1. INTRODUCTION
The COVID‐19 pandemic has had a profound and direct impact on the health and lives of the global population and has placed a huge burden on healthcare systems (Mayor, 2020; CDC, 2020a, 2020b,2020a, 2020b; WHO, 2020a). As the pandemic spread, many countries took drastic actions aiming to curtail the spread of the disease and to avoid overwhelming local, regional and national health care services. Much of routine health care diverted to COVID‐19; non‐COVID primary care and specialty care services were downscaled or completely halted. (Jones et al., 2020; The Lancet Oncology, 2020a; WHO, 2020a). Fear of contracting COVID‐19 also led to patient reluctance towards attending in‐person visits (CRUK, 2020; Jones et al., 2020). Consequently, timely cancer diagnosis may have been affected, with early reports suggesting that the decreased number of general practitioner (GP) consultations, combined with the minimalised capacity for non‐COVID care, may have had a serious impact on the diagnostic pathway (CRUK, 2020; Richards et al., 2020; Dinmohamed et al., 2020; EHRN, 2020; WHO, 2020a). For example, in the Netherlands, the number of new weekly diagnoses of all cancers (except skin) dropped to 73% of normal and to 39% of normal for skin cancers (Dinmohamed et al., 2020). In the United States, a data set that included 2.7 million patients from 39 health systems, showed that appointments for cervical, colon and breast cancer screening decreased by 86%–95% in March 2020 (EHRN, 2020). In Australia, despite relatively few COVID‐19 cases, anecdotal accounts suggest while overall GP consultations have not fallen dramatically, the switch to telemedicine has been associated with reduced pathology and radiology requests, reduced referrals for suspected cancer, and early evidence of reduced cancer incidence (Cunningham, 2020). In countries with organised cancer screening programmes, services were suspended or reduced in capacity, and in some cases, staff and laboratory resources pivoted to support COVID‐19 efforts (CRUK, 2020; Dinmohamed et al., 2020).
Cancer is the leading cause of death in many countries and its prognosis and burden are highly dependent on disease stage at diagnosis (De Angelis et al., 2014). In countries with a gatekeeper healthcare system around 86% of cancer patients are diagnosed after initial symptomatic presentation to the GP (Hansen, Vedsted, Sokolowski, Søndergaard, & Olesen, 2011). Over the past decade, there have been concerted efforts to promote early presentation of patients to primary care and timely referral by GPs to specialty care, aiming to reduce diagnostic delays (Emery et al., 2014; Hamilton, Walter, Rubin, & Neal, 2016; Rubin et al., 2015). The COVID‐19 pandemic likely already has and will continue to have an effect on all intervals of the diagnostic pathway (Dobson, Russell, & Rubin, 2014; Weller et al., 2012). Specifically, it is likely to impact the patient interval, as patients postpone presentation of symptoms; the primary care interval, as GP evaluations are delayed, unavailable or substandard by re‐organisation and use of telemedicine, and the secondary care interval, as reallocation of specialised diagnostic and care capacity increases times to consultation, testing and diagnosis (CRUK, 2020; Dobson et al., 2014; Jones, 2020). Consequences for cancer‐related burden and mortality seem inevitable and will likely be long‐lasting (Richards et al., 2020).
The COVID‐19 pandemic is at different stages around the world. Regardless of the prevailing status, it is critical that the cancer diagnostic pathway is preserved. This means we must find mechanisms to prepare for the first wave's impact, adapt to its impact, and/or recover while preparing for the next. To this end, we need to support effective and well‐balanced COVID‐19 measures that support the role of the GP as the protector of the healthcare system and as the provider of holistic care for the population. Finally, we need to learn from these experiences, as COVID‐19 may become a continuous obstacle for organising safe and effective cancer diagnostic care.
As members of the Cancer and Primary Care Research International Network (Ca‐PRI), we propose the following essential components that should be part of an ongoing approach to iteratively and continuously find the optimal balance between minimising the negative impact of the COVID‐19 pandemic while safeguarding timely cancer diagnosis. Figure 1.
