Abstract
Background
The aim of this clinical trial was to evaluate the effects of febuxostat (FBX) in comparison with hydroxychloroquine (HCQ) on clinical symptoms, laboratory tests and chest CT findings in outpatients with moderate symptoms of COVID‐19 infection.
Methods
We conducted a clinical trial involving adult outpatients with the moderate respiratory illness following COVID‐19 infection. Patients were randomly assigned to receive either FBX or HCQ for 5 days. The measured variables were needs to hospitalisation, clinical and laboratory data including fever, cough, breathing rate, C‐Reactive Protein level, lymphocytes count at onset of admission and was well as at 5 days of treatments. In addition, CT findings were evaluated on admission and 14 days after initiation of treatment.
Results
Sixty subjects were enrolled in the study with a 1 to 1 ratio in FBX and HCQ groups. On admission, fever (66.7%), cough (87%), tachypnoea (44.4%), dyspnoea (35%), elevated CRP value (94.4%) and lung involvement according to chest CT (100%) were documented in enrolled patients with insignificant difference between FBX and HCQ groups. Fever, cough and tachypnoea were significantly mitigated in both groups after five days of treatments without any significant differences between groups. The mean percentages of lung involvement were significantly reduced to 7.3% and 8% after 14 days of treatment with FBX and HCQ, respectively. In adult outpatients with moderate COVID‐19 infection, the effectiveness of FBX and HCQ was not different in terms of resolution of clinical manifestations, laboratory tests and lung CT findings.
Conclusion
This trial suggests that FBX is as an alternative treatment to HCQ for COVID‐19 infection and may be considered in patients with a contraindication or precaution to HCQ.
What’s known
Coronavirus (COVID‐19)—caused respiratory tract illness may result in severe progressive pneumonia, multiorgan failure and death in critically ill patients.
Inflammatory response and storm cytokine production are involved in pulmonary tissue damage in patient with COVID‐19.
There is no specific antiviral treatment against COVID‐19 infection.
What’s new
Fever, cough and tachyponea significantly mitigated in patients with COVID‐19 after febuxostat treatment, in addition, the lymphocytes count significantly increased after febuxostat treatment.
The mean percentages of CT abnormalities were significantly reduced to 47% compared to baseline after febuxostat treatment.
The efficacy of febuxostat and hydroxychloroquine in treatment of outpatients with COVID‐19 infection was not significantly different.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) causes respiratory tract illness which may result to severe progressive pneumonia, multiorgan failure and death in critically ill patients. 1 , 2 There is no specific antiviral treatment against COVID‐19 infection but some antiviral drugs have been used as empirical. 3 The current therapy for COVID‐19 infection focuses on symptomatic treatment and supportive care. Patients with severe coronavirus disease have lower lymphocytes count, higher leukocytes count and lower percentages of monocytes, eosinophils and basophils. Elevation in inflammatory cytokines including IL‐2R, IL‐6, IL‐8, IL‐10 and TNF‐α and dysregulation of immune system has been observed. 4 The expression levels of interleukin‐2 receptor (IL‐2R) and serum level of IL‐6 were associated with severity of disease. 5 In addition, virus infection causes nuclear factor kappa B (NF‐κB) overexpression, which plays central role in massive overproduction of pro‐inflammatory cytokines as well as triggering a variety of cellular responses, including cell phagocytosis, maturation of dendritic cells and chemotaxis of cells. 6 The uncontrolled inflammatory response may result in pulmonary tissue damage, functional impairment and reduced lung capacity. Hence, it is proposed that an excessive production of IL‐6 is associated with severe lung damage and acute respiratory illness in patients with COVID‐19 infection. Febuxostat (FBX) is a novel non‐purine xanthine oxidase (XO) inhibitor that has been approved for treating hyperuricemia in patients with gout. Several studies have already demonstrated the anti‐inflammatory, 7 anti‐oxidant 8 and anti‐apoptosis effects of FBX. 9 Several preclinical studies showed that FBX inhibits inflammatory responses through reducing the levels of pro‐inflammatory mediators such as tumour necrosis factor (TNF)‐α, interleukin (IL)‐1β, IL‐6 and NF‐κB. 10 , 11 , 12 It protects animal against toxic‐induced lung inflammation through downstream inflammatory mediators and oxidative stress. 8 , 13 , 14 FBX markedly accelerates pulmonary endothelial barrier recovery and improves survival in lipopolysaccharide‐induced murine sepsis. 15 This clinical trial was conducted to assess the effects of FBX and hydroxychloroquine (HCQ) on clinical symptoms, laboratory tests and chest CT findings of patients with COVID‐19 infection.
