Abstract
Background
The detection of serum antibodies to the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is emerging as a new tool for the coronavirus disease 2019 (COVID‐19) diagnosis. Since many coronaviruses are sensitive to heat, heating inactivation of samples at 56°C prior to testing is considered a possible method to reduce the risk of transmission, but the effect of heating on the measurement of SARS‐CoV‐2 antibodies is still unclear.
Methods
By comparing the levels of SARS‐CoV‐2 antibodies before and after heat inactivation of serum at 56°C for 30 minutes using a quantitative fluorescence immunochromatographic assay
Results
We showed that heat inactivation significantly interferes with the levels of antibodies to SARS‐CoV‐2. The IgM levels of all the 34 serum samples (100%) from COVID‐19 patients decreased by an average level of 53.56%. The IgG levels were decreased in 22 of 34 samples (64.71%) by an average level of 49.54%. Similar changes can also be observed in the non–COVID‐19 disease group (n = 9). Of note, 44.12% of the detected IgM levels were dropped below the cutoff value after heating, suggesting heat inactivation can lead to false‐negative results of these samples.
Conclusion
Our results indicate that heat inactivation of serum at 56°C for 30 minutes interferes with the immunoanalysis of antibodies to SARS‐CoV‐2. Heat inactivation prior to immunoanalysis is not recommended, and the possibility of false‐negative results should be considered if the sample was pre‐inactivated by heating.
Keywords: antibodies, COVID‐19, heat inactivation, immunoanalysis, SARS‐CoV‐2
1. INTRODUCTION
The current outbreak of coronavirus disease 2019 (COVID‐19) caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is posing a serious threat to public health. 1 , 2 , 3 Early diagnosis of suspect cases is critical to reduce and interrupt the transmission of COVID‐19 from person to person. 4 Currently, laboratory testing of viral nucleic acid by real‐time reverse transcriptase–polymerase chain reaction (RT‐PCR) assay is the “gold standard” for COVID‐19 diagnosing. 5 However, the requirement of sophisticated instruments and laboratory conditions, tedious experimental procedures, and longer detection time significantly hamper its widespread applicability. 4 Antibodies produced in the blood after COVID‐19 infection are emerging as a promising class of biomarkers. 6 The antibodies to SARS‐CoV‐2 are specific, sensitive, and more importantly, their detection can be much faster and simpler than RT‐PCR, which allows rapid screening of suspect cases to be possible. 7
All the biological specimens for COVID‐19 testing should be considered to be potentially infectious. Therefore, the test must be performed by medical professionals with protective equipment in a qualified laboratory. To further reduce the risk of exposure to infectious agents, viral inactivation before sample handling is usually be recommended. 8 , 9 While the sensitivity of SARS‐CoV‐2 to the conditions of inactivation is unknown, it is reported that many coronaviruses such as SARS are heat‐sensitive and can be killed at 56°C for 30 minutes. 10 , 11 , 12 , 13 , 14 It is thus inferred that heating at 56°C could be an effective approach for SARS‐CoV‐2 inactivation. 15 However, the effect of heating at 56°C on COVID‐19 antibody detection is unclear. The objective of this study was to compare the levels of COVID‐19 antibody before and after heat inactivation.
2. METHODS
A total of 34 serum samples with positive SARS‐CoV‐2 antibody results from patients with COVID‐19 infections, and 9 serum samples from non–COVID‐19 diseases were collected from Hankou Hospital, Wuhan city, with approval of the ethics committee (hkyy2020‐004). All patients with COVID‐19 infections were confirmed by RT‐PCR. The antibody detection kits for SARS‐CoV‐2 were obtained from Kingfocus Biomedical Engineering Co., Ltd, (AIE/quantum dot‐based fluorescence immunochromatographic assay, AFIA). The immunoassay quantitatively measures IgM and IgG antibodies to SARS‐CoV‐2. Serum samples before and after heat inactivation at 56°C for 30 minutes were analyzed according to the protocol. Briefly, 100 µL of serum was dropped on the test card and the fluorescence signal was measured after 15 minutes. Detection values above the cutoff threshold are considered positive for COVID‐19.