FIGURE 1.
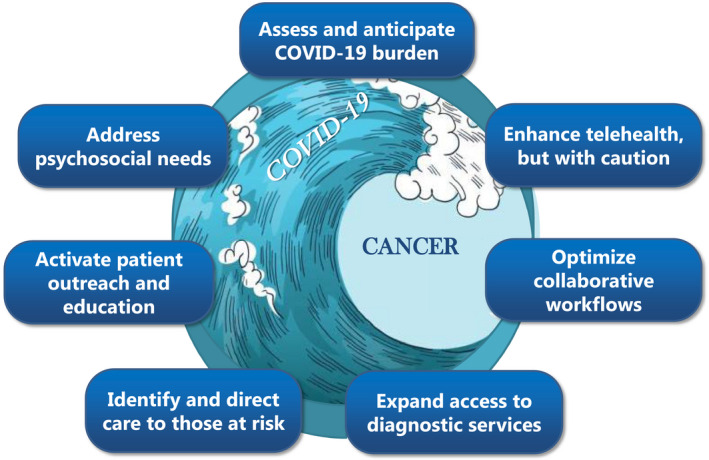
Essential components of an iterative, continuous approach to find optimal balance between impact of COVID‐19 pandemic while safeguarding timely cancer diagnosis
1.1. Assess and anticipate COVID‐19 burden
Measures taken during the COVID‐19 pandemic require real‐time, accurate evidence regarding local and national COVID‐19 disease burden. We propose that healthcare communities working with their local or national public health agencies regularly estimate, share and evaluate information regarding the status (both current and anticipated) of the COVID‐19 disease burden in their respective populations. Collaboratively charting and preparing for the anticipated impact enables a well‐balanced strategy that takes into account the expected changes in the incidence and prevalence of mild, serious and complicated COVID‐19 infections. The COVID‐19 burden should then be balanced with the diagnostic and clinical care capacity for cancer and other serious conditions. The World Health Organization (WHO) provides helpful tools to guide COVID‐19‐related measures (WHO, 2020b, c).
1.2. Enhance telemedicine in primary care, but with caution
Expanding telemedicine seems vital during the COVID‐19 pandemic; however, it also has potential risks that must be considered (Gray, Joseph, & Olayiwola, 2020). First, cancer diagnosis often requires a physical examination, hence telemedicine has clear limitations. Second, there are known issues regarding digital inequality, or disparities in both the ability to access and to use telemedicine, within local, regional, national, and global populations (Gray et al., 2020; Helsper, in press; Nouri, Khoong, Lyles, & Karliner, 2020; Velasquez & Mehrotra, 2020) and efforts to overcome these disparities are needed (Citizens online, 2020; Gray et al., 2020; Velasquez & Mehrotra, 2020). We recommend that access to primary care is optimised and expanded with the use of telemedicine, with attention for these potential negative consequences. We propose that practices not solely rely on telemedicine but also plan for in‐person office or home visits. This may be done following a screening or triage telemedicine encounter, which assesses for COVID‐19 symptoms and the need for and type of physical examination. COVID‐19 evaluations should be diverted to other settings, thus allowing for reduced spread of disease, efficient use of primary care capacity as well as optimising the use of personal protective materials (e.g. masks) and facilitating in‐person consultations with individuals concerned about contacting COVID‐19. The in‐person or home visits should follow recommended measures to prevent contamination, such as minimal “touch points” with the office staff and clinicians (WHO, 2020d). It is also important to note that telemedicine does require preparation and training. Strategies for telemedicine have been described, including guides for its adoption in primary care (BJGP Life, 2020; Shaw et al., 2020). Further, technological as well as financial incentives and reimbursement may support increased use of telemedicine (Shachar, Engel, & Elwyn, 2020). With these considerations in mind, we believe that telemedicine can be used in ways that support cancer diagnosis.