2. METHODS
2.1. Study design
A clinical trial was conducted on outpatients with COVID‐19 infections from March 16, 2020, to April 10, 2020, at Mostafavian Fever Clinic in Sari (Iran). This study was an open label clinical trial, with blinded outcome assessment. This study was approved by the Ethical Committee (ID; IR.MAZUMS.REC.1398.7294) and Research Council of Mazandaran University of Medical Sciences and was submitted and approved by the Iranian Registry of Clinical Trials (ID; IRCT2019072704434N1, the full trial protocol can be accessed at: http://www.irct.ir). The study was performed in accordance with declaration of Helsinki. All patients signed the informed consent form. Sample size was determined as 30 patients in both group based on effect size = 0.3 for difference in response rate as a primary endpoint, power = 80% and alpha = 0.05 for this study, as a two‐sided superiority trial. 16 , 17
2.2. Patients
Inclusion criteria were as following 1; chest CT finding compatible with COVID‐19 infection along with other symptoms of coronavirus infection. Bilateral and peripheral ground‐glass and consolidative pulmonary opacities were the hallmarks of CT findings. 2; any symptoms of respiratory tract involvement including cough, dyspnoea or tachypnoea along with a history of contact with a known case of COVID‐19 3; creatinine clearance greater than 60 mL/min. The exclusion criteria include: 1; Suspicious patients for COVID‐19 pneumonia who had severe underlying diseases such as cardiovascular, lung and kidney diseases, 2; patients with severe pneumonia needing hospitalisation, 3; patient who were unable to take oral medications and 4; concurrent use of azathioprine, didanosine, mercaptopurine or pegloticase (due to drug interaction with FBX).
2.3. Interventions
Patients were randomised using the balance block method to receive HCQ (30 patients) or FBX (30 patients). HCQ were administered one tablet of HCQ 200 mg twice daily (Amin Pharmaceutical Company, Iran). Patients in FBX group took one tablet of FBX 80 mg per day (Jalinus Pharmaceutical Company, Iran). All patients were taken acetaminophen 325 mg, as needed, for controlling the fever. The pharmaceutical companies were neither involved in the design nor in the financial support of the study. Study drugs were purchased from an official Iranian pharmacy. Amin and Jalinus pharmaceutical companies did not access to the data of the study during trial and prior publication. The treatment duration was five days. Both patients and physician did not know the contents of tables.
2.4. Outcome measures
The primary outcome of this study was the rate of hospitalisation. Secondary outcomes were clinical improvements (eg, resolution of fever, cough and dyspnoea) and improvement of CT findings at days 14 after initiation of the treatment. Patients were assessed clinically (eg, temperature, respiratory rate, cough, and dyspnoea) and paraclinically (eg, CBC‐diff and C‐Reactive Protein) at onset of admission and at 5th day of treatment. In addition, the chest CT scans were done at first and 14 days after the onset of treatment. For each patient, the chest CT scan was evaluated for the presence of ground‐glass opacities and/or consolidation. Each five lobe of the lung was assessed and the overall lung involvement was reached by summing the five lobe scores (range of possible scores, 0‐20 for each lobe and total lung involvement of possible score of 0‐100 percent). The Chest CT scan was repeated in day 14 and compared with the initial finding. Reduced lung CT involvement; not adjusted" values were computed according to this equation:
Reduced lung CT involvement; adjusted values was computed with the following equation that included the initial total lung involvement in the denominator:
2.5. Statistical analysis
Normality of data was checked with Shapiro‐Wilk Test. Independent sample t‐test and Mann‐Whitney U test (comparison of continuous variables between two groups), Wilcoxon matched‐pair signed‐rank test (comparison of continuous variables before and after treatment), and Chi‐square test (comparing the qualitative data) were used for analysis. The method of analysis was intention‐to‐treat. The SPSS software version 21.0 (SPSS, Inc, Chicago, IL) was applied for statistical analysis.
3. RESULTS
3.1. Patients
Sixty subjects were enrolled including FBX (N = 30) and HCQ groups (N = 30) (Figure 1). Six patients (one patient in FBX group and five patients in HCQ group) were excluded, because patients were not interest to continue the treatment (Figure 1). Table 1 shows baseline demographic and clinical characteristics of the 54 patients who were enrolled in the study. The mean age of patients was 57.7 ± 8.4 years, and 59% of the patients were men. Among these patients, the most common symptom was fever, followed by cough and shortness of breath. On admission, fever temperature ≥37.8°C (66.7%), cough (87%), respiratory rate >20/min (44.4%), dyspnoea (35%), elevated CRP value (94.4%) and lung involvement according to chest CT (100%) were documented in enrolled patients. The WBC counts were 4578 ± 1539/µL. On admission, lymphopenia (lymphocytes count <1500/µL) was found in 44 (81.5%) of patients and most patients had a moderate pneumonia as documented by clinical manifestations and CT findings. There were no between‐group significant differences in baseline demographic characteristics, laboratory data (eg, CRP, WBC and lymphocytes counts) and CT scores of lung lesions (Table 1).