3. RESULTS
In the patients with COVID‐19, the IgM signals of all the 34 serum samples (100%) decreased (Figure 1, Table 1) by an average level of 53.56% ([95% CI, 7.64%‐99.49%]; P < .013) after heat inactivation. The IgG signals were decreased in 22 of 34 samples (64.71%) by an average level of 49.54% ([95% CI, 8.76%‐90.32%]), and 12 samples (35.29%) increased with a median percentage of 24.22%. 44.12% of the IgM signals from COVID‐19 patients were below the cutoff value after heat inactivation. In the non–COVID‐19 group, the IgM levels were decreased in 7 of 9 samples (77.78%) by an average of 43.31% (Figure 2, Table 2) after heat inactivation and 2 samples (22.22%) increased with an average level of 29.84% ([95% CI, 5.44%‐54.23%]). The IgG signals were decreased in 7 of 9 samples (77.78%) by an average level of 79.42% ([95% CI, 44.54%‐114.31%]), and 2 samples (22.22%) increased with an average level of 44.00% ([95% CI, 21.37%‐66.63%]). None of the measured antibodies became higher than the cutoff value after heating.
Figure 1.
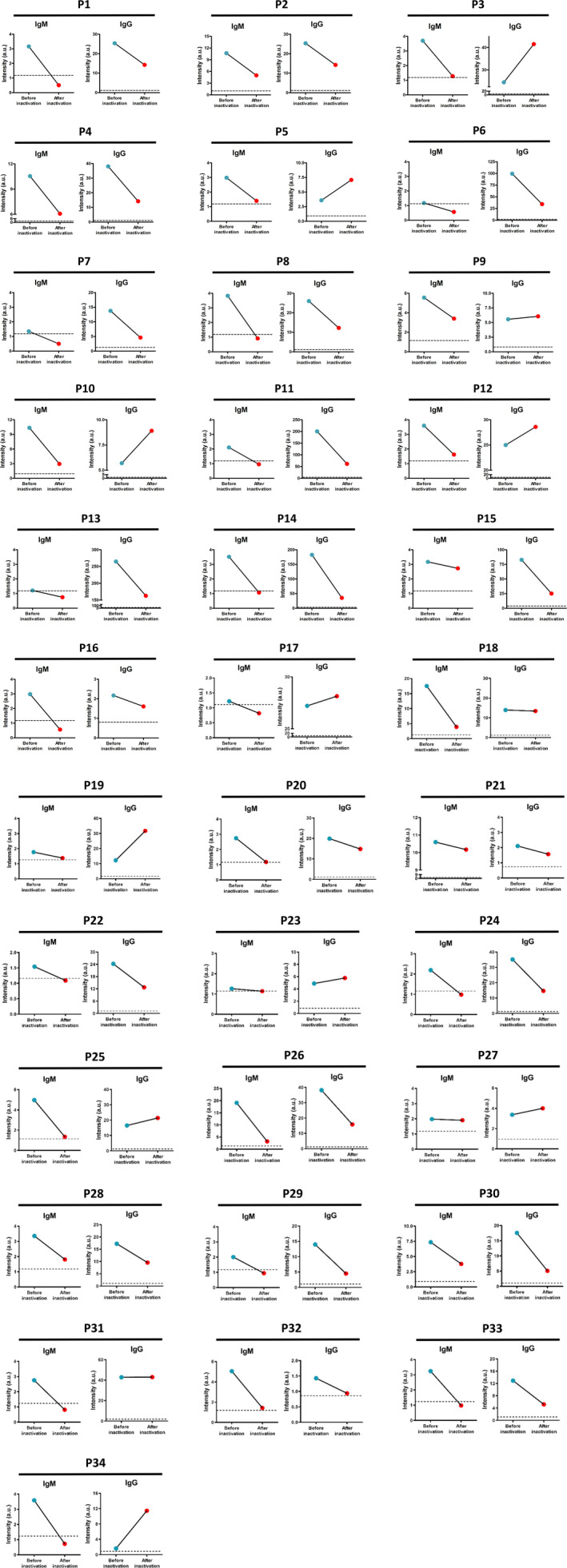
The changes in the IgM and IgG levels of the 34 serum samples from patients with COVID‐19 infections detected by AFIA before (blue dot) and after heat inactivation (red dot). Dash line indicates the cutoff value of the assay
Table 1.
