Abstract
The normal development of the pulmonary system is critical to transitioning from placental‐dependent fetal life to alveolar‐dependent newborn life. Human lung development and disease have been difficult to study due to the lack of an in vitro model system containing cells from the large airways and distal alveolus. This article describes a system that allows human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs) to differentiate and form three‐dimensional (3D) structures that emulate the development, cytoarchitecture, and function of the lung (“organoids”), containing epithelial and mesenchymal cell populations, and including the production of surfactant and presence of ciliated cells. The organoids can also be invested with mesoderm derivatives, differentiated from the same human pluripotent stem cells, such as alveolar macrophages and vasculature. Such lung organoids may be used to study the impact of environmental modifiers and perturbagens (toxins, microbial or viral pathogens, alterations in microbiome) or the efficacy and safety of drugs, biologics, and gene transfer. © 2020 Wiley Periodicals LLC.
Basic Protocol: hESC/hiPSC dissection, definitive endoderm formation, and lung progenitor cell induction
Keywords: ciliated cells, differentiation, endoderm, human pluripotent stem cells, lung development, lung organoid, pulmonary disease modeling, SARS‐CoV‐2 viral infection, smooth muscle, surfactant, type 1 alveolar cells, type 2 alveolar cells
INTRODUCTION
This article describes a protocol for the differentiation of human pluripotent stem cells (hPSCs)—both human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs)—into three‐dimensional (3D) arrangements of the multiple cell types that constitute the fully functioning, mature lung (i.e., lung “organoids”). Enzymatic disruption of hPSCs and subsequent growth in inductive media specifying endodermal differentiation on a two‐dimensional (2D) matrix allows the cells to proceed to lung progenitor cells (LPC), expressing the transcription factor NKX2‐1. At that point, the LPCs are enzymatically disrupted again and suspended in a Matrigel matrix on transwell inserts as 3D “organoids” which follow lung branching morphogenesis and maturation into a combination of lung epithelial and mesenchymal cells from both the proximal airway and distal alveolus. If and only if, the 3D configuration is maintained do surfactant‐producing alveolar cells develop. If exposed to an air‐liquid interface, the organoids develop ciliated cells. Further, the organoids can be invested with appropriate non‐endoderm, non‐pulmonary lineages derived from the same hiPSCs (i.e., isogenic) by protocols not described here but available in other articles in Current Protocols, in order to emulate even more completely the structure of the mature lung—e.g., mesoderm‐derived alveolar macrophages and vasculature. This model can then be used to examine lung disease due to SARS‐CoV‐2 or any other respiratory virus.
hESC/hiPSC DISSECTION, DEFINITIVE ENDODERM FORMATION, AND LUNG PROGENITOR CELL INDUCTION
This protocol will begin with pluripotent stem cell dissection and definitive endoderm (DE) formation, followed by anterior foregut endoderm (AFE) and lung progenitor cell (LPC) differentiation. It will then proceed into 3D lung organoid development and analysis. hPSCs are routinely passaged by using RLeSR (StemCell Technologies, cat. no. 05872) or mechanical dissection.
NOTE: The following procedures are performed in a Class II biological hazard flow hood or a laminar‐flow hood.
NOTE: All solutions and equipment coming into contact with live cells must be sterile, and proper aseptic technique should be used accordingly.
NOTE: All incubations are performed in a humidified 37°C, 5% CO2 incubator unless otherwise specified.
Materials
Matrigel human growth factor−reduced matrix (Corning, cat. no. 354230)
DMEM/F12 medium (Gibco, cat. no. 10565042)
hESCs/iPSCs (human pluripotent stem cells or hPSCs) grown on feeder‐free Matrigel in plate wells at 70% confluence
mTeSR Plus defined feeder‐free medium (StemCell Technologies, cat. no. 05825)
10 mM Y‐27632 stock (Tocris, cat. no. 1254)
Phosphate‐buffered saline (PBS; Gibco, cat. no. 10010023)
Accutase (Innovative Cell Technologies, cat. no. AT104)
Stem cell passage medium (see recipe)
mTeSR Plus medium (StemCell Technologies, cat. no. 05825) supplemented with 10 μM Y‐27632 (Tocris, cat. no. 1254)
DE induction medium (see recipe)
AFE induction medium (see recipe)
Quenching medium (see recipe)
LPC induction medium (see recipe)
3D organoid induction medium (see recipe)
3D organoid branching medium (see recipe)
3D organoid maturation medium (see recipe)
Reagents for detecting endodermal and lung markers (Table 1)
4% paraformaldehyde
Table 1.