1.3. Optimise primary care and specialty care workflows
Healthcare settings should evaluate primary and specialty care capacity and explore potential for collaborative workflows, including those that allow for timely evaluation of potential cancer symptoms. For example, within the primary care setting, GP practices may consider reorganising on a regional level, thereby expanding their operations as a network to jointly provide care for their patient populations (including COVID‐19 and non‐COVID‐19). This could include dividing GP practices into those that do and do not see patients with suspected COVID‐19, thus saving protective resources and enabling the continuation of “normal” (face‐to‐face) primary care practice. The feasibility of such re‐organisation is likely dependent on local, regional or national policies, health insurance (for some countries), access to medical records, and geography, among others. In a shared effort between primary and secondary care, the efficient and flexible use of diagnostic and referral capacity should be developed. This collaboration may include agreements on prioritisation and triage of patients with and without COVID‐19 as well as strategies to remove barriers for diagnostic workup related to the availability of specialised cancer diagnostic equipment or personnel. For example, this could include facilitated access to personal contact between the GP and specialist in order to triage priority for individual patients, or direct primary care use of diagnostic equipment which would normally need referral to secondary care (additionally addressed below).
1.4. Expand access to diagnostic services
We propose that access to specialty care diagnostic services is systematic but flexible. A tailored diagnostic pathway, which takes into account the reduced availability of some investigations during the pandemic, should be guided by triage guidelines (adapted for use during the pandemic) for cancer symptoms in order to identify those who are at most urgent need of additional evaluation. For example, a breast lump, haemoptysis or rectal bleeding may warrant expeditious referrals to secondary care; whereas less specific symptoms such as fatigue or weight loss may warrant additional telemedicine consultation. Further, some diagnostic tests not typically used by GPs in some countries (e.g. faecal immunochemical test for colorectal cancer) may be expanded. The use of specialty care diagnostic services may also be broadened by joint consultations (with both primary care and patients) using telemedicine, or by promoting direct contact between GPs and secondary care physicians. Strengthening the GPs’ gatekeeper function by allowing direct access to diagnostic facilities that are currently only available to secondary care may also expedite diagnostic workup without burdening secondary care services. Another consideration may be to set up temporary diagnostic sites, staffed by specialised teams, to prevent overcrowding of regular facilities and to reassure patients avoiding regular care for fear of infection. This approach could work if it systematically developed, and performed in a flexible manner, tailored for the urgency in each patient and with appropriate training. Handling patients who need investigation in this way may reduce the potential backlog and further delays in diagnosis.
1.5. Identify and direct care to patients at risk
We must build capacity to identify patients at risk of suboptimal or delayed diagnostic workup for cancer. This may include those who are more likely to “no show” or postpone GP visits, or those who may have already been “lost” in the diagnostic pathway (Jefferson et al., 2019; Sheridan et al., 2019). Patients with chronic diseases and older age may warrant extra attention, as they are at higher risk of cancer but paradoxically are also advised to practise social distancing and remain at home. These patients at risk should be identified in each community and GP practice as these individuals may differ between practices, regions and/or countries. For example, patients who are more likely to “no show” or postpone GP visits may include those who are less willing or unable to access the GP through telemedicine, those without insurance (particularly in countries without universal health care), those in underserved communities, those with underlying psychiatric conditions (likely to worsen during COVID‐19), and those with cultural and/or language barriers (Jefferson et al., 2019; Sheridan et al., 2019). Those already lost in the diagnostic pathway may include patients who were scheduled for specialty care consultation or testing but whose appointments were cancelled due to COVID‐19, those who relayed concerning symptoms to primary care during the pandemic peak and were not referred for additional evaluation, and lastly those who may had symptoms but never reported them. Identifying these patients requires a multifaceted approach, including reviewing appointment logs, primary care records (using both diagnostic coding and free text coding), and individual physician, practice or healthcare system panels. Direct outreach with these patients at risk, in‐person or on a larger scale, advising regular “check‐ins” using telemedicine will be critical. Expansion of home visits, using required precautions, may be needed.
