Introduction
Ventricular arrhythmias are common after left ventricular assist device (LVAD) implantation and have been associated with increased mortality.1 The burden of ventricular arrhythmias is high in these patients, with observational studies suggesting that as many as 20%–50% of patients experience ventricular arrhythmias after LVAD implantation.2,3 Mechanisms of arrhythmogenesis include physical contact between the inflow cannula and myocardium, myocardial scar, and electrolyte disturbances. Ventricular tachycardia (VT) can be difficult to manage medically, and catheter ablation is often necessary.4 Catheter ablation has previously been shown to be safe and effective in LVAD patients with drug-refractory VT.5,6 However, the presence of diffuse endocardial scar and multiple VT morphologies during intracardiac mapping make the diagnosis of clinical VT difficult.6 Furthermore, invasive electrophysiological testing and ablation are associated with significant risks in LVAD patients, including access site bleeding that could mandate interruption in oral anticoagulation. Therefore, tools that allow noninvasive electrocardiographic mapping (ECM) and localization of the clinically relevant VT prior to attempted ablation could be particularly helpful. The CardioInsight Vest (Medtronic, Minneapolis, MN) has previously been successful in identifying the focus of VT prior to invasive electrophysiologic study7,8; however, the application of this technology in a patient with an LVAD and the associated electrical interference seen with these devices has not been previously reported.
Case report
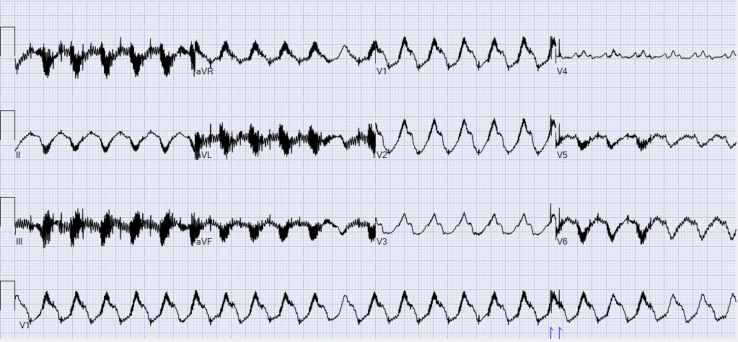
A 73-year-old male patient with a history of ischemic cardiomyopathy who underwent placement of a HeartMate 3 (Abbott, Chicago, IL) LVAD (HM3) approximately 20 months prior to presentation was admitted to our facility in VT storm after sustaining 15 appropriate implantable cardioverter-defibrillator (ICD) shocks for monomorphic VT in the setting of recent gastrointestinal illness and associated hypokalemia. After repletion of electrolytes, he was started on amiodarone followed by the addition of mexiletine due to ongoing episodic nonsustained VT. Unfortunately, 3 days after hospitalization he had 2 appropriate ICD shocks for VT despite adherence with medical therapy. His beta blockade was increased and he was discharged after 48 hours of observation. Approximately 1 week later, he presented to the Emergency Department with palpitations and LVAD low-flow alarms. He was found to be in sustained monomorphic VT (Figure 1). VT episodes had variable cycle length (400–530 ms), although the morphology was consistent. Given the medically refractory nature of the VT, associated low-flow alarms, and recurrent hospitalizations, VT ablation was considered. Based on the 12-lead electrocardiogram, it was thought that the VT likely originated from the endocardial surface near the site of the apical inflow cannula. However, to further assess the feasibility of endocardial ablation as well as decrease intracardiac mapping time, the decision was made to employ noninvasive ECM prior to VT ablation.
Figure 1.
Twelve-lead electrocardiogram of clinical ventricular tachycardia in patient with HeartMate 3 (Abbott, Chicago, IL) left ventricular assist device. Right bundle branch morphology with rightward superior axis and V3/V4 transition suspicious for exit site near the apical inflow cannula.
