Abstract
Background:
We need high-quality data to assess the determinants for COVID-19 severity in people with MS (PwMS). Several studies have recently emerged but there is great benefit in aligning data collection efforts at a global scale.
Objectives:
Our mission is to scale-up COVID-19 data collection efforts and provide the MS community with data-driven insights as soon as possible.
Methods:
Numerous stakeholders were brought together. Small dedicated interdisciplinary task forces were created to speed-up the formulation of the study design and work plan. First step was to agree upon a COVID-19 MS core data set. Second, we worked on providing a user-friendly and rapid pipeline to share COVID-19 data at a global scale.
Results:
The COVID-19 MS core data set was agreed within 48 hours. To date, 23 data collection partners are involved and the first data imports have been performed successfully. Data processing and analysis is an on-going process.
Conclusions:
We reached a consensus on a core data set and established data sharing processes with multiple partners to address an urgent need for information to guide clinical practice. First results show that partners are motivated to share data to attain the ultimate joint goal: better understand the effect of COVID-19 in PwMS.
Keywords: Multiple sclerosis, pandemics, COVID-19, data collection, registries, coronavirus 2, humans
Introduction
There is uncertainty as to the specific risks of COVID-19 in people with multiple sclerosis (PwMS), in particular, whether taking immunosuppressant or immune-modifying medications affect COVID-19 course or outcome. With the current lack of robust data, approaches to the clinical management of MS during the pandemic diverge between countries. To overcome the insecurity, now and in the future, various COVID-19 data collection initiatives have recently been developed.1 However, because of the relatively low prevalence of MS,2 the absolute number of symptomatic COVID-19 infection in people also affected by MS is likely to be relatively low. This reduces the ability of single registries to achieve significant insights via individual national efforts. International data sharing is likely to enable more rapid evidence generation that will help to guide clinical management during the pandemic and may support future research. The Multiple Sclerosis International Federation (MSIF: https://www.msif.org/) and the Multiple Sclerosis Data Alliance (MSDA: https://msdataalliance.com/) have teamed up with multiple partners to establish a global data sharing initiative (in short ‘initiative’). This paper explains the steps taken and direction proposed for this effort.
The overall approach of the global data sharing initiative
The ultimate driving research question of the initiative is to understand the effect of individual disease-modifying therapies (DMTs) on COVID-19 outcomes. We aim to feed back results as rapidly as possible to the community and to inform evidence-based global guidance for PwMS and healthcare professionals during the pandemic. Time is of the essence here. Therefore, a pragmatic approach was defined and developed. Figure 1 summarizes the high-level overview of our approach. The approach we propose is compliant with all legal and ethical restrictions relating to data collection and data sharing.
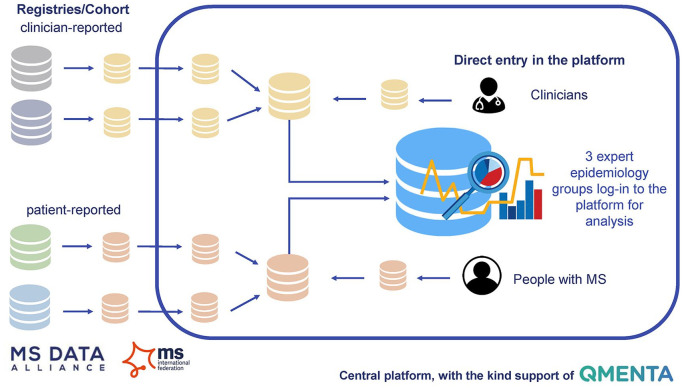
Figure 1.
High-level overview of the COVID-19 in MS-global data sharing initiative: we recommend the implementation of the COVID-19 in MS core data set in as many different data initiatives (registries/cohorts) as possible. All initiatives are invited to regularly share de-identified COVID-19 in MS core data sets into the central platform. In parallel, direct entry of individual PwMS and clinicians in the central platform is possible. The data sources are combined within the platform and the expert epidemiology groups check the quality of the data sources. Once a certain threshold of trustworthiness of the data and the results is met, the platform will become interactive and feed back results to the community.
Global alignment on a COVID-19 in MS core data set
To prepare the draft COVID-19 in MS core data set, we started with identifying common areas of interest in emerging and active data collection efforts on COVID-19 in MS. A final list of variables was agreed to within 48 hours in a global consensus-building teleconference. The core variable set can be downloaded via the MSDA website (https://msdataalliance.com/covid-19/for-ms-registers-and-data-custodians/). The following groups of variables were deemed important: COVID-19 infection, COVID-19 severity, COVID-19 treatment, demographic information, MS history and severity, information on DMT use and comorbidities and selected lifestyle behaviours, particularly smoking. During the consensus-building teleconference, we agreed on the importance of including both patient-reported and clinician-reported data to facilitate the inclusion of both milder and more severe COVID-19 cases in MS.