Antibodies Used in FACS and Immunofluorescence
| Primary antibodies | Company | Cat. no. | Dilution rate |
|---|---|---|---|
| CXCR4‐PE | R&D Systems | FAB170P | 1:200 (F) |
| EPCAM‐488 | BD Bioscience | 347197 | 1:500 (F) |
| FOXJ1 | Invitrogen | 14‐9965‐82 | 1:300 |
| HOPX | Santa Cruz Biotech | sc‐398703 | 1:200 |
| HTII‐280 | Terrace Biotech | TB‐27AHT2‐280 | 1:150 |
| ID2 | Abcam | ab52093 | 1:300 |
| KRT5 | abcam | ab52635 | 1:200 |
| MUC5AC | Millipore | MAB2011 | 1:200 |
| NKX2‐1 | abcam | ab76013 | 1:300 |
| NKX2‐1‐APC | LS‐BIO | LS‐C264437 | 1:1000 (F) |
| p63 | Boster | PB9152 | 1:250 |
| PDPN | abcam | ab10288 | 1:500 |
| pSPC | abcam | ab40871 | 1:250 |
| SCGB3A2 | abcam | ab181853 | 1:300 |
| SOX2 | Invitrogen | MA1‐014 | 1:200 |
| SOX9 | R&D Systems | AF3075 | 1:200 |
| SPB | abcam | ab40876 | 1:250 |
| SPB (mature) | 7 Hills | 48604 | 1: 1500 (F) 1:500 (W) a |
| SPC (mature) | LS Bio | LS‐B9161 | 1:100 (F); 1:500 (W) a |
F, FACS; W, western blot.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Sterile 12‐ or 6‐well tissue culture plates
Repeat pipettor and filtered pipet tips (P‐10, P‐20, P‐200, and P‐1000)
Disposable pipets (2, 5, 10, 25 and 50 ml)
Sterile 15‐ and 50‐ml conical tubes
Humidified 37°C incubator with 5% CO2
12‐well transwells, 0.4‐µm pore size (Corning)
Additional reagents and equipment for basic cell culture techniques including cell counting (see Current Protocols article: Phelan & May, 2017)
Endoderm induction and differentiation to lung progenitor cells (LPCs)
-
1
Thaw growth factor−reduced Matrigel on ice and dilute 1:1 (volume/volume) in cold DMEM/F12 medium.
-
2
Add 1 ml of DMEM/F12:Matrigel per well of a 6‐well plate or 0.5 ml per well of 12‐well plate, ensure that it covers the entire bottom of the well, then remove remaining volume of DMEM/F12:Matrigel with a cold P‐1000 pipet tip and place into the next well. Be careful not to add bubbles. Once desired number of wells are coated, remove any excess DMEM/F12:Matrigel and/or bubbles from wells and place plate on ice for 20 min. Transfer plate to a humidified 37°C, 5% CO2 incubator overnight and use the next day.
-
3
Add 10 μM of Y‐27632 to wells of hPSCs in mTESR Plus medium that are 70% confluent, and continue incubation for 1 hr.
-
4
Aspirate off mTESR Plus medium and wash wells once with PBS.
-
5
Add Accutase (1 ml/well for 6‐well plate; 0.5 ml/well for 12‐well plate) and incubate for 20 min at 37°C in a 5% CO2 incubator.
-
6
Add stem cell passage medium directly to the Accutase at an equal volume and gently pipet cells up and down using a P‐1000 tip to make a single‐cell suspension.