The signal intensity of the IgM and IgG levels of the 34 serum samples from patients with COVID‐19 infections detected by AFIA before and after heat inactivation
| Patient ID | IgM | IgG | ||
|---|---|---|---|---|
| Before inactivation | After inactivation | Before inactivation | After inactivation | |
| P1 | 3.16 | 0.51 | 25.36 | 14.25 |
| P2 | 10.68 | 5.00 | 109.02 | 88.66 |
| P3 | 3.70 | 1.28 | 24.52 | 41.56 |
| P4 | 10.54 | 6.07 | 38.08 | 14.22 |
| P5 | 2.98 | 1.40 | 3.59 | 7.08 |
| P6 | 1.18 | 0.57 | 99.46 | 34.40 |
| P7 | 1.35 | 0.50 | 13.77 | 4.63 |
| P8 | 3.83 | 0.91 | 26.02 | 12.23 |
| P9 | 5.56 | 3.40 | 5.55 | 6.06 |
| P10 | 10.36 | 2.94 | 5.70 | 8.91 |
| P11 | 2.11 | 0.96 | 200.56 | 61.56 |
| P12 | 3.60 | 1.62 | 25.00 | 28.59 |
| P13 | 1.21 | 0.75 | 265.06 | 162.96 |
| P14 | 3.52 | 1.07 | 182.76 | 35.36 |
| P15 | 3.17 | 2.73 | 82.78 | 25.12 |
| P16 | 2.98 | 0.56 | 2.17 | 1.61 |
| P17 | 1.22 | 0.82 | 24.41 | 26.28 |
| P18 | 17.53 | 3.90 | 13.94 | 13.43 |
| P19 | 1.77 | 1.38 | 12.21 | 31.69 |
| P20 | 2.74 | 1.18 | 19.88 | 14.83 |
| P21 | 10.60 | 10.17 | 2.11 | 1.57 |
| P22 | 1.54 | 1.09 | 24.24 | 12.76 |
| P23 | 1.26 | 1.14 | 4.89 | 5.78 |
| P24 | 2.19 | 0.98 | 35.14 | 14.69 |
| P25 | 4.97 | 1.34 | 16.51 | 21.42 |
| P26 | 19.08 | 3.22 | 38.14 | 15.81 |
| P27 | 1.98 | 1.91 | 3.37 | 4.00 |
| P28 | 3.36 | 1.81 | 17.23 | 9.57 |
| P29 | 2.01 | 0.95 | 13.99 | 4.52 |
| P30 | 7.35 | 3.79 | 17.57 | 5.10 |
| P31 | 2.76 | 0.82 | 42.84 | 42.98 |
| P32 | 5.06 | 1.43 | 1.43 | 0.94 |
| P33 | 3.24 | 0.98 | 12.93 | 5.19 |
| P34 | 3.59 | 0.73 | 1.63 | 11.45 |
Figure 2.