Working through the global MSIF movement to encourage widespread recording of COVID-19 in PwMS
The success of this initiative requires widespread activation of the global MS community to record the COVID-19 status of PwMS. The global recommendations for PwMS and healthcare professionals can be consulted on our websites. Our communications approach centers on working with national and regional MS organizations across the global MSIF movement to create and share specific content for their stakeholder networks. We invite all MS societies and patient organizations to join the initiative by encouraging PwMS and healthcare professionals across their networks to record data.
Implementation of the COVID-19 in MS core data set in as many existing and emerging registries or cohorts as possible
Registries and cohorts are able to deliver the highest data quality. Such high-quality data are needed to deliver fine-tuned results during, but particularly after, the pandemic. Some of these initiatives are already available for international COVID-19 and MS data collection and have implemented standardized safety protocols to address post-authorization safety studies (PASS).3 Table 1 summarizes the current list of initiatives that have or are planning to implement the COVID-19 in MS core data set (as of 10 May 2020). This list is expanding rapidly and is continuously updated on our websites.
Table 1.
Currently participating COVID-19 in MS data collection initiatives.
| COVID-19 in MS data collection initiative | Patient-reported data collection | Clinician-reported data collection |
|---|---|---|
| ABEM | Yes | No |
| AMSLS | Yes | No |
| Australia and New Zealand COVID-19 data set | No | Yes |
| Bulgarian SmartMS COVID-19 data set for patients or clinicians | Yes | Yes |
| Cleveland Clinic MS COVID-19 Registry | Yes | Yes |
| COViMS Registry, a North American COVID-19 and MS Reporting Database | No | Yes |
| EMA COVID-19 survey | Yes | No |
| French COVISEP4 | No | Yes |
| German MS Register, by the German MS Society COVID-19 Survey | Yes | Yes |
| HOLISM | Yes | No |
| Icompanion | Yes | Yes |
| iConquer MS COVID-19 Survey | Yes | No |
| LEOSS Registry | No | Yes |
| MSBase COVID-19 Sub-study | No | Yes |
| NeuroTransData | Yes | Yes |
| OptimiseMS | No | Yes |
| REDONE | No | Yes |
| RELACOEM | No | Yes |
| Swedish MS Registry COVID-19 Module | No | Yes |
| The Danish Multiple Sclerosis Registry | No | Yes |
| The Spanish MS Registry | No | Yes |
| UK MS Register COVID-19 CRF | Yes | Yes |
COVID-19: Coronavirus Disease 2019; MS: multiple sclerosis; ABEM: Brazilian Multiple Sclerosis Association; AMSLS: Australian MS Longitudinal Study; COViMS: COVID-19 infections in MS database; EMA: Esclerosis Multiple Argentina; COVISEP: part of French MS Registry collecting data on COVID-19; HOLISM: Health Outcomes and Lifestyle In a Sample of people with Multiple sclerosis; LEOSS: Lean European Open Survey on SARS-CoV-2 infected patients; REDONE: Brazilian Registry of multiple sclerosis and neuromyelitis optica spectrum disorders; RELACOEM: part of RelevarEM registry that will collect the data of COVID-19; CRF: case report form.Currently, there are 11 registries/cohorts collecting (or preparing to collect) patient-reported data and 18 registries/cohorts collecting (or preparing to collect) clinician-reported data.
We also provide an option to directly enter data into our central platform (a distinction is made between a clinician-reported fast module for data entry by healthcare professionals (https://platform.qmenta.com/covid19_ms_clinician) and a patient-reported fast module for data entry by PwMS (link: https://platform.qmenta.com/covid19_ms_patient)). The clinician-reported fast module might be of benefit to healthcare professionals who only have the resources to collect the core data set.
Regular uploads of de-identified COVID-19 in MS core data sets into a central platform
We invite all MS registries and cohorts to regularly share their COVID-19 core data set (‘export’) into the central platform, kindly provided by QMENTA (https://www.qmenta.com/). QMENTA is a cloud-based platform that allows for the aggregation, standardization, management and visualization of any form of data, with a focus on clinical and imaging data sets. The protocol of this study is approved by the ethical committee of Hasselt University (reference no. CME2020/025). A disclosure risk assessment was performed by an independent third party (P-95: https://www.p-95.com/). To minimize the risk of patient’s identity disclosure, we implemented a strict user access management as well as restricted data access to a minimal number of dedicated researchers. Next to this, the data are stored centrally in a secured environment.