1.6. Activate patient outreach and education
To prevent persistent underutilisation of primary care services, there must be a concerted effort to educate patients about the need to report suspicious symptoms that may indicate cancer and address them with their primary care clinicians. Improving outreach and education involves collaboration with local and national cancer societies, charitable organisations, public health officials and the media. Public service advertisements may be shared on traditional media channels, public spaces, as well as social media. Educating patients also means educating families, promoting social awareness to keep an eye on (symptoms among) loved ones, and encouraging help‐seeking behaviours. Campaigns are needed to encourage individuals to seek medical attention and reassure them that (even if the symptom has improved or disappeared), they can safely visit their GP (with recommended safeguards outlined by the WHO applied by patients (e.g. washing hands) and by practices (e.g. using protective materials)) (WHO, 2020d).
1.7. Address psychosocial needs
Care during the COVID‐19 pandemic requires focused attention on and proactive monitoring of psychological, financial and interpersonal issues (Dubey et al., 2020), especially since they are often pervasive among those with cancer. Patterns of COVID‐19 incidence and mortality have already shown stark disparities across the globe (WHO, 2020e), and the impact of COVID‐19 will likely continue to be felt most acutely and long‐term among at risk patient populations. It is critical that GPs recognise that there will be delayed cancer diagnoses, and for some individuals this will result in diagnosis of late‐stage disease, and possibly at a non‐curative stage. Whether or not this late‐stage diagnosis was directly attributable to COVID‐19 for an individual patient may be difficult to determine, but this may agonise patients and their families. Patients may blame themselves for waiting to contact their GP or be angry at the perceived “closing down” of routine health services, while at the same time feeling guilty because they know many others needed priority care for COVID‐19. Distress related to a perceived or bona fide delayed diagnosis, coupled with the social isolation and increased community stress due to COVID‐19, may exacerbate the psychological burden among patients (Röhr et al., 2020). Integrating psychosocial screening in routine GP care will be critical in order to assess and address these needs for months and years to come (Holland, Watson, & Dunn, 2011).
2. CONCLUSION
COVID‐19 has drastically affected health care around the world, and its effects on cancer diagnosis are undeniable. In attempts to recover and mitigate these effects, we recommend an approach to iteratively and continuously find the optimal balance between minimising the negative impact of the COVID‐19 pandemic while safeguarding the diagnostic cancer pathway. This effort should be collaborative and include primary care, secondary care, patient organisations and policy makers. Changes in disease burden and healthcare system capacity should be regularly measured and deliberated between the secondary and primary care community. Knowledge should continuously be used to improve systematic, flexible and efficient triage of patients and iteratively improve the balance between referrals that are deemed urgent and delaying others that can be safely deferred. The use of telemedicine should be enhanced, but with careful consideration of its potential risks. Psychosocial care needs and patient education must remain at the forefront of the ongoing efforts.
The approach we have outlined is a starting point to guide efforts to promote timely cancer diagnosis during COVID‐19 and is not meant to be comprehensive in scope. We emphasise that in this crisis situation, situations change and evidence accumulates daily, so we will need to continuously examine, measure and refine our strategies. Our recommendations focus on cancer diagnosis, though we acknowledge the effects of the COVID‐19 pandemic on other phases of the cancer care continuum (Chan et al., 2020; Gosain et al., 2020; Mehta & Smith, 2020; Nekhlyudov et al., 2020; The Lancet Oncology, 2020b; WHO, 2020a) and that ongoing management of chronic medical conditions among those with cancer should not be overlooked. Further, these recommendations may be most applicable for high‐income and middle‐income countries and thus may be of less benefit for low‐income countries without sufficient primary and specialty care and limited infrastructure including electricity and internet access. As we already noted, relying on telemedicine in less resourced communities may worsen disparities.