FIGURE 1.
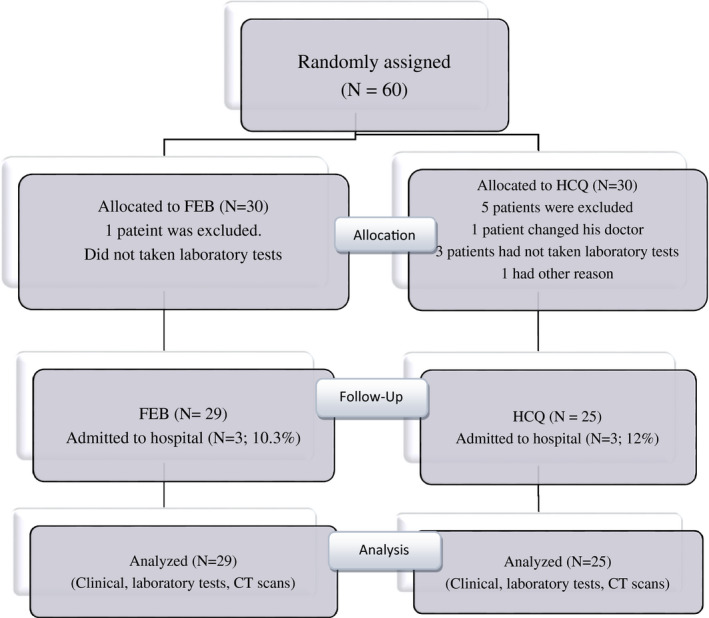
CONSORT diagram, including the number of patients who started and continued trial treatment, and stopped
TABLE 1.
Demographic and clinical characteristics of the patients at Baseline
| Characteristic | Total (N = 54) | Febuxostat (N = 29) | Hydroxychloroquine (N = 25) |
|---|---|---|---|
| Age (Mean ± SEM) | 57.7 ± 1.26 | 58 ± 1.47 | 57.3 ± 2.2 |
| Male gender; no (%) | 32 (59.3) | 16 (55.2) | 16 (64) |
| Current smoking; no. (%) | 1 (1.9) | 1 (3.6) | 0 (0) |
| Coexisting conditions | |||
| Diabetes; no. (%) | 15 (27.8) | 8 (27.6) | 7 (28) |
| Lung disease; no. (%) | 1 (1.9) | 0 (0) | 1 (4) |
| Fever (T > 37.8°C) | 36 (66.7) | 16 (55.2) | 20 (80) |
| Body temperature; °C | |||
| Mean ± SEM | 37.7 ± 0.07 | 37.6 ± 0.09 | 37.9 ± 0.09 |
| Respiratory rate | |||
| Mean ± SEM | 19.7 ± 0.24 | 19.8 ± 0.32 | 19.6 ± 0.37 |
| Respiratory rate ≥ 20/min; no. (%) | 24 (44.4) | 14 (50) | 10 (40) |
| Cough; no. (%) | 47 (87%) | 27 (93.1) | 20 (80) |
| Dyspnoea; no. (%) | 19 (35.2%) | 10 (35) | 9 (36) |
| White Blood Cell count | |||
| Mean ± SEM | 4578 ± 211 | 4689 ± 321 | 4444 ± 265 |
| Lymphocyte count | |||
| Mean ± SEM | 1285 ± 76 | 1308 ± 114 | 1258 ± 100 |
| Lymphopenia (<1500/µL) | 44 (81.5) | 23 (79.3) | 21 (84) |
| CRP (Elevated value); no. (%) | 51 (94.4) | 28 (96.6) | 23 (92) |
| Lung CT (%involvement) | |||
| Mean ± SEM | 17.5 ± 1.35 | 16 ± 1.2 | 19.2 ± 2.6 |
There were not any significant differences between two groups in baseline demographic and clinical characteristics.
3.2. Oucomes
The rate of hospitalisation, the primary end‐point of the study, was not different among groups. Six patients (11%, 3 patients in each group) were hospitalised because of developing more severe symptoms. The rate of intensive care unit (ICU) care and also mortality rate was not different between patients received FBX or HCQ. All hospitalised patients were released from hospitals between 1 and 7 days of hospitalisation and did not require ICU care.