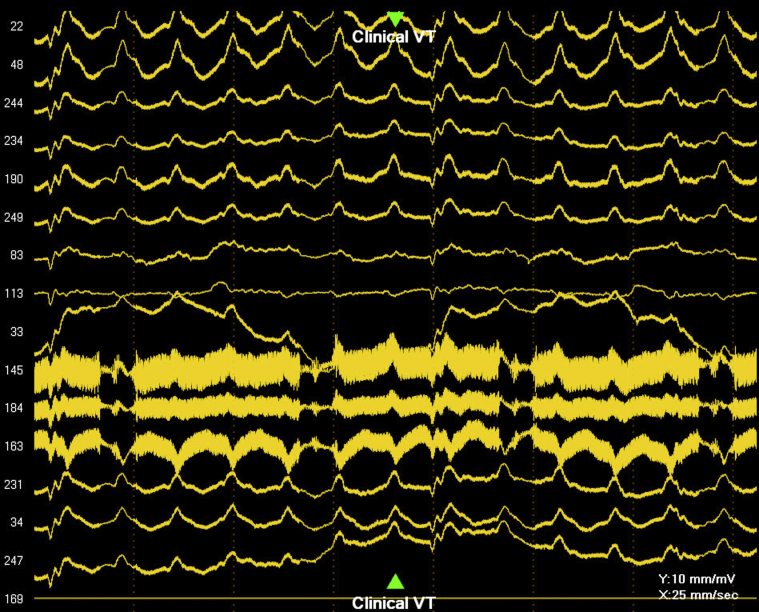
The patient was prepped and the CardioInsight Vest was applied to the torso. He underwent a noncontrast computed tomography scan of the chest per protocol to provide anatomic data for mapping. In the presence of the LVAD, there was considerable noise acquired by the surface electrodes (Figure 2). Despite the noise in the signals, potential and activation maps (Figure 3A and B, Video 1) were successfully created.
Figure 2.
Epicardial potentials recorded by the 252-electrode CardioInsight Vest (Medtronic, Minneapolis, MN) in a patient with a left ventricular assist device. Note the presence of significant high-frequency noise embedded within each signal. VT = ventricular tachycardia.
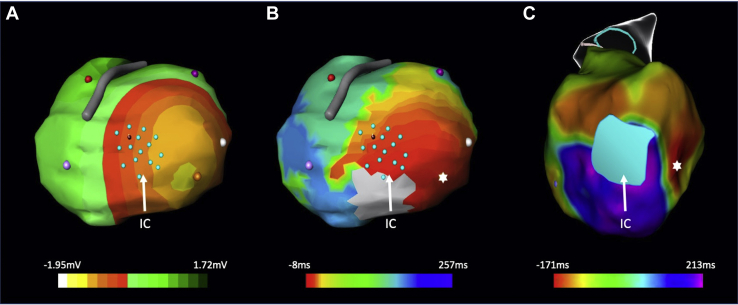
Figure 3.
CardioInsight-derived (Medtronic, Minneapolis, MN) epicardial potential (A) and activation maps (B) with corresponding invasive endocardial activation map (C) in patient with HeartMate 3 (Abbott, Chicago, IL) left ventricular assist device. Both systems localize the earliest activation to the lateral margin of the inferior wall (∗) near the site of the apical inflow cannula (IC, designated in light blue). After ablation in this region, clinical ventricular tachycardia was no longer inducible with double or triple extrastimulus testing.
Following application of the CardioInsight Vest, the patient subsequently underwent an invasive electrophysiologic study. Voltage and activation maps were created using the CARTO 3 (Biosense Webster Inc, Irvine, CA) electroanatomic mapping system and the multielectrode PentaRay catheter (Biosense Webster Inc, Irvine, CA). Clinical VT was induced with catheter ectopy with tachycardia cycle length of 500 ms. CardioInsight and endocardial activation mapping were both consistent with exit at the lateral margin of the inferior wall near the site of the apical inflow cannula (Figure 3). Extensive ablation was performed along the lateral margin of the scar as well as substrate modification along all sites of local abnormal ventricular activity. At conclusion of the case, ventricular tachycardia was noninducible with double and triple extrastimulus testing.
Discussion
Noninvasive ECM with the CardioInsight Vest was able to successfully localize the origin of VT in a patient with an HM3 LVAD. Electromagnetic interference from LVADs resulting in pacemaker or ICD malfunction has been well described in the literature; however, less is known about the impact of LVAD-associated interference on surface electrocardiographic signals and their interpretation.9,10 Moreover, the use of noninvasive ECM in patients with LVADs in situ has never been studied. A small single-center study analyzing the electrocardiographic signals in 3 patients with a HeartWare (Medtronic, Minneapolis, MN) LVAD found oscillating electrical interference at roughly 40 Hz, with subtle differences thought to be associated with speed changes.11 The patient presented in this case had a HM3 LVAD, which similarly to the HeartWare LVAD is a centrifugal flow pump, but is fully magnetically levitated12 and likely has a similar range of oscillating electrical interference frequencies.