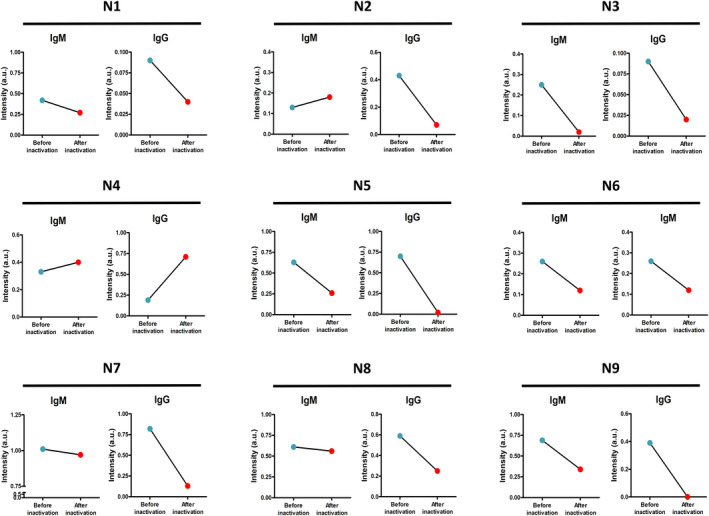
The changes in the IgM and IgG levels of 9 serum samples from non–COVID‐19 group detected by AFIA before (blue dot) and after heat inactivation (red dot)
Table 2.
The signal intensity of the IgM and IgG levels of 9 serum samples from non–COVID‐19 group detected by AFIA before and after heat inactivation
| Patient ID | IgM | IgG | ||
|---|---|---|---|---|
| Before inactivation | After inactivation | Before inactivation | After inactivation | |
| N1 | 0.42 | 0.27 | 0.09 | 0.04 |
| N2 | 0.13 | 0.18 | 0.43 | 0.07 |
| N3 | 0.25 | 0.02 | 0.09 | 0.02 |
| N4 | 0.33 | 0.4 | 0.19 | 0.71 |
| N5 | 0.63 | 0.26 | 0.7 | 0.02 |
| N6 | 0.26 | 0.12 | 0.21 | 0.57 |
| N7 | 1.01 | 0.97 | 0.82 | 0.13 |
| N8 | 0.61 | 0.56 | 0.59 | 0.25 |
| N9 | 0.69 | 0.34 | 0.39 | 0.00 |
4. DISCUSSION
This study analyzed the changes in SARS‐CoV‐2 antibody concentration before and after heat inactivation at 56°C for 30 minutes. We found that after heat inactivation, all of the serum IgM (100%) demonstrated significantly lower levels. For IgG, 64.71% of the sample levels dropped after heat inactivation.
All immunological assays are highly dependent on the recognition and binding of antigens to antibodies. The decrease in SARS‐CoV‐2 antibody levels may be related to their structural change in denaturation and aggregation. 16 , 17 Previous studies have shown that antibodies can be denatured and lose their antigen‐binding activities after heating, 18 and IgM is reported to be less thermally stable than IgG 19 , 20 due to their different compositions and structures of heavy chains. 21 This is consistent with our results that SARS‐CoV‐2 IgM concentration decreased more significantly than IgG after heating. In addition, the IgG levels in 12 samples (35.29%) increased with a median of 24.22% after heating, which may be due to the increases in immunogenicity caused by the formation of IgG aggregates heating at 56°C. 16 , 22 , 23 It is noteworthy that after heat inactivation, 44.12% of the IgM levels from COVID‐19 patients were below the cutoff value. These results suggest that heat inactivation of serum can lead to false‐negative results in these samples.
5. CONCLUSIONS
Heat inactivation of serum at 56°C for 30 minutes interferes with the immunoanalysis of antibodies to SARS‐CoV‐2. For highly suspected cases, the possibility of false‐negative results should be considered if the sample was inactivated by heating.
ACKNOWLEDGMENTS
This work was supported by funds from the National Natural Science Foundation of China (81601819), Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2016J013), and Medical Science and Technology Research Foundation of Guangdong Province (A2016280).
Hu X, An T, Situ B, et al. Heat inactivation of serum interferes with the immunoanalysis of antibodies to SARS‐CoV‐2. J Clin Lab Anal. 2020;34:e23411 10.1002/jcla.23411
Xiumei Hu, Taixue An and Bo Situ contributed equally to this work.