Next steps
Regular updates of the progress of the initiative and the data counts are provided publicly on the QMENTA website (https://www.qmenta.com/covid19-patients_ms-table/). Current global COVID-19 advice for PwMS is based on expert opinion but will be revised as soon as data of sufficient quality are available. PwMS and healthcare professionals have a broad variety of questions that need to be addressed as soon as possible. To scope the initiative, we considered the following initial questions expressed as important among patients and organizations:
Are PwMS at greater risk for severe COVID-19 outcomes compared to the general population?
Is the pattern of risk factors for COVID-19 outcomes similar compared to the general population? (e.g. age, comorbidities, etc.); Does the severity of MS have an effect on COVID-19 outcomes?
Is there a difference in COVID-19 outcomes between untreated PwMS and PwMS on DMT?
Does the type of treatment have an effect on COVID-19 outcomes?
With the data being collected, which will focus on reported cases of COVID-19 among patients and not assess the background populations, we need to give priority to analyses on cases only, most importantly to identify factors predicting outcome once an infection is suspected or confirmed. In contrast, we believe in-depth analyses of population-based data sources including history and follow-up information will be required to address some research questions, such as risks of COVID-19 in the MS population and contributing factors such as DMTs or the long-term effect on MS progression of having had a COVID-19 infection, which will require a longer term follow-up. We aim to achieve methodological insights during this global data sharing initiative that can stimulate this on-going and future scientific research led by others within the MS community.
Conclusion
The COVID-19 pandemic represents a humanitarian crisis on a colossal global scale and there is evidence that the crisis will not be solved soon, so initiatives to minimize the risks of COVID-19 are fundamental. For people living with MS, it is not only the threat to their health imposed by the virus itself but also the wider impact of reduced access to healthcare and medications that are essential for managing their symptoms, preventing relapses and reducing disability. PwMS and the healthcare professionals providing their care are in urgent need of evidence on which to base challenging clinical decisions. Uncertainty over the true threat of COVID-19 to PwMS has stimulated a cautious approach to preventing infection, including from the clinical community, patients themselves and many governments as well. PwMS are frequently classified as ‘high risk’ and advised to follow the strictest social distancing measures. These measures have reduced access to routine healthcare, including rehabilitation, exercise and sources of emotional support and as such come with significant risks to physical and mental health. There are several advantages of formulating global recommendations for data collection as rapidly as possible. These include, for example, providing a framework to enable data collection in a wider number of countries and regions; enabling comparative analysis of treatment regimens and outcomes across different countries; and reducing the time and cost of future collaborative research using COVID-19 and MS case data (compared to using retrospective data harmonization efforts).
The prompt establishment of a global data sharing initiative due to the natural urgency is an ambitious undertaking. Nevertheless, we were able to motivate numerous stakeholders in the field of MS (neurologists, patient organizations, researchers, registries, etc.) to join our effort in bringing together data from patients, clinicians, registries and other COVID-19 in MS initiatives. We aim to continuously improve our approach while we move ahead. We hope the data sharing approach used here can be used as a template for different stakeholders to work together on an international scale also outside of the scope of the COVID-19 crisis. Indeed, there are many other urgent research questions that need to be addressed that require scaling-up real-world data research at a global scale.