The CardioInsight Vest consists of a 252-electrode system with a 1 kHz sampling frequency applied to the patient’s torso. A computed tomography scan is acquired in order to provide anatomic data for mapping purposes. By utilization of the Laplace equation, epicardial potentials are calculated based on the relationship between torso potentials and the thoracic anatomy.13,14 Data are acquired over a single beat and therefore a sustained arrhythmia is not required for a complete map. Epicardial potentials, electrograms, repolarization patterns, and activation mapping are displayed on this anatomic map. Because epicardial potentials are derived from torso potentials, small errors in torso measurements can be magnified in the computed epicardial potentials.13 Furthermore, the system derives epicardial potentials and must make several additional assumptions when calculating endocardial or intramural potentials, although previous work has shown that the system can relatively accurately provide information regarding intramural potential and reentry.15
In order to address noise in the acquired signals, the CardioInsight system utilizes several different filtering strategies to process the raw data. The ABCD (assisted bad channel detection) filter detects noisy channels and will flag these channels for review during the mapping process. Ultimately, the user is given the option of including or excluding these data. A low-pass filter is also employed that can be turned on and off, although the cutoff values for this filter cannot be adjusted by the user. Lastly, a 60-Hz line noise filter is applied to all signals (outside the United States this is set to 50 Hz). Signal averaging can also be used to improve signal-to-noise ratio, although this is not routinely needed in ventricular mapping owing to the relatively high-amplitude signals in these cases. During this case, no specific changes had to be made in order to account for the electromagnetic noise introduced by the LVAD.
Conclusion
In the case described here, the origin of the exit site of ventricular tachycardia was successfully identified using the CardioInsight system in a patient with an HM3 LVAD. Despite the electromagnetic interference and noise introduced by the LVAD, the standard filtering algorithm was able to generate a reproducible map of VT adjacent to the inflow cannula that was concordant with the endocardial activation map obtained at the time of ablation. Radiofrequency ablation at this site was successful, with no further inducible VT. To our knowledge this is the first reported case utilizing the CardioInsight system to noninvasively map VT in a patient with an LVAD. As the number of patients undergoing LVAD implantations continues to rise, so will the number of medically refractory ventricular arrhythmias in this patient population. The method described in this case may prove to be a safe and useful adjunct to endocardial mapping in this population, although future analyses including application to different types of LVADs will be needed to verify these findings.
Key Teaching Points.
-
•
Noninvasive electrocardiographic mapping of ventricular tachycardia (VT) can be performed in a patient with a HeartMate 3 (Abbott, Chicago, IL) magnetically levitated left ventricular assist device (LVAD) using the CardioInsight Vest (Medtronic, Minneapolis, MN).
-
•
The CardioInsight system applies a standard set of filters, including the ABCD (assisted bad channel detection), low-pass, and 60-Hz line noise filters to process signals for construction of activation and potential maps. Signal averaging can be applied to improve signal-to-noise ratio, although this is rarely necessary for ventricular signals.
-
•
Noninvasive mapping of VT in a patient with a LVAD may be a reasonable first step prior to performing an invasive procedure in the electrophysiology lab in order to better understand the origin of the arrhythmia.
Footnotes
Conflict of Interest: Michael Rehorn receives grant support for clinical research from Boston Scientific and Pfizer. Eric Black-Maier receives grant support for clinical research from Boston Scientific. Adam Barnett, Jason Koontz, and Jacob Schroder report no relevant disclosures. Jonathan Piccini is supported by R01HL128595 from the National Heart, Lung and Blood Institute. He receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, Myokardia, Sanofi, Philips, and Up-to-Date. Zak Loring is supported by NIH T32 grant #5T32HL069749 and receives grant support for clinical research from Boston Scientific. Albert Sun reports the following relationships with industry: (1) research grants to Duke University from Medtronic and Boston Scientific; (2) honoraria for lectures or consultation from Biosense-Webster, Boston Scientific, Medtronic, Merit Medical, and St. Jude.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2020.03.015.
Appendix. Supplementary data
Potential map of VT in a patient with a HeartMate 3 left ventricular assist device acquired with the CardioInsight Vest (location of inflow cannula annotated by blue dots).