In summary, COVID‐19 has led to challenges for an efficient diagnostic pathway and in the provision of health care overall. It has further complicated the promise of early cancer diagnosis. As the pandemic continues to affect global societies, this is an opportunity to overcome the challenges by joining forces to carefully expedite the development and use of promising solutions, implement collaborative changes, continuously learn and adapt our interventions. For primary care, COVID‐19 has offered an opportunity to find innovative ways to restructure practices, adapt to telemedicine and digital triaging systems, and to launch collaborative methods to work with specialty care, community groups and patient populations. Challenges will undoubtedly remain, but we need to begin the dialogue and use this newly acquired potential to balance the negative impact of the COVID‐19 pandemic with safeguarding timely cancer diagnosis.
Helsper CW, Campbell C, Emery J, et al. Cancer has not gone away: A primary care perspective to support a balanced approach for timely cancer diagnosis during COVID‐19. Eur J Cancer Care. 2020;29:e13290. 10.1111/ecc.13290
REFERENCES
- BJGP Life (2020). Video consultations: a guide for practice. Retrieved from https://bjgplife.com/2020/03/18/video-consultations-guide-for-practice/ [Google Scholar]
- Cancer Research UK (CRUK) (2020). How coronavirus is impacting cancer services in the UK. Retrieved from https://scienceblog.cancerresearchuk.org/2020/04/21/how-coronavirus-is-impacting-cancer-services-in-the-uk/ [Google Scholar]
- Chan, A. , Ashbury, F. , Fitch, M. I. , Koczwara, B. , & Chan, R. J. (2020). Cancer survivorship care during COVID‐19—perspectives and recommendations from the MASCC survivorship study group. Supportive Care in Cancer, 28(8), 3485–3488. 10.1007/s00520-020-05544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citizens online (2020). Age and digital exclusion risk: a map of GP surgeries in England. Retrieved from https://www.citizensonline.org.uk/gp-map/ [Google Scholar]
- Cunningham, M. (2020). Fears seriously ill people going unchecked as cancer referrals plummet. The Sydney Morning Herald. Retrieved from https://www.smh.com.au/national/fears-seriously-ill-people-going-unchecked-as-cancer-referrals-plummet-20200426-p54n95.html [Google Scholar]
- De Angelis, R. , Sant, M. , Coleman, M. P. , Francisci, S. , Baili, P. , Pierannunzio, D. , … Capocaccia, R. (2014). Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE–5‐a population‐based study. The Lancet Oncology, 15(1), 23–34. 10.1016/S1470-2045(13)70546-1 [DOI] [PubMed] [Google Scholar]
- Dinmohamed, A. G. , Visser, O. , Verhoeven, R. H. A. , Louwman, M. W. J. , van Nederveen, F. H. , Willems, S. M. , … Siesling, S. (2020). Fewer cancer diagnoses during the COVID‐19 epidemic in the Netherlands. The Lancet Oncology, 21(6), 750–751. 10.1016/S1470-2045(20)30265-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, C. M. , Russell, A. J. , & Rubin, G. P. (2014). Patient delay in cancer diagnosis: What do we really mean and can we be more specific? BMC Health Services Research, 14, 387. 10.1186/1472-6963-14-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey, S. , Biswas, P. , Ghosh, R. , Chatterjee, S. , Dubey, M. J. , Chatterjee, S. , … Lavie, C. J. (2020). Psychosocial impact of COVID‐19. Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 14(5), 779–788. 10.1016/j.dsx.2020.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery, J. D. , Shaw, K. , Williams, B. , Mazza, D. , Fallon‐Ferguson, J. , Varlow, M. , & Trevena, L. J. (2014). The role of primary care in early detection and follow‐up of cancer. Nature Reviews Clinical Oncology, 11(1), 38–48. 10.1038/nrclinonc.2013.212 [DOI] [PubMed] [Google Scholar]