Acknowledgments
We would like to thank the sponsors of the Multiple Sclerosis Data Alliance and the Multiple Sclerosis International Federation for providing financial support. Special thanks goes to QMENTA for kindly providing us with the central platform. The authors thank everyone who actively participated in any of the global teleconferences, brainstorms and task force meetings. We would like to thank all the PwMS and healthcare professionals who already contributed to the different data collection efforts. We also want to thank Jan Samyn, Margo Heremans, Angela Hooper, Jorina Nickmans, Gunther Meyer and Victoria Gilbert to support us with ensuring the operational power we needed to speed-up our progress.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: L.M.P. has no personal pecuniary interests to disclose, other than being the chair of The MS Data Alliance (MSDA), which receives income from a range of corporate sponsors, recently including Biogen, Bristol-Myers Squibb (formerly Celgene), Canopy Growth Corporation, Genzyme, Icometrix, Merck, Mylan, Novartis, QMENTA, Quanterix and Roche. C.W. has no personal pecuniary interests to disclose, other than being an employee of MSIF, which receives income from a range of corporate sponsors, recently including Biogen, Bristol-Myers Squibb (formerly Celgene), Genzyme, Med-Day, Merck, Mylan, Novartis and Roche. T.K. served on scientific advisory boards for Roche, Sanofi-Genzyme, Novartis, Merck and Biogen, steering committee for Brain Atrophy Initiative by Sanofi-Genzyme; received conference travel support and/or speaker honoraria from WebMD Global, Novartis, Biogen, Sanofi-Genzyme, Teva, BioCSL and Merck; and received research support from Biogen. G.E. has received consulting/speaking fees/research support from Bayer, Novartis, Teva, Sanofi-Genzyme, Merck-Serono, Biogen Idec and Roche. P.R.R. is shareholder, employee and member of board of directors of QMENTA. J.H. has received honoraria for serving on advisory boards for Biogen, Celgene, Sanofi-Genzyme, Merck KGaA, Novartis and Sandoz and speaker’s fees from Biogen, Novartis, Merck KGaA, Teva and Sanofi-Genzyme. He has served as P.I. for projects or received unrestricted research support from Biogen, Celgene, Merck KGaA, Novartis, Roche and Sanofi-Genzyme. His MS research was funded by the Swedish Research Council and the Swedish Brain foundation. Accelerated Cure Project for MS (ACP) has received grants, collaboration funding, payments for use of assets or in-kind contributions from the following companies: EMD Serono, Sanofi-Genzyme, Biogen, Genentech, AbbVie, Octave, GlycoMinds, Pfizer, Med-Day, AstraZeneca, Teva, Mallinckrodt, MSDx, Regeneron Genetics Centre, BC Platforms and Celgene. ACP has also received funding from the Patient-Centred Outcomes Research Institute (PCORI) and the National MS Society (NMSS). R.M. has received consulting payments from EMD Serono, which have been donated to ACP. A.B. has received consulting fees from advisory board/speaker/other activities for NeuroTransData and project management/clinical studies and travel expenses from Novartis and Servier. A.S. has no personal pecuniary interests to disclose, other than being the lead of the German MS Registry, which receives funding from a range of public and corporate sponsors, recently including The German Innovation Fund (G-BA), The German MS Trust, Biogen, German MS Society, Celgene (BMS), Merck and Novartis. A.S. is statistical editor for Circulation: Cardiovascular Imaging. J.I.R. has received honoraria from Novartis as a scientific advisor. He has received travel grants and attended courses and conferences on behalf of Merck-Serono Argentina and Novartis Argentina. A.v.d.W. has received honoraria and unrestricted research funding from Novartis, Biogen, Roche, Merck and Sanofi. H.B. Institution has received honoraria for steering committee activities, advisory board and meeting activities from Roche, Biogen, Novartis and Merck. H.B. has received consulting fees from Finstat (IQVIA) and Oxford Health policy Forum. J.A.C. has received personal compensation for consulting for Adamas, Convelo, Med-Day, Mylan and Population Council and is serving as an Editor of Multiple Sclerosis Journal. W.V.H. is shareholder, employee and member of board of directors of Icometrix. OPTIMISEMS has received funding from Biogen, Merck and Celgene. R.D. has received honoraria from Biogen, Merck and Teva. M.M. has served on scientific advisory board for Biogen, Sanofi, Roche, Novartis, Merck and AbbVie; received honoraria for lecturing from Biogen, Merck, Novartis, Sanofi-Genzyme; and received research support and support for congress