References
- 1.Makki N., Mesubi O., Steyers C., Olshansky B., Abraham W.T. Meta-analysis of the relation of ventricular arrhythmias to all-cause mortality after implantation of a left ventricular assist device. Am J Cardiol. 2015;116:1385–1390. doi: 10.1016/j.amjcard.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 2.Gopinathannair R., Cornwell W.K., Dukes J.W. Device therapy and arrhythmia management in left ventricular assist device recipients: a scientific statement from the American Heart Association. Circulation. 2019;139:e967–e989. doi: 10.1161/CIR.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 3.Yoruk A., Sherazi S., Massey H.T. Predictors and clinical relevance of ventricular tachyarrhythmias in ambulatory patients with a continuous flow left ventricular assist device. Heart Rhythm. 2016;13:1052–1056. doi: 10.1016/j.hrthm.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Griffin J.M., Katz J.N. The burden of ventricular arrhythmias following left ventricular assist device implantation. Arrhythm Electrophysiol Rev. 2014;3:145–148. doi: 10.15420/aer.2014.3.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho G., Braun O.Ö., Adler E.D., Feld G.K., Pretorius V.G., Birgersdotter-Green U. Management of arrhythmias and cardiac implantable electronic devices in patients with left ventricular assist devices. JACC Clin Electrophysiol. 2018;4:847–859. doi: 10.1016/j.jacep.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Moss J.D., Flatley E.E., Beaser A.D. Characterization of ventricular tachycardia after left ventricular assist device implantation as destination therapy: a single-center ablation experience. JACC Clin Electrophysiol. 2017;3:1412–1424. doi: 10.1016/j.jacep.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Mountantonakis S.E., Vaishnav A.S., Jacobson J.D. Conduction patterns of idiopathic arrhythmias from the endocardium and epicardium of outflow tracts: new insights with noninvasive electroanatomic mapping. Heart Rhythm. 2019;16:1562–1569. doi: 10.1016/j.hrthm.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Betancourt J.E., Noheria A., Cooper D., Orme G., Sodhi S., Steyers C., Cuculich P. Accuracy of cardioinsight noninvasive electrocardiographic imaging compared with invasive mapping for determining location of ventricular arrhythmias. J Am Coll Cardiol. 2019;73:458. [Google Scholar]
- 9.Mozes A., DeNofrio D., Pham D.T., Homoud M.K. Inappropriate implantable cardioverter-defibrillator therapy due to electromagnetic interference in patient with a HeartWare HVAD left ventricular assist device. Heart Rhythm. 2011;8:778–780. doi: 10.1016/j.hrthm.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Sehatbakhsh S., Kushnir A., Kabach M., Kolek M., Chait R., Ghumman W. A case of electromagnetic interference between HeartMate 3 LVAD and implantable cardioverter defibrillator. Pacing Clin Electrophysiol. 2018;41:218–220. doi: 10.1111/pace.13210. [DOI] [PubMed] [Google Scholar]
- 11.Schettle S., Kassi M., Asleh R., Pereira N., Maltais S., Stulak J., Boilson B. LVAD ECG artifact reflecting HeartWare pump speed. J Am Coll Cardiol. 2018;71:A816. [Google Scholar]
- 12.Wiegmann L., Thamsen B., de Zélicourt D. Fluid dynamics in the HeartMate 3: influence of the artificial pulse feature and residual cardiac pulsation. Artif Organs. 2019;43:363–376. doi: 10.1111/aor.13346. [DOI] [PubMed] [Google Scholar]
- 13.Rudy Y. Noninvasive electrocardiographic imaging of arrhythmogenic substrates in humans. Circ Res. 2013;112:863–874. doi: 10.1161/CIRCRESAHA.112.279315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah A.J., Hocini M., Xhaet O. Validation of novel 3-dimensional electrocardiographic mapping of atrial tachycardias by invasive mapping and ablation: a multicenter study. J Am Coll Cardiol. 2013;62:889–897. doi: 10.1016/j.jacc.2013.03.082. [DOI] [PubMed] [Google Scholar]
- 15.Burnes J.E., Taccardi B., Ershler P.R., Rudy Y. Noninvasive electrocardiogram imaging of substrate and intramural ventricular tachycardia in infarcted hearts. J Am Coll Cardiol. 2001;38:2071–2078. doi: 10.1016/s0735-1097(01)01653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Potential map of VT in a patient with a HeartMate 3 left ventricular assist device acquired with the CardioInsight Vest (location of inflow cannula annotated by blue dots).