-
7
Transfer individual wells into one 15‐ml conical tube and centrifuge for 5 min at 300 × g, room temperature.
-
8
Aspirate medium and resuspend the cell pellet with 1 ml of mTESR Plus supplemented with 10 μM Y‐27632.
-
9
Perform a cell count using a hemocytometer and trypan blue (see Current Protocols article: Phelan & May, 2017), then aliquot out the appropriate volume to obtain 2.0 × 105 cells/ml and plate onto a 12‐well Matrigel‐coated plate prepared in steps 1 and 2. Bring total volume of well up to 1 ml with mTESR Plus supplemented with 10 μM Y‐27632.
Cell number must be optimized per cell line. 24 hr after plating, wells should be 50%‐70% confluent.
-
10
Incubate at 37°C for 24 hr in a humidified 5% CO2 incubator.
-
11
24 hr later, aspirate mTESR Plus and add DE induction medium.
CHIR99021 should be removed after 20‐24 hr for successful DE induction.
-
12
On days 2 and 3, add DE induction medium supplemented with 100 ng/ml of human activin A only.
DE differentiation should not exceed a total of 72 hr, or efficacy will decrease.
DE differentiation can be analyzed after day 3.
-
13
On day 4, change medium to AFE induction medium. Change AFE induction medium daily (days 4, 5, and 6)
AFE differentiation can be analyzed after day 6.
-
14
On day 7, aspirate out AFE medium and wash wells with PBS.
-
15
Add 1 ml of Accutase for 10 min at 37°C.
-
16
Add 1 ml of quenching medium. Pipet up and down gently and make sure all cells are dislodged.
-
17
Transfer individual wells into one 15‐ml conical tube and centrifuge for 5 min at 300 × g, room temperature.
-
18
Resuspend in LPC induction medium with all additives plus 10 μM of Y‐27632.
-
19
Perform a cell count (see Current Protocols article: Phelan & May, 2017), then aliquot out the appropriate volume to obtain 2.5 × 105 cells/ml and plate onto a 12‐well Matrigel‐coated plate prepared in steps 1 and 2. Bring total volume of well up to 1 ml with LPC induction medium supplemented with 10 µM Y‐27632.
In 24 hr, the confluence of the cells should be 70%‐90% for successful LPC induction.
-
20
A day after, change to LPC induction medium without Y‐27632. Change the medium every other day for 9‐11 days (1.5 ml of LPC induction medium/well of a 12‐well plate).
LPC differentiation can be analyzed on days 15, 16, or 17.
3D lung organoid formation, branching, and maturation
-
21
Thaw growth factor−reduced Matrigel on ice and place pipet tips in the freezer.
-
22
Add 10 μM of Y‐27632 to each well from step 20 containing LPC induction medium.
-
23
Aspirate LPC induction medium and wash 1× with PBS.
-
24
Add Accutase (0.5 ml/12‐well) and incubate for 10 min at 37°C in a 5% CO2 incubator.
-
25
Add 1 ml of quenching medium. Pipet up and down forcefully and make sure all cells are dislodged.
Epithelial cells are very “sticky,” so make sure that all cells are dissociated from the well.
-
26
Transfer individual wells into one 15‐ml conical tube and centrifuge for 5 min at 300 × g, room temperature.
-
27
Resuspend in quenching medium plus 10 μM Y‐27632.
-
28
Perform a cell count (see Current Protocols article: Phelan & May, 2017) and aliquot out the desired volume of quenching medium/Y‐27632 with cells into 1.5‐ml microcentrifuge tubes to obtain 4.0 × 104 cells per transwell (see steps below) for ESCs and 8.0 × 104 cells for iPSCs.
Cell number must be optimized per cell line; the cells should not become overly confluent within 18 days of differentiation.
-
29
Microcentrifuge the tubes for 5 min at 300 × g, room temperature.