participation from Biogen, Genzyme, Roche, Merck and Novartis. R.A. has received honoraria from Novartis as a scientific advisor. He has received travel grants and attended courses and conferences on behalf of Merck-Serono Argentina, Biogen Argentina, Genzyme Argentina, Roche Argentina and Novartis Argentina. R.N. has received honoraria from Novartis, Roche and Biogen for advisory boards. J.d.S. has received Honoraria from Biogen, Roche, Genzyme, Celgene, Alexion and Novartis. C.L. has received consulting or travel fees from Biogen, Novartis, Roche, Sanofi, Teva and Merck-Serono. G.C. has received consulting and speaking fees from Novartis, Teva Pharmaceutical Industries Ltd, Teva Italia Srl, Sanofi-Genzyme Corporation, Genzyme Europe, Merck KGgA, Merck-Serono SpA, Celgene Group, Biogen Idec, Biogen Italia Srl, F. Hoffman-La Roche, Roche SpA, Almirall SpA, Forward Pharma, Med-Day and Excemed. N.R. has no personal pecuniary interests to disclose, other than being an employee of MSIF, which receives income from a range of corporate sponsors, recently including Biogen, Bristol-Myers Squibb (formerly Celgene), Genzyme, Med-Day, Merck, Mylan, Novartis and Roche. RMM has reviewed no personal funding from any sources, the UK MS Register is funded by the MS Society and has received funding for specific projects from Novartis, Sanofi-Genzyme and Merck KGaA. T.P., L.G., Y.M., E.D.B., D.R., A.P., S.S.-Y., L.D.R., Y.D., C.G., L.M., N.L., L.F., T.S., S.B., B.F.B., I.v.d.M., R.I., K.H., G.S.d.O., D.G.B., J.B. and A.C. report no disclosures.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The operational costs linked to this study are funded by the Multiple Sclerosis International Federation (MSIF) and the Multiple Sclerosis Data Alliance (MSDA), acting under the umbrella of the European Charcot Foundation (ECF). The MSDA receives income from a range of corporate sponsors, recently including Biogen, Bristol-Myers Squibb (formerly Celgene), Canopy Growth Corporation, Genzyme, Icometrix, Merck, Mylan, Novartis, QMENTA, Quanterix and Roche. MSIF receives income from a range of corporate sponsors, recently including Biogen, Bristol-Myers Squibb (formerly Celgene), Genzyme, Med-Day, Merck, Mylan, Novartis and Roche.
ORCID iDs: Liesbet M Peeters  https://orcid.org/0000-0002-6066-3899
https://orcid.org/0000-0002-6066-3899
Ashkan Pirmani  https://orcid.org/0000-0003-4549-1002
https://orcid.org/0000-0003-4549-1002
Steve Simpson-Yap  https://orcid.org/0000-0001-6521-3056
https://orcid.org/0000-0001-6521-3056
Clément Gautrais  https://orcid.org/0000-0001-8486-9616
https://orcid.org/0000-0001-8486-9616
Tim Spelman  https://orcid.org/0000-0001-9204-3216
https://orcid.org/0000-0001-9204-3216
Rodden Middleton  https://orcid.org/0000-0002-2130-4420
https://orcid.org/0000-0002-2130-4420
Amber Salter  https://orcid.org/0000-0002-1088-110X
https://orcid.org/0000-0002-1088-110X
Anneke van der Walt  https://orcid.org/0000-0002-4278-7003
https://orcid.org/0000-0002-4278-7003
Jeffrey A Cohen  https://orcid.org/0000-0001-9245-9772
https://orcid.org/0000-0001-9245-9772
Ruth Dobson  https://orcid.org/0000-0002-2993-585X
https://orcid.org/0000-0002-2993-585X
Contributor Information
Liesbet M Peeters, Biomedical Research Institute and Data Science Institute, Hasselt University, Diepenbeek, Belgium.
Tina Parciak, Department of Medical Informatics, University Medical Center Göttingen, Göttingen, Germany.
Clare Walton, MS International Federation, London, UK.
Lotte Geys, Biomedical Research Institute and Data Science Institute, Hasselt University, Diepenbeek, Belgium.
Yves Moreau, ESAT-STADIUS, KU Leuven, Leuven, Belgium.
Edward De Brouwer, ESAT-STADIUS, KU Leuven, Leuven, Belgium.
Daniele Raimondi, ESAT-STADIUS, KU Leuven, Leuven, Belgium.
Ashkan Pirmani, Biomedical Research Institute and Data Science Institute, Hasselt University, Diepenbeek, Belgium/ESAT-STADIUS, KU Leuven, Leuven, Belgium.
Tomas Kalincik, Clinical Outcomes Research (CORe) Unit, The University of Melbourne, Melbourne, VIC, Australia/Melbourne MS Centre, Department of Neurology, Royal Melbourne Hospital, Melbourne, VIC, Australia.
Gilles Edan, Department of Neurology, CHU Pontchaillou, Rennes, France.
Steve Simpson-Yap, Neuroepidemiology Unit, Melbourne School of Population & Global Health, The University of Melbourne, Melbourne, VIC, Australia/Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia.
Luc De Raedt, Department of Computer Science and Leuven.AI, KU Leuven, Leuven, Belgium.
Yann Dauxais, Department of Computer Science and Leuven.AI, KU Leuven, Leuven, Belgium.
Clément Gautrais, Department of Computer Science and Leuven.AI, KU Leuven, Leuven, Belgium.