Patients were re‐evaluated at five days after admission and using FBX or HCQ. Fever, cough and tachypnoea significantly mitigated in patients treated with either FBX or HCQ after five days of treatment (P < .01 compared to baseline of each group) (Table 2). No significant difference in clinical symptoms was observed after 5 days of treatment between FBX and HCQ groups. Also, the lymphocytes counts increased significantly in both treatment groups. The mean lymphocytes counts were 1308 ± 617 and 1258 ± 498 for FBX and HCQ groups at first days of admission whereas they increased to 1962 ± 478 and 1911 ± 798 after 5 days with FBX and HCQ treatments, respectively. No significant differences were observed between FBX and HCQ in lymphocytes counts. The CRP values dropped in normal range (non‐elevated value) in most of patients after receiving FBX or HCQ treatment. FBX and HCQ treatments showed insignificant difference in the percentages of non‐elevated CRP values after 5 days of treatments.
TABLE 2.
Outcomes in the febuxostat and hydroxychloroquine treatments
| Characteristic | Febuxostat (N = 29) | Hydroxychloroquine (N = 25) | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 5 | P‐value (within group) | Day 1 | Day 5 | P‐value (within group) | |
| Fever (T > 37.8°C); n (%) | 16 (55.2) | 0 (0) | NAζ | 20 (80) | 0 (0) | NAζ |
| Body temperature; °C | ||||||
| Mean ± SEM | 37.6 ± 0.09 | 37.1 ± 0.05 | .001 | 37.9 ± 0.09 | 37 ± 0.05 | <.001 |
| Respiratory rate; | ||||||
| Mean ± SEM | 19.8 ± 0.32 | 17.3 ± 0.48 | <.001 | 19.6 ± 0.37 | 17.4 ± 0.48 | .001 |
| Respiratory rate ≥ 20/min; no. (%) | 14 (50) | 2 (6.9) | <.001 | 10 (40) | 3 (12) | .04 |
| Cough; n (%) | 27 (93.1) | 13 (44.8) | <.001 | 20(80) | 10 (43.5) | .008 |
| Dyspnoea; n (%) | 10 (34.5) | 3 (10.7) | .07 | 9 (36) | 4 (17.4) | .06 |
| White‐cell count; | ||||||
| Mean ± SEM | 4689 ± 321 | 6111 ± 285 | <.001 | 4444 ± 265 | 6130 ± 310 | .001 |
| Lymphocyte count; Mean ± SEM | 1308 ± 114 | 1962 ± 141 | <.001 | 1258 ± 100 | 1911 ± 166 | .002 |
| Lymphopenia (<1500/µL) Positive; n (%) | 23 (79.3) | 8 (28.6) | <.001 | 21 (84) | 7 (30.4) | <.001 |
| CRP (Elevated value); n (%) | 28 (96.6) | 14 (50) | <.001 | 23 (92) | 12 (60) | .03 |
| Lung CT (% involvement); | <.001 | |||||
| Mean ± SEM | 16 ± 1.2 | 7.3 ± 2.17 | .004 | 19.2 ± 2.6 | 8 ± 2.4 | |
| Range | 5‐25 | 0‐50 a | 5‐50 | 0‐50 a | ||
| Reduced lung CT involvement; not adjusted a ; | ||||||
| Mean ± SEM | −8.4 ± 2.4 | −10.8 ± 2.3 | ||||
| Range | −25 to +30 a | −45 to +10 a | ||||
| Reduced lung CT involvement; adjusted a ; | ||||||
| Mean ± SEM | 47.4 ± 17 | 58.3 ± 13.7 | ||||
| Range | −200 to +100 a | −200 to +100 a | ||||
| CT day14 involvement, Negative; n (%) | 9 (31) | 8 (32) | ||||
| Hospitalisation no. (%) | 3 (10.3) | 3 (12.5) | ||||
Indicate the Lung CT data on day 14 compared to Day 1; P‐value for between groups differences were not significant for any of variables both on day 1 and day 14.
Finding of chest CT scans at baseline and day 14 indicated that the percent of lung involvement were 16% and 19.2% in FBX and HCQ groups at admission, while these scores significantly reduced to 7.3% and 8% after 14 days of treatment, respectively, in which the adjusted reduction of lung involvement were 47.4% and 58.3% as compared to the initial CT findings (P = .004 and <.001, respectively).