-
30
Aspirate off the medium and re‐suspend cells in cold Matrigel, adding 200 µl Matrigel for every 4.0 × 104 cells per transwell for ESCs and 8.0 × 104 cells for iPSCs. Keep Matrigel with cells on ice.
-
31
Add 200 µl of Matrigel/cell mixture on to a 12‐well transwell insert suspended in a 12‐well plate.
-
32
Place plate into the 5% CO2 humidified incubator at 37°C for 30‐60 min to allow gelling of Matrigel.
-
33
Add 1 ml of 3D organoid induction medium with all additive plus 10 μM of Y‐27632 to the lower chamber of the well and change the medium every other day for 6 days.
At the end of 3D organoid induction, there should be multiple clumps of cells beginning to show “sprouts.”
-
34
On day 23, change the medium to 3D organoid branching medium.
-
35
Change medium every other day for 6 days using 3D organoid branching medium.
At day 6 of 3D branching differentiation, there should be multiple branching organoids (see Fig. 2).
Figure 2.
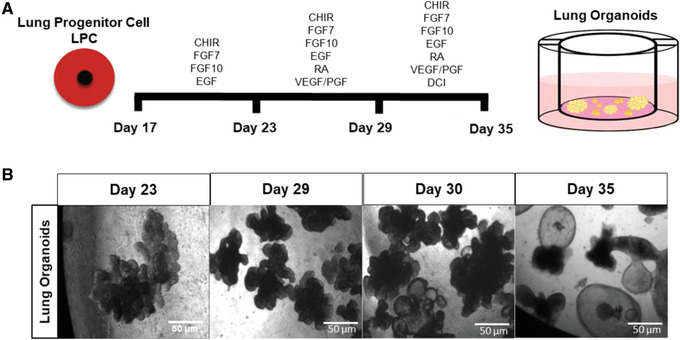
Timeline of differentiation of LPCs to 3D lung organoids. (A) Timeline showing monolayer culture of LPCs passaged on to 3D Matrigel on transwells. (B) Phase‐contrast images of live organoids as they appear at the end of each step. All images were obtained at 10× power. Scale bars, 50 µm.
Figure 1.
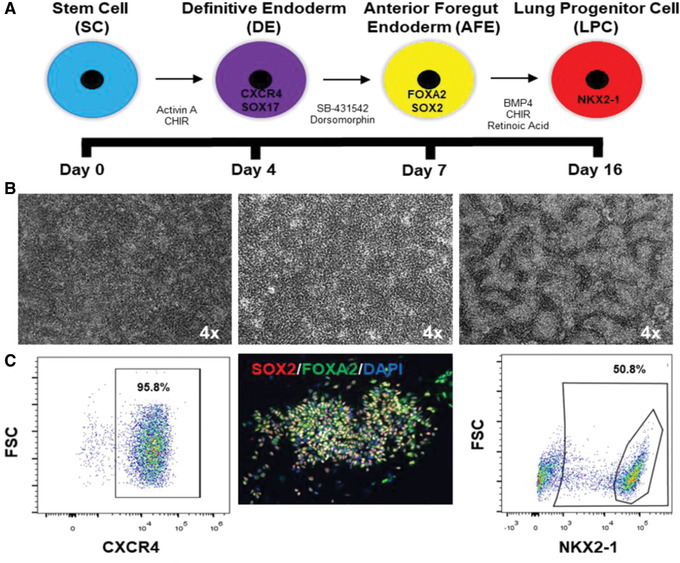
Timeline of differentiation of hPSC to LPC. (A) Timeline showing monolayer culture steps from hPSC to definitive endoderm (DE), anterior foregut endoderm (AFE), and lung progenitor cells (LPCs). Also shown are the markers expressed by the respective cell types and the combination of growth factors and small molecules used for their differentiation. (B) Representative phase‐contrast images of Day 3 DE, Day 6 AFE, and Day 16 LPCs. (C) Representative fluorescence‐activated cell sorting (FACS) plots of DE (96% efficiency); also shown is an immunofluorescence (IF) image of AFE cells that are dual SOX2 and FOXA2 immunopositive, and a FACS plot of NKX2‐1 LPCs (yielded with 51% efficiency).
