci. Food Saf. 2017;16:617–631. doi: 10.1111/1541-4337.12267. [DOI] [PubMed] [Google Scholar]
- 91.Park D., Shahbaz H.M., Kim S.-H., Lee M., Lee W., Oh J.-W., Lee D.-U., Park J. Inactivation efficiency and mechanism of UV-TiO2 photocatalysis against murine norovirus using a solidified agar matrix. Int. J. Food Microbiol. 2016;238:256–264. doi: 10.1016/j.ijfoodmicro.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 92.Bae S.-C., Park S.Y., Kim A.-N., Oh M.-H., Ha S.-D. Survival of hepatitis A virus on various food-contact surfaces during 28 days of storage at room temperature. Food Res. Int. 2014;64:849–854. doi: 10.1016/j.foodres.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 93.Park S.Y., Ha S.-D. Assessment of cold oxygen plasma technology for the inactivation of major foodborne viruses on stainless steel. J. Food Eng. 2018;223:42–45. [Google Scholar]
- 94.Polo D., Álvarez C., Díez J., Darriba S., Longa Á., Romalde J.L. Viral elimination during commercial depuration of shellfish. Food Control. 2014;43:206–212. [Google Scholar]
- 95.Kim S.-H., Shahbaz H.M., Park D., Chun S., Lee W., Oh J.-W., Lee D.-U., Park J. A combined treatment of UV-assisted TiO2 photocatalysis and high hydrostatic pressure to inactivate internalized murine norovirus. Innov. Food Sci. Emerg. Technol. 2017;39:188–196. [Google Scholar]
- 96.Cho M., Cates E.L., Kim J.-H. Inactivation and surface interactions of MS-2 bacteriophage in a TiO2 photoelectrocatalytic reactor. Water Res. 2011;45:2104–2110. doi: 10.1016/j.watres.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 97.Cheng R., Shen L., Wang Q., Xiang S., Shi L., Zheng X., Lv W. Photocatalytic membrane reactor (PMR) for virus removal in drinking water: Effect of humic acid. Catalysts. 2018;8:284. [Google Scholar]
- 98.Lee J.E., Zoh K.D., Ko G.P. Inactivation and UV disinfection of murine norovirus with TiO2 under various environmental conditions. Appl. Environ. Microbiol. 2008;74:2111–2117. doi: 10.1128/AEM.02442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheng R., Kang M., Shen Z.-P., Shi L., Zheng X. Visible-light-driven photocatalytic inactivation of bacteriophage f2 by Cu-TiO2 nanofibers in the presence of humic acid. J. Environ. Sci. 2019;77:383–391. doi: 10.1016/j.jes.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 100.Venieri D., Gounaki I., Binas V., Zachopoulos A., Kiriakidis G., Mantzavinos D. Inactivation of MS2 coliphage in sewage by solar photocatalysis using metal-doped TiO2. Appl. Catal. B. 2015;178:54–64. [Google Scholar]
- 101.Zhang C., Li Y., Zhang W., Wang P., Wang C. Metal-free virucidal effects induced by g-C3N4 under visible light irradiation: Statistical analysis and parameter optimization. Chemosphere. 2018;195:551–558. doi: 10.1016/j.chemosphere.2017.12.122. [DOI] [PubMed] [Google Scholar]
- 102.Zhang C., Li Y., Shuai D., Zhang W., Niu L., Wang L., Zhang H. Visible-light-driven, water-surface-floating antimicrobials developed from graphitic carbon nitride and expanded perlite for water disinfection. Chemosphere. 2018;208:84–92. doi: 10.1016/j.chemosphere.2018.05.163. [DOI] [PubMed] [Google Scholar]
- 103.Akhavan O., Choobtashani M., Ghaderi E. Protein degradation and RNA efflux of viruses photocatalyzed by graphene-tungsten oxide composite under visible light irradiation. J. Phys. Chem. C. 2012;116:9653–9659. [Google Scholar]
- 104.Giannakis S., Liu S., Carratala A., Rtimi S., Talebi Amiri M., Bensimon M., Pulgarin C. Iron oxide-mediated semiconductor photocatalysis vs. heterogeneous photo-Fenton treatment of viruses in wastewater. Impact of the oxide particle size. J. Hazard. Mater. 2017;339:223–231. doi: 10.1016/j.jhazmat.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 105.Moor K.J., Kim J.H. Simple synthetic method toward solid supported C60 visible light-activated photocatalysts. Environ. Sci. Technol. 2014;48:2785–2791. doi: 10.1021/es405283w. [DOI] [PubMed] [Google Scholar]
- 106.Moor K.J., Valle D.C., Li C., Kim J.H. Improving the visible light photoactivity of supported fullerene photocatalysts through the use of [C70] fullerene. Environmental Science & Technology49. 2015:6190–6197. doi: 10.1021/es505888d. [DOI] [PubMed] [Google Scholar]
- 107.Yamaguchi Y., Usuki S., Kanai Y., Yamatoya K., Suzuki N., Katsumata K.I., Terashima C., Suzuki T., Fujishima A., Sakai H., Kudo A., Nakata K. Selective inactivation of bacteriophage in the presence of bacteria by use of ground Rh-doped SrTiO3 photocatalyst and visible light. ACS Appl. Mater. Interfaces. 2017;9:31393–31400. doi: 10.1021/acsami.7b07786. [DOI] [PubMed] [Google Scholar]