4. DISCUSSION
This open label clinical trial found that the efficacy of FBX and HCQ in treatment of patients with COVID‐19 infection is not significantly different in terms of resolution of clinical manifestations and para‐clinical abnormalities in outpatients with suspected COVID‐19 infection. Six patients including 3 of 29 (10%) patients in FBX group and 3 of 25 (12%) patients in HCQ were admitted to hospital because of progressing symptoms, but they did not need ICU admission, totally 11% of patients needed to be hospitalised. In China, 15%‐20% of cases required hospitalisation, around 15% had severe disease and 5% needed critical care. 18 In Italy, approximately 40% of patients have been hospitalised, whereas nearly 7% admitted to ICU. 19 There is significant variation in the rate of hospitalisation of patients with COVID‐19 in the world. However, sample size of our clinical trial was smaller and patients with significant comorbidities such as severe cardiovascular and renal diseases were excluded in our trial. Clinical symptoms such as fever, cough and shortness of breath were observed in a large proportion of patients at admission, but these manifestations markedly reduced or resolved (eg, fever and dyspnoea) after 5 days following use of FBX or HCQ. No statistically difference was observed in mitigating of clinical symptoms between FBX and HCQ treatments. Low lymphocyte count has been consistently reported in patients with COVID‐19 infection (in 80% of cases) and may indicate the severity of disease and serve as a predictor of prognosis. 20 More than half of patients showed elevated values of CRP. Patients with severe disease had more prominent laboratory abnormalities than those with non‐severe disease. 21 In our study, lymphopenia was observed in 81.5% of patients at onset of admission and significantly increased after 5 days of treatments into normal range. It is shown that both treatments significantly improved lymphocyte counts during 5 days of treatments. Elevated CRP values were observed in majority of patients [FBX (96.6%) and HCQ (92%)] at onset of admission, while it significantly decreased to non‐elevated range in 50% and 40% of patients who received FBX and HCQ, respectively. The differences between two groups in terms of changes in CRP after 5 days were not significant. Chest CT images from patients with COVID‐19 typically demonstrate bilateral, peripheral ground glass opacities. In China report, of 975 CT scans that were performed at the time of admission, 86.2% revealed abnormal results. 21 In our study, diagnosis was based on clinical symptoms, laboratory findings, history of exposure to a patient with COVID‐19 infection and lung CT abnormalities consistent with coronavirus pneumonia at early phase. Due to a high sensitivity of lung CT in detecting lung involvement and also the importance of initiating the treatment as soon as possible, we included patients according to chest CT findings. The real time Reverse Transcription Polymerase Chain Reaction (rRT‐PCR) was not performed in our trial. We believe that this limitation would not affect the validity of our results. The value of high sensitivity chest CT in diagnosis of COVID‐19 infection was frequently reported in previous studies. Long et al, reported 97.2% sensitivity for CT whereas the sensitivity of initial rRT‐PCR was only 83.3%. Considering the false‐negative results of rRT‐PCR and the relatively long assay time, they suggested that patients with typical CT findings but negative rRT‐PCR results should be isolated and a repeated rRT‐PCR was conducted to avoid misdiagnosis. 22 In our study, all patients showed chest CT abnormalities (100%) at admission, while FBX or HCQ treatments significantly cleared CT abnormalities in 31% and 32% of patients after 14 days of treatments, respectively. All data show that FBX is affective as HCQ in improvement of clinical symptoms and also laboratory and radiographic abnormalities in outpatients with suspected COVID‐19 infection. To date, no FDA‐approved drug has demonstrated safety and efficacy in randomised controlled trials for patients infected with COVID‐19. Several drugs with in vitro antiviral activity against SARS‐CoV‐2 and/or immunomodulatory effects have been suggested to be clinically beneficial in patients with COVID‐19. In our protocol, HCQ is prescribed in combination with acetaminophen for controlling fever. However, antiviral drug(s) may be added to HCQ in spite of some challenges regarding the effectiveness or safety of available antiviral medicines for management of patients with COVID‐19 disease. Considering these challenges and low severity of disease, we have not prescribed antiviral medicines in our study. Several clinical studies used chloroquine (CQ) or HCQ in treatment of SARS‐CoV‐2‐caused pneumonia in China. The first results of a trial that included 100 patients showed the superiority of CQ compared with control group in terms of reduction in exacerbation of pneumonia, duration of symptoms and delay of viral clearance, without severe side effects. This has led to include CQ in the recommendations regarding the prevention and treatment of COVID‐19 caused pneumonia. The antiviral and anti‐inflammatory activities of CQ may account for its efficacy in treating patients with COVID‐19 caused pneumonia. 23 , 24 , 25 In a trial conducted in France, a higher frequency of SARS‐CoV‐2 clearance was noticed after 6 days of treatment with HCQ alone or HCQ+ azithromycin (AZM) vs the untreated control group (70% vs 12.5%; P < .001). In that study, PCR results of nasopharyngeal samples and clearing viral nasopharyngeal carriage of SARS‐CoV‐2 in COVID‐19 patients were evaluated. The clinical symptoms, laboratory tests and lung CT features were reported to be similar in both groups of patients. 