-
36
On day 29, change the medium to 3D organoid maturation medium.
-
37
Change medium every other day for 6 days using 3D organoid maturation medium.
24 hr after 3D maturation, the branching organoids should inflate into large, clear spheres.
At the end of 3D maturation, the majority of organoids should be clear, large spheres (see Supporting Information, Movie #1).
Analyze differentiation
-
38
To analyze DE efficiency, perform flow cytometry for markers CXCR4 and EPCAM or perform ICC for SOX17 (day 3).
-
39
To analyze AFE efficiency, perform immunocytochemistry (ICC) for SOX2 and FOXA2 (day 6).
-
40
To analyze LPC efficiency, perform immunocytochemistry (ICC) for NKX2‐1 or flow cytometry for CD47hi/CD36low or CPM (days 15‐17).
-
41
For 3D organoid analysis, add the fixative 4% PFA directly on to the Matrigel in the transwell, as well as to the bottom well, for 1 hr at 4°C. Embed in paraffin wax and dehydrate per published protocols. Perform antigen retrieval prior to staining.
Airway markers include KRT5, MUC5AC, and SCGB3A2. Alveolar markers include SP‐C, SP‐B, HTII‐280, and AGER
REAGENTS AND SOLUTIONS
Stem cell passaging medium (day 0)
500 ml DMEM/F12 (Gibco, 10565042)
129 ml KSR (Life Technologies, 10828028)
6.5 ml Glutamax (Life Technologies, 35050061)
6.5 ml NEAA (Life Technologies, 11140050)
1.3 ml 2‐mercaptoethanol (Sigma‐Aldrich, M3148)
6.5 ml pen/strep (Lonza, 17‐602F)
Store up to 2 weeks at 4°C
Quenching medium
49 ml DMEM/F12 (Gibco, 10565042)
1 ml FBS (Gibco, 10082139)
Store up to 1 month at 4°C
DE induction medium (day 1‐3)
48.5 ml RPMI1640 + Glutamax (Life Technologies, 12633012)
1 ml B27 without retinoic acid (ThermoFisher, 12587010)
500 µl HEPES (1%) (Gibco, 15630‐080)
500 µl pen/strep (Lonza, 17‐602F)
Store with above ingredients up to 2 weeks at 4°C
Supplemented with:
Human activin A (R&D Systems; 100 ng/ml)
-
CHIR99021 (Stemgent; 5 μM)
Add the two growth factors above immediately prior to use.
Serum‐free basal medium
375 ml Iscove's Modified Dulbecco's Medium (IMDM) + Glutamax (Invitrogen, 31980030)
125 ml Ham's F12 (Invitrogen, 11765‐054)
5 ml B27 without retinoic acid (ThermoFisher, 12587010)
2.5 ml N2 (ThermoFisher, 17502048)
500 μl ascorbic acid, 50 mg/ml (Sigma, A4544)
19.5 μl monothioglycerol, 500 μg/ml (Sigma, M6145)
3.75 ml bovine serum albumin (BSA) Fraction V, 7.5% solution (Gibco, 15260‐037)
500 μl pen/strep (Lonza, 17‐602F)
Store up to 2 weeks at 4°C
AFE induction medium (day 4‐6)
Serum‐free basal medium (see recipe) supplemented with:
10 μM SB431542 (R&D Systems)
2 μM dorsomorphin (Stemgent)
Add the two growth factors above immediately prior to use.
LPC induction medium (day 7‐16)
Serum‐free basal medium (see recipe) supplemented with:
10 ng/ml of human recombinant BMP4 (R&D Systems)
0.1 μM of all‐trans retinoic acid (RA) (Sigma‐Aldrich)
3 μM of CHIR99021 (Stemgent)
Add the three growth factors above immediately prior to use.