- Epic Health Research Network (EHRN) (2020). Preventive cancer screenings during COVID‐19 Pandemic. Retrieved from https://ehrn.org/wp-content/uploads/Preventive-Cancer-Screenings-during-COVID-19-Pandemic.pdf [Google Scholar]
- Gosain, R. , Abdou, Y. , Singh, A. , Rana, N. , Puzanov, I. , & Ernstoff, M. S. (2020). COVID‐19 and Cancer: A Comprehensive Review. Current Oncology Reports, 22(5), 53. 10.1007/s11912-020-00934-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, D. M. , Joseph, J. , & Olayiwola, J. N. (2020). Strategies for Digital Care of Vulnerable Patients in a COVID‐19 World—Keeping in Touch. JAMA Health Forum, 1(6), e200734. 10.1001/jamahealthforum.2020.0734 [DOI] [PubMed] [Google Scholar]
- Hamilton, W. , Walter, F. M. , Rubin, G. , & Neal, R. D. (2016). Improving early diagnosis of symptomatic cancer. Nature Reviews. Clinical Oncology, 13(12), 740–749. 10.1038/nrclinonc.2016.109 [DOI] [PubMed] [Google Scholar]
- Hansen, R. P. , Vedsted, P. , Sokolowski, I. , Søndergaard, J. , & Olesen, F. (2011). Time intervals from first symptom to treatment of cancer: A cohort study of 2,212 newly diagnosed cancer patients. BMC Health Services Research, 11, 284. 10.1186/1472-6963-11-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsper, E. J. (in press). Digital disconnect: The social causes and consequences of digital inequalities. London: SAGE. [Google Scholar]
- Holland, J. , Watson, M. , & Dunn, J. (2011). The IPOS new International Standard of Quality Cancer Care: Integrating the psychosocial domain into routine care. Psycho‐Oncology, 20(7), 677–680. 10.1002/pon.1978 [DOI] [PubMed] [Google Scholar]
- Jefferson, L. , Atkin, K. , Sheridan, R. , Oliver, S. , Macleod, U. , Hall, G. , … Knapp, P. (2019). Non‐attendance at urgent referral appointments for suspected cancer: A qualitative study to gain understanding from patients and GPs. The British Journal of General Practice: The Journal of the Royal College of General Practitioners, 69(689), e850–e859. 10.3399/bjgp19X706625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. , Neal, R. D. , Duffy, S. , Scott, S. E. , Whitaker, K. L. , & Brain, K. (2020). Impact of the COVID‐19 pandemic on the symptomatic diagnosis of cancer: the view from primary care. The Lancet Oncology, 21(6), 748–750. 10.1016/S1470-2045(20)30242-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor, S. (2020). COVID‐19: Impact on cancer workforce and delivery of care. The Lancet Oncology, 21(5), 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta, A. K. , & Smith, T. J. (2020). Palliative Care for Patients With Cancer in the COVID‐19 Era. JAMA Oncology. 10.1001/jamaoncol.2020.1938 [DOI] [PubMed] [Google Scholar]
- Nekhlyudov, L. , Duijts, S. , Hudson, S. V. , Jones, J. M. , Keogh, J. , Love, B. , … Feuerstein, M. (2020). Addressing the needs of cancer survivors during the COVID‐19 pandemic. Journal of Cancer Survivorship, 1–6. 10.1007/s11764-020-00884-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri, S. , Khoong, E. C. , Lyles, C. R. , & Karliner, L. (2020). Addressing equity in telemedicine for chronic disease management during the Covid‐19 Pandemic. NEJM Catalyst. https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0123 [Google Scholar]