16 In other study, Gautret et al conducted an uncontrolled non‐comparative observational study in a cohort of 80 mildly infected inpatients treated with a combination of HCQ and AZT over a period of at least three days. A rapid fall of nasopharyngeal viral load tested by qPCR was noted, with 83% negative at Day 7, and 93% at Day 8. Authors concluded that they have provided evidence of a beneficial effect of co‐administration of HCQ with AZT in the treatment of COVID‐19 and its potential effectiveness in the early stages of contagiousness. 26 To date, despite some promising results associated with efficacy and safety of HCQ in COVID‐19, the evidence regarding its effect remains limited. 27 Infrequent and rare side effects include retinal toxicity, cardiac toxicity, QT interval prolongation and agranulocytosis in patients receiving HCQ or CQ. Life threatening arrhythmias following use of CQ and HCQ appear to be rare but cardiac monitoring is necessary if the drug is being used more extensively. 28 , 29 HCQ inhibits IL‐6, TNF‐α, IL‐1β and NF‐κB, 30 , 31 , 32 , 33 , 34 and it has immunomodulatory and anti‐inflammatory effects, 31 , 35 , 36 which may be beneficial in patients with COVID‐19 whose inflammatory response and storm cytokine production plays a major role in damaging the lung tissue. 36 There are complex interactions between inflammation and thrombosis, that inflammation is causing a thrombotic tendency. The suspected contribution of thrombotic events to morbidity and mortality in COVID‐19 patients, it is recommended to use medicine with anti‐inflammatory and antithrombotic properties for prevention or management of thrombosis in patients with COVID‐19 infection. 37 Recently in the recovery trialthe use of dexamethasone was associated with mortality benefit in patients with severe form of COVID‐19 infection. 38 NF‐κB plays a central role in inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease and autoimmunity. NF‐κB is activated through microbial products and also through pro‐inflammatory cytokines, as well as endogenous ligands that function as its trigger during tissue injury, the latter of which may promote inflammation in the absence of infection. 39 , 40 , 41 In addition, viral infections cause NF‐κB overexpression, which plays a crucial role in the production of pro‐inflammatory cytokine storms and triggers various cellular responses including cell phagocytosis, dendritic cell maturation, chemotaxis and lipopolysaccharide‐induced pulmonary inflammation. 6 , 42 , 43 It seems that downregulation of NF‐κB results in the attenuation of inflammatory cytokine signalling and may be a promising target for lung protection. 44 FBX is in a class of medications called xanthine oxidase (OX) inhibitors leading to decrease in uric acid production. Beside decreasing serum uric acid in gout by FBX, there are well documents demonstrating FBX suppresses pro‐inflammatory cytokines such as IL‐1β, IL‐6 MCP‐1 and TNF‐α as well as inhibits the oxidative stress and inflammatory responses through NF‐κB pathway in animal models. 10 , 12 , 45 , 46 FBX is able to improve lung damage induced by toxic agents through down‐regulation of oxidative stress pathway and suppression of inflammatory mediators. 7 , 8 , 14 , 15 To date, there is not any report associated to anti‐viral activity of FBX. Xanthine oxidase, which is responsible for the generation of oxygen free radicals, was elevated in serum and lung tissue of mice infected with influenza virus. 47 , 48 , 49 Due to the crucial roles of cytokines and pro‐inflammatory mediators in severe acute respiratory syndrome induced by COVID‐19, with respect to beneficial effects of FBX in blockading the activation of cytokines and NF‐κB pathway, this medicine seems to be an effective drug for the prevention and treatment of lung inflammation in patients with COVID‐19 insignificant efficacy with HCQ. However, further studies are needed to find the exact mechanism of FBX in treatment of COVID‐19 infection. The adverse effects associated with FBX therapy include nausea, diarrhoea, arthralgia, headache, increased hepatic serum enzyme levels, rash, and cardiovascular problems. These side effects were not observed in our study, which may be due to the short time consuming of FBX. It is also notable that we excluded patients with cardiovascular and chronic kidney diseases. The limitation of our study was the absence of placebo group. Considering ethical issues, we designed this study without a placebo group due to the life‐threatening nature of COVID‐19 infection.
5. CONCLUSION
Our trial suggests a beneficial effect of administration of FBX in patients with suspected mild‐to‐moderate COVID‐19 infection. The effects of FBX and HCQ was not different in terms of need to hospitalisation, improvement in clinical symptoms and CT findings pointing out the lung involvement. FBX may be considered in patients who are not a good candidate of HCQ due to underlying cardiovascular diseases.
DISCLOSURE
The authors declare no potential conflicts of interest with respect to authorship, and/or publication of this study.
ACKNOWLEDGEMENTS
This study was supported by a grant from Mazandaran University of Medical Science, Sari, Iran (ID#7294). We thank all patients who participated in this trial and their families. We thank Adel Heidari for assistance in data gathering. We appreciated the health care staffs who lost their lives in the care of patients with COVID‐19.