3D organoid induction medium (day 17‐22)
Serum‐free basal medium (see recipe) supplemented with:
FGF7 (R&D Systems; 10 ng/ml)
FGF10 (R&D Systems; 10 ng/ml)
CHIR99021 (Stemgent; 3 μM)
EGF (R&D Systems; 10 ng/ml)
Add the growth factors above immediately prior to use.
3D organoid branching medium (day 23‐28)
Serum‐free basal medium (see recipe) supplemented with:
FGF7 (R&D Systems; 10 ng/ml)
FGF10 (R&D Systems; 10 ng/ml)
CHIR99021 (Stemgent; 3 μM)
all‐trans retinoic acid (RA; Sigma‐Aldrich; 0.1 μM)
EGF (R&D Systems; 10 ng/ml)
VEGF/PIGF (R&D Systems; 10 ng/ml)
Add the growth factors above immediately prior to use.
3D organoid maturation medium (day 29‐34)
3D organoid branching medium (see recipe) supplemented with:
Dexamethasone (Sigma‐Aldrich; 50 nM)
cAMP (Sigma‐Aldrich; 100 μM)
IBMX (Sigma‐Aldrich; 100 μM)
Add the growth factors above immediately prior to use.
COMMENTARY
Background Information
hPSCs have the ability to differentiate into most cell types of the body when exposed to induction signals that emulate what occurs during normal gastrulation and then normal development and lineage commitment (Takahashi et al., 2007; Thomson et al., 1998). Because they can be differentiated into these many cell types (Shi, Inoue, Wu, & Yamanaka, 2017), they can provide a platform for studying human development and disease under exceptional scrutiny, and with the ability to systematically manipulate, for experimental purposes, the genetic and environmental conditions. We are coming to appreciate, however, that having the correct complement of cells of a given organ is not sufficient. The normal human body plan has multiple cell types, in the correct ratio, not in monolayer but in three dimensions where cell‐cell contact, mechanical forces, differences in diffusion gradients, and oxygen tension all play a role in normal functioning. Hence, the stem cell field has begun to evolve such that it has become possible for the hPSC‐derived cell types to form three‐dimensional aggregates that might more authentically reproduce a given organ in vitro—a “mini‐organ” or “organoid.”
To generate lung organoids, many groups have focused on directed differentiation of PSCs into lung epithelial cells (Dye et al., 2015; Gotoh et al., 2014; Green et al., 2011; Hawkins et al., 2017; Huang et al., 2014; Konishi et al., 2016; Leibel et al., 2019; McCauley et al., 2017) via the addition of regulators of cell signaling pathways. First is the induction of definitive endoderm (D'Amour et al., 2005), then anterior foregut endoderm (Green et al., 2011), and finally specification into lung progenitor cells (LPCs) expressing the transcription factor NKX2‐1(Minoo et al., 2007). Some groups sort at this stage to purify the LPCs and make “epithelial‐only” lung organoids (Gotoh et al., 2014; Hawkins et al., 2017), while others utilize fully formed spheres that bud off the monolayer cultures (Dye et al., 2015; Huang et al., 2014). Subsequent lung differentiation occurs in 3D culture systems, usually on Matrigel, and can be used to make specific cell types including alveoli (Gotoh et al., 2014, Jacob et al., 2017), proximal airways (Konishi et al., 2016; McCauley et al., 2017), or a mix of both (Huang et al., 2015; Leibel et al., 2019). Smooth muscle also emerges in the organoids which, at times, endows them with the ability to evince respiratory contractions (Movie 2 in Supporting Information).
Such lung organoids can be used to help decipher normal and aberrant human lung development, study pulmonary disease, and test therapies (whether through gene transfer, use of drugs, or exposure to biologics). Indeed, some tests of the efficacy of a therapy may only be proven using lung organoids. For example, the viral vector‐mediated genetic correction of the lethal inborn error of surfactant B production (due to a deletion mutation in the surfactant B gene) could be demonstrated recently only in lung organoids, because only in that configuration could Type 2 alveolar cells (the lung's surfactant‐producing cells) robustly emerge (Leibel et al., 2019).