- Richards, M. , Anderson, M. , Carter, P. , Ebert, B. L. & Mossialos, E. (2020). The impact of the COVID‐19 pandemic on cancer care. Nat Cancer, 1, 565–567. 10.1038/s43018-020-0074-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhr, S. , Müller, F. , Jung, F. , Apfelbacher, C. , Seidler, A. , & Riedel‐Heller, S. G. (2020). Psychosocial impact of quarantine measures during serious coronavirus outbreaks: a rapid review. Psychiatrische Praxis, 47(4), 179–189. 10.1055/a-1159-5562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, G. , Berendsen, A. , Crawford, S. M. , Dommett, R. , Earle, C. , Emery, J. , … Zimmermann, C. (2015). The expanding role of primary care in cancer control. The Lancet Oncology, 16(12), 1231–1272. 10.1016/S1470-2045(15)00205-3 [DOI] [PubMed] [Google Scholar]
- Shachar, C. , Engel, J. , & Elwyn, G. (2020). Implications for telehealth in a postpandemic future. JAMA, 323(23), 2375. 10.1001/jama.2020.7943. [DOI] [PubMed] [Google Scholar]
- Shaw, S. E. , Seuren, L. M. , Wherton, J. , Cameron, D. , A'Court, C. , Vijayaraghavan, S. , … Greenhalgh, T. (2020). Video consultations between patients and clinicians in diabetes, cancer, and heart failure services: Linguistic ethnographic study of video‐mediated interaction. Journal of Medical Internet Research, 22(5), e18378. 10.2196/18378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan, R. , Oliver, S. E. , Hall, G. , Allgar, V. , Melling, P. , Bolton, E. , … Knapp, P. (2019). Patient non‐attendance at urgent referral appointments for suspected cancer and its links to cancer diagnosis and one year mortality: A cohort study of patients referred on the Two Week Wait pathway. Cancer Epidemiology, 63, 101588. 10.1016/j.canep.2019.101588 [DOI] [PubMed] [Google Scholar]
- The Centers for Disease Control and Prevention (CDC) (2020a). People who need to take extra precautions. Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html. [Google Scholar]
- The Centers for Disease Control and Prevention (CDC) (2020b). Severe outcomes among patients with coronavirus disease 2019 (COVID‐19) ‐ United States, February 12‐March 16, 2020. Morbidity and Mortality Weekly Report (MMWR), 2020(69), 343–346. 10.15585/mmwr.mm6912e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet Oncology (2020a). COVID‐19: Global consequences for oncology. The Lancet. Oncology, 21(4), 467. 10.1016/S1470-2045(20)30175-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet Oncology (2020b). Safeguarding cancer care in a post‐COVID‐19 world. The Lancet Oncology, 21(5), 603. 10.1016/S1470-2045(20)30243-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez, D. , & Mehrotra, A. (2020). Ensuring the growth of telehealth during COVID‐19 does not exacerbate disparities in care. https://www.healthaffairs.org/do/10.1377/hblog20200505.591306/full/
- Weller, D. , Vedsted, P. , Rubin, G. , Walter, F. M. , Emery, J. , Scott, S. , … Neal, R. D. (2012). The Aarhus statement: Improving design and reporting of studies on early cancer diagnosis. British Journal of Cancer, 106(7), 1262–1267. 10.1038/bjc.2012.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2020a). COVID‐19 AND NCDs ‐ Rapid assessment of service delivery for NCDs during the COVID‐19 pandemic. Retrieved from https://www.who.int/teams/ncds/covid-19. [Google Scholar]
- World Health Organization (WHO) (2020b). Country & Technical Guidance ‐ Coronavirus disease (COVID‐19). Retrieved from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance [Google Scholar]
- World Health Organization (WHO) (2020c). Health Workforce Estimator (HWFE). Retrieved from http://www.euro.who.int/en/health-topics/Health-systems/pages/strengthening-the-health-system-response-to-covid-19/surge-planning-tools/health-workforce-estimator-hwfe [Google Scholar]
- World Health Organization (WHO) (2020d). Coronavirus disease (COVID‐19) advice for the public. Retrieved from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public [Google Scholar]
- World Health Organization (WHO) (2020e). WHO Coronavirus Disease (COVID‐19) Dashboard. https://covid19.who.int/ [Google Scholar]