Davoodi L, Abedi SM, Salehifar E, et al. Febuxostat therapy in outpatients with suspected COVID-19: A clinical trial. Int J Clin Pract. 2020;74:e13600. 10.1111/ijcp.13600
The authors confirm that the Principal Investigator for this paper is Dr Lotfollah Davoodi and that he had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID‐19). Treasure Island (FL): StatPearls; 2020. [PubMed] [Google Scholar]
- 4. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen L, Liu HG, Liu W, et al. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:203‐208 (Abstract). [DOI] [PubMed] [Google Scholar]
- 6. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kataoka H, Yang K, Rock KL. The xanthine oxidase inhibitor Febuxostat reduces tissue uric acid content and inhibits injury‐induced inflammation in the liver and lung. Eur J Pharmacol. 2015;746:174‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahmed MA, El Morsy EM, Ahmed AA. Protective effects of febuxostat against paraquat‐induced lung toxicity in rats: impact on RAGE/PI3K/Akt pathway and downstream inflammatory cascades. Life Sci. 2019;221:56‐64. [DOI] [PubMed] [Google Scholar]
- 9. Wang S, Li Y, Song X, et al. Febuxostat pretreatment attenuates myocardial ischemia/reperfusion injury via mitochondrial apoptosis. J Transl Med. 2015;13:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amirshahrokhi K. Febuxostat attenuates ulcerative colitis by the inhibition of NF‐kappaB, proinflammatory cytokines, and oxidative stress in mice. Int Immunopharmacol. 2019;76:105884. [DOI] [PubMed] [Google Scholar]
- 11. Hao G, Duan W, Sun J, Liu J, Peng B. Effects of febuxostat on serum cytokines IL‐1, IL‐4, IL‐6, IL‐8, TNF‐alpha and COX‐2. Exp Ther Med. 2019;17:812‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan SI, Malhotra RK, Rani N, et al. Febuxostat modulates MAPK/NF‐kappaBp65/TNF‐alpha signaling in cardiac ischemia‐reperfusion injury. Oxid Med Cell Longevity. 2017;2017:8095825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacob S, Herndon DN, Hawkins HK, Enkhbaatar P, Cox RA. Xanthine oxidase contributes to sustained airway epithelial oxidative stress after scald burn. Int J Burns Trauma. 2017;7:98‐106. [PMC free article] [PubMed] [Google Scholar]
- 14. Fahmi AN, Shehatou GS, Shebl AM, Salem HA. Febuxostat protects rats against lipopolysaccharide‐induced lung inflammation in a dose‐dependent manner. Naunyn Schmiedebergs Arch Pharmacol. 2016;389:269‐278. [DOI] [PubMed] [Google Scholar]
- 15. Damarla M, Johnston LF, Liu G, et al. XOR inhibition with febuxostat accelerates pulmonary endothelial barrier recovery and improves survival in lipopolysaccharide‐induced murine sepsis. Physiol Rep. 2017;5:e13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020:105949. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial. medRxiv. preprint: https://wwwmedrxivorg/content/101101/2020032220040758v32020 Accessed May 15, 2020.
- 18. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 19. Lazzerini M, Putoto G. COVID‐19 in Italy: momentous decisions and many uncertainties. Lancet Global Health. 2020;8:e641‐e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guan WJ, Ni ZY, Hu Y, et al.; China Medical Treatment Expert Group for C . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiong Z, Fu L, Zhou H, et al. Construction and evaluation of a novel diagnosis process for 2019‐Corona Virus Disease. Zhonghua yi xue za zhi. 2020;100:E019. [DOI] [PubMed] [Google Scholar]
- 23. Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72‐73. [DOI] [PubMed] [Google Scholar]
- 24. multicenter collaboration group of Department of S, Technology of Guangdong P, Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus p . [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:185‐188 (Abstract). [DOI] [PubMed] [Google Scholar]
- 25. Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID‐19. Int J Antimicrob Agents. 2020;55:105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gautret P, Lagier JC, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID‐19 patients with at least a six‐day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34:101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gbinigie K, Frie K. Should chloroquine and hydroxychloroquine be used to treat COVID‐19? A rapid review. BJGP Open. 2020. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bauman JL, Tisdale JE. Chloroquine and hydroxychloroquine in the era of SARS ‐ CoV2: caution on their cardiac toxicity. Pharmacotherapy. 2020;40:387‐388. [DOI] [PubMed] [Google Scholar]