Most recently, lung organoids are being used to assess the pathophysiology of such environmental perturbagens as vaping toxins, nicotine, and viral infections such as SAR2‐CoV‐2. Because the lung organoids can be invested with hPSC derivatives other than solely from endoderm but nevertheless representative of the true human lung in situ—e.g., alveolar macrophages and vasculature—the true panoply of the viral‐mediated pathology can be assessed.
In addition, because the lung organoids can be derived from hiPSCs generated from a wide spectrum of individuals of both sexes and from a broad spectrum of racial backgrounds, ages, and genetic predispositions to disease, “patient‐specific lung organoids” can also be created. Such lung organoids may be used to help explain disparities is disease susceptibility—for example, in response to infection with viruses such as SARS‐CoV‐2—as well as help to adjust the doses of therapeutic compounds that might obviate such disparities.
Critical Parameters and Troubleshooting
The starting cell population is extremely important for a successful differentiation. The hPSCs need to be of high quality, with minimal differentiation. Timing of growth factor addition is critical and must be monitored closely. The medium can become acidotic (yellow), and therefore must be changed daily to ensure healthy differentiating cells. Cell number at each passage is crucial, as is the addition of Y‐27632 after every passage. The most critical step is achieving an efficient LPC differentiation. If the yield of NKX2‐1 or CD47hi/CD36low or CPM is less than 30%, we do not recommend continuing with the 3D lung organoid induction. We usually check the yield on day 15 or 16, and then passage the cells into organoid induction medium if there is >30% NKX2‐1.
Anticipated Results
Generally, if the protocol is followed with a close eye on timing of growth factors and cell number, there should be a yield of >30% NKX2‐1+ LPCs, which can then be transferred into 3D Matrigel for lung organoid induction. The 3D culture protocol is easy to follow, and the organoids should go through the stages of branching and budding as seen in Figure 2 and Supporting Information Movie 1. Once mature, the organoids can be passaged, frozen. or used in various assays.
While the amount of time to generate authentic organoids seems protracted, it is likely required to yield truly mature organoids that mimic true development. However, we are working on making the process more automated, higher‐throughput, less labor‐intensive, and with greater yield via parallel processing.
Time Considerations
This assay requires up to 35 days for completion, so proper planning of experiments is important. We recommend setting up the experiment on a Monday, undergoing endoderm induction on a Tuesday, and changing medium all weekend for AFE. Then, the cells can be passaged into LPCs on the following Monday and changed every 2 days until ready for analysis and 3D induction. We recommend evaluation of NKX2‐1 expression on Day 9‐10 of LPC generation, then passaging the cells when >30% LPCs are present into 3D Matrigel on the following day.
Supporting information
Time‐lapse video micrograph of the last 24 hr of the branching and maturation of the hPSC‐derived 3D lung organoids. 24 hr after 3D maturation, the branching organoids inflate into large, clear spheres as shown with the majority of the organoids appearing as clear, large spheres.
The lung organoids ultimately come to contain smooth muscle which, at times, endows them with the ability to evince respiratory contractions.
Acknowledgments
We thank James Short for helping to create the movie of the lung organoids developing.
Leibel, S. L. , McVicar, R. N. , Winquist, A. M. , Niles, W. D. , & Snyder, E. Y. (2020). Generation of complete multi−cell type lung organoids from human embryonic and patient‐specific induced pluripotent stem cells for infectious disease modeling and therapeutics validation. Current Protocols in Stem Cell Biology, 54, e118. doi: 10.1002/cpsc.118
Contributor Information
Sandra L. Leibel, Email: saleibel@health.ucsd.edu.
Evan Y. Snyder, Email: esnyder@sbp.edu.