Contributor Information
Dan Ding, Email: dingd@nankai.edu.cn.
Lei Zheng, Email: nfyyzhenglei@smu.edu.cn.
REFERENCES
- 1. Mahase E. Coronavirus: covid‐19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ. 2020;368:m641. [DOI] [PubMed] [Google Scholar]
- 2. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. J Am Med Assoc. 2020;323(8):709. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Laboratory testing for coronavirus disease 2019 (COVID‐19) in suspected human cases: interim guidance. 2 March 2020.
- 5. General office of the National Health Commission . Guide of diagnosis and treatment of novel coronavirus (nCoV) pneumonia, 6th Version. 18 February 2020.
- 6. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM‐IgG combined antibody test for SARS‐CoV‐2 infection diagnosis. J Med Virol. 2020. 10.1002/jmv.25727. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected: interim guidance. 25 January 2020.
- 9. Darnell MER, Subbarao K, Feinstone SM, Taylor DR. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS‐CoV. J Virol Methods. 2004;121(1):85‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leclercq I, Batéjat C, Burguière AM, Manuguerra JC. Heat inactivation of the middle east respiratory syndrome coronavirus. Influenza Other Respir Viruses. 2014;8(5):585‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yunoki M, Urayama T, Yamamoto I, Abe S, Ikuta K. Heat sensitivity of a SARS‐associated coronavirus introduced into plasma products. Vox Sang. 2004;87(4):302‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone‐iodine, physical conditions and chemical reagents. Dermatology. 2006;212(1):119‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr HW. Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. 2005;194:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duan SM, Zhao XS, Wen RF, et al. SARS research team, stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed Environ Sci. 2003;16(3):246‐255. [PubMed] [Google Scholar]
- 15. Chang L, Yan Y, Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. 2020. 10.1016/j.tmrv.2020.02.003. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang W, Singh S, Zeng DL, King K, Nema S. Antibody structure, instability, and formulation. J Pharm Sci. 2007;96(1):1‐26. [DOI] [PubMed] [Google Scholar]
- 17. Dominguez E, Perez MD, Calvo M. Effect of heat treatment on the antigen‐binding activity of anti‐peroxidase immunoglobulins in bovine colostrum. J Dairy Sci. 1997;80(12):3182‐3187. [DOI] [PubMed] [Google Scholar]
- 18. Goldsmith SJ, Dickson JS, Barnhart HM, Toledo RT, Eiten‐Miller RR. IgA, IgG, IgM and lactoferrin contents of human milk during early lactation and the effect of processing and storage. J Food Prot. 1983;46(1):4‐7. [DOI] [PubMed] [Google Scholar]
- 19. Li SQ, Bomser JA, Zhang QH. Effects of pulsed electric fields and heat treatment on stability and secondary structure of bovine immunoglobulin G. J Agric Food Chem. 2005;53(3):663‐670. [DOI] [PubMed] [Google Scholar]
- 20. Mainer G, Sánchez L, Ena JM, Calvo M. Kinetic and thermodynamic parameters for heat denaturation of bovine milk IgG, IgA and IgM. J Food Sci. 1997;62(5):1034‐1038. [Google Scholar]
- 21. Akazawa‐Ogawa Y, Nagai H, Hagihara Y. Heat denaturation of the antibody, a multi‐domain protein. Biophys Rev. 2018;10(2):255‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Soltis RD, Hasz D, Morris MJ, Wilson ID. The effect of heat inactivation of serum on aggregation of immunoglobulins. Immunology. 1979;36(1):37‐45. [PMC free article] [PubMed] [Google Scholar]
- 23. Hermeling S, Crommelin DJ, Schellekens H, Jiskoot W. Structure‐immunogenicity relationships of therapeutic proteins. Pharm Res. 2004;21(6):897‐903. [DOI] [PubMed] [Google Scholar]