Paulo R Rodrigues, QMENTA, Barcelona, Spain.
Landon McKenna, QMENTA, Barcelona, Spain.
Nikola Lazovski, QMENTA, Barcelona, Spain.
Jan Hillert, Department of Clinical Neuroscience, Swedish MS Registry, Stockholm, Sweden.
Lars Forsberg, Department of Clinical Neuroscience, Swedish MS Registry, Stockholm, Sweden.
Tim Spelman, Department of Clinical Neuroscience, Swedish MS Registry, Stockholm, Sweden.
Robert McBurney, iConquerMS People-Powered Research Network, Accelerated Cure Project for MS, Waltham, MA, USA.
Hollie Schmidt, iConquerMS People-Powered Research Network, Accelerated Cure Project for MS, Waltham, MA, USA.
Arnfin Bergmann, NeuroTransData Study Group, NeuroTransData, Neuburg, Germany.
Stefan Braune, NeuroTransData Study Group, NeuroTransData, Neuburg, Germany.
Alexander Stahmann, MS Forschungs- und Projektentwicklungs-gGmbH, Hannover, Germany.
Rodden Middleton, UK MS Register, Swansea, UK.
Amber Salter, COViMS, St Louis, USA/ Division of Biostatistics, Washington University in St. Louis, St. Louis, MO, USA.
Bruce F Bebo, COViMS, USA/National Multiple Sclerosis Society, Portland, OR, USA.
Juan I Rojas, Neurology Department, Hospital Universitario de CEMIC, Buenos Aires, Argentina/RELACOEM, Buenos Aires, Argentina.
Anneke van der Walt, MSBase Registry, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia.
Helmut Butzkueven, MSBase Registry, Department of Neuroscience, Central Clinical School, Monash University, Melbourne, VIC, Australia.
Ingrid van der Mei, The Australian MS Longitudinal Study (AMSLS), Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia.
Rumen Ivanov, Bulgarian SmartMS COVID-19 Dataset, Sofia, Bulgaria.
Kerstin Hellwig, LEOSS, Department of Neurology, Katholisches Klinikum Bochum, Bochum, Germany.
Guilherme Sciascia do Olival, Brazilian Multiple Sclerosis Association (ABEM), São Paulo, Brazil.
Jeffrey A Cohen, Cleveland Clinic MS COVID-19 Registry, Mellen MS Center, Cleveland Clinic, Cleveland, OH, USA.
Wim Van Hecke, Icometrix – Icompanion, Leuven, Belgium.
Ruth Dobson, OptimiseMS, Preventive Neurology Unit, Queen Mary University of London, London, UK.
Melinda Magyari, The Danish Multiple Sclerosis Registry, Department of Neurology, University Hospital Rigshospitalet, Glostrup, Denmark.
Doralina Guimarães Brum, Universidade Estadual Paulista (Unesp), Faculdade de Medicina, Botucatu/REDONE – Brazilian Registry of Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders, São Paulo, Brazil.
Ricardo Alonso, RELACOEM, Buenos Aires, Argentina/Multiple Sclerosis University Center, Ramos Mejia Hospital – EMA, Buenos Aires, Argentina.
Richard Nicholas, UK MS Register, Swansea, UK/Imperial College London, London, UK/Swansea University, Swansea, UK.
Johana Bauer, Mental Health Area, EMA, Buenos Aires, Argentina.
Anibal Chertcoff, MS and Demyelinating Diseases, Hospital Británico de Buenos Aires – EMA, Buenos Aires, Argentina.
Jérôme de Sèze, Department of Neurology, Strasbourg University Hospital, Strasbourg, France/ COVISEP, France.
Céline Louapre, COVISEP, France/Institut du Cerveau ICM, APHP – Hôpital Pitié Salpêtrière, Sorbonne University, Paris, France.
Giancarlo Comi, Institute of Experimental Neurology, Ospedale San Raffaele, Milan, Italy.
Nick Rijke, MS International Federation, London, UK.
References
- 1. Sormani MP. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol 2020; 19(6): 481–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2016. Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18(3): 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hillert J, Trojano M, Magyari M, et al. Big multiple sclerosis data – A registry basis for post authorization safety studies (PASS) for multiple sclerosis. Poster presented at: ECTRIMS. 35th Congress of the European Committee for Treatment and Research in Multiple Sclerosis and 24th annual conference of rehabilitation in MS, Stockholm, 11–13 September 2019.f [Google Scholar]
- 4. Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. Epub ahead of print 26 June 2020. DOI: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]