- 29. Bell JS, Bell JA, Creek DJ. Off‐label prescribing in the midst of a pandemic: the case of hydroxychloroquine. Aust J Gen Pract. 2020;49. (In press). [DOI] [PubMed] [Google Scholar]
- 30. Zeidi M, Kim HJ, Werth VP. Increased myeloid dendritic cells and TNF‐alpha expression predicts poor response to hydroxychloroquine in cutaneous lupus erythematosus. J Invest Dermatol. 2019;139:324‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muller‐Calleja N, Manukyan D, Canisius A, Strand D, Lackner KJ. Hydroxychloroquine inhibits proinflammatory signalling pathways by targeting endosomal NADPH oxidase. Ann Rheum Dis. 2017;76:891‐897. [DOI] [PubMed] [Google Scholar]
- 32. Sun Y, Zhang J, Li X, Sun E. [Hydroxychloroquine alleviates 5‐fluorouracil‐induced enteritis in mice and its mechanism]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020;36:1‐8. [PubMed] [Google Scholar]
- 33. Silva JC, Mariz HA, Rocha LF Jr, et al. Hydroxychloroquine decreases Th17‐related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics (Sao Paulo). 2013;68:766‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu L, Ren J, He Z, et al. Cholesterol‐modified hydroxychloroquine‐loaded nanocarriers in bleomycin‐induced pulmonary fibrosis. Sci Rep. 2017;7:10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu C, Lu L, Wan JP, Wen C. The pharmacological mechanisms and therapeutic activities of hydroxychloroquine in rheumatic and related diseases. Curr Med Chem. 2017;24:2241‐2249. [DOI] [PubMed] [Google Scholar]
- 36. Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155‐166. [DOI] [PubMed] [Google Scholar]
- 37. Bikdeli B, Madhavan MV, Gupta A, et al; Global C‐TCG . Pharmacological agents targeting thromboinflammation in COVID‐19: review and implications for future research. Thrombosis Haemostasis. 2020. 10.1055/s-0040-1713152 (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilkinson E. RECOVERY trial: the UK covid‐19 study resetting expectations for clinical trials. BMJ. 2020;369.m1626. (In press). [DOI] [PubMed] [Google Scholar]
- 39. Baker RG, Hayden MS, Ghosh S. NF‐kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lawrence T. The nuclear factor NF‐kappaB pathway in inflammation.Cold Spring Harbor Perspectives in Biology. 2009;1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pordanjani SM, Hosseinimehr SJ. The role of NF‐kB inhibitors in cell response to radiation. Curr Med Chem. 2016;23:3951‐3963. [DOI] [PubMed] [Google Scholar]
- 42. Jiang K, Guo S, Yang C, et al. Barbaloin protects against lipopolysaccharide (LPS)‐induced acute lung injury by inhibiting the ROS‐mediated PI3K/AKT/NF‐kappaB pathway. Int Immunopharmacol. 2018;64:140‐150. [DOI] [PubMed] [Google Scholar]
- 43. Jin Y, Qian J, Ju X, et al. Osthole protects against acute lung injury by suppressing NF‐kappaB‐dependent inflammation. Mediators Inflamm. 2018;2018:4934592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Linard C, Marquette C, Mathieu J, Pennequin A, Clarençon D, Mathé D. Acute induction of inflammatory cytokine expression after γ‐irradiation in the rat: effect of an NF‐κB inhibitor. Int J Radiat Oncol Biol Phys. 2004;58:427‐434. [DOI] [PubMed] [Google Scholar]
- 45. Omori H, Kawada N, Inoue K, et al. Use of xanthine oxidase inhibitor febuxostat inhibits renal interstitial inflammation and fibrosis in unilateral ureteral obstructive nephropathy. Clin Exp Nephrol. 2012;16:549‐556. [DOI] [PubMed] [Google Scholar]
- 46. Krishnamurthy B, Rani N, Bharti S, et al. Febuxostat ameliorates doxorubicin‐induced cardiotoxicity in rats. Chem Biol Interact. 2015;237:96‐103. [DOI] [PubMed] [Google Scholar]
- 47. Oda T, Akaike T, Hamamoto T, Suzuki F, Hirano T, Maeda H. Oxygen radicals in influenza‐induced pathogenesis and treatment with pyran polymer‐conjugated SOD. Science. 1989;244:974‐976. [DOI] [PubMed] [Google Scholar]
- 48. Ungheri D, Pisani C, Sanson G, et al. Protective effect of n‐acetylcysteine in a model of influenza infection in mice. Int J Immunopathol Pharmacol. 2000;13:123‐128. [PubMed] [Google Scholar]
- 49. Yokozawa T, Sekiya M, Cho EJ, Kurokawa M, Shiraki K. Effect of Wen‐Pi‐Tang extract on lung damage by influenza virus infection. Phytomedicine. 2004;11:625‐632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