Literature Cited
- D'Amour, K. A. , Agulnick, A. D. , Eliazer, S. , Kelly, O. G. , Kroon, E. , & Baetge, E. E. (2005). Efficient differentiation of human embryonic stem cells to definitive endoderm. Nature Biotechnology, 23(12), 1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Dye, B. R. , Hill, D. R. , Ferguson, M. A. , Tsai, Y. H. , Nagy, M. S. , Dyal, R. , … Spence, J. R. (2015). In vitro generation of human pluripotent stem cell derived lung organoids. Elife, 4, e05098. doi: 10.7554/eLife.05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh, S. , Ito, I. , Nagasaki, T. , Yamamoto, Y. , Konishi, S. , Korogi, Y. , … Mishima, M. (2014). Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Reports, 3(3), 394–403. doi: 10.1016/j.stemcr.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. D. , Chen, A. , Nostro, M. C. , d'Souza, S. L. , Schaniel, C. , Lemischka, I. R. , … Snoeck, H. W. (2011). Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nature Biotechnology, 29(3), 267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins, F. , Kramer, P. , Jacob, A. , Driver, I. , Thomas, D. C. , McCauley, K. B. , … Kotton, D. N. (2017). Prospective isolation of NKX2‐1‐expressing human lung progenitors derived from pluripotent stem cells. The Journal of Clinical Investigation, 127(6), 2277–2294. doi: 10.1172/JCI89950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. X. , Islam, M. N. , O'Neill, J. , Hu, Z. , Yang, Y. G. , Chen, Y. W. , … Snoeck, H. W. (2014). Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nature Biotechnology, 32(1), 84–91. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. X. L. , Green, M. D. , de Carvalho, A. T. , Mumau, M. , Chen, Y.‐W. , D'Souza, S. L. , & Snoeck, H.‐W. (2015). The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nature Protocols, 10(3), 413–425. doi: 10.1038/nprot.2015.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob, A. , Morley, M. , Hawkins, F. , McCauley, K. B. , Jean, J. C. , Heins, H. , … Kotton, D. N. (2017). Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell, 21(4), 472–488.e410. doi: 10.1016/j.stem.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi, S. , Gotoh, S. , Tateishi, K. , Yamamoto, Y. , Korogi, Y. , Nagasaki, T. , … Mishima, M. (2016). Directed induction of functional multi‐ciliated cells in proximal airway epithelial spheroids from human pluripotent stem cells. Stem Cell Reports, 6(1), 18–25. doi: 10.1016/j.stemcr.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibel, S. L. , Winquist, A. , Tseu, I. , Wang, J. , Luo, D. , Shojaie, S. , … Post, M. (2019). Reversal of surfactant protein B deficiency in patient specific human induced pluripotent stem cell derived lung organoids by gene therapy. Scientific Reports, 9(1), 13450. doi: 10.1038/s41598-019-49696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley, K. B. , Hawkins, F. , Serra, M. , Thomas, D. C. , Jacob, A. , & Kotton, D. N. (2017). Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of wnt signaling. Cell Stem Cell, 20(6), 844–857.e846. doi: 10.1016/j.stem.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo, P. , Hu, L. , Xing, Y. , Zhu, N. L. , Chen, H. , Li, M. , … Li, C. (2007). Physical and functional interactions between homeodomain NKX2.1 and winged helix/forkhead FOXA1 in lung epithelial cells. Molecular and Cellular Biology, 27(6), 2155–2165. doi: 10.1128/MCB.01133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Y. , Inoue, H. , Wu, J. C. , & Yamanaka, S. (2017). Induced pluripotent stem cell technology: A decade of progress. Nature Reviews Drug Discovery, 16(2), 115–130. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K. , Tanabe, K. , Ohnuki, M. , Narita, M. , Ichisaka, T. , Tomoda, K. , & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson, J. A. , Itskovitz‐Eldor, J. , Shapiro, S. S. , Waknitz, M. A. , Swiergiel, J. J. , Marshall, V. S. , & Jones, J. M. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time‐lapse video micrograph of the last 24 hr of the branching and maturation of the hPSC‐derived 3D lung organoids. 24 hr after 3D maturation, the branching organoids inflate into large, clear spheres as shown with the majority of the organoids appearing as clear, large spheres.
The lung organoids ultimately come to contain smooth muscle which, at times, endows them with the ability to evince respiratory contractions.


