Dear Editor,
In December 2019, the cases of pneumonia of unknown etiology were reported in Wuhan city, Hubei province of China. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pathogen, a novel coronavirus, was detected from the lower respiratory tract samples of infected patients, and this virus‐caused respiratory tract infection was termed Coronavirus Disease 2019 (COVID‐19). The pandemic was declared by World Health Organization on 11 March 2020 and as of 21 May 21 2020, more than 5 million cases and more than 300 thousand deaths were reported. 1 The disease has been reported to show different types of cutaneous findings including urticarial, maculopapular, erythema multiforme‐like, varicella‐like, purpuric, livedoid, and thrombotic‐ischemic lesions. 2 , 3 Given the high mortality rate of the disease, prompt and accurate identification of the relevant skin manifestations may play a significant role in the early diagnosis and appropriate management of the entity.
A 37‐year‐old female patient with confirmed COVID‐19 pneumonia presented with erythematous targetoid lesions distributed over the ventral and dorsal sides of the hands, and elbows (Figures 1 and 2). There were also painful ulcerations on the lip, tongue, and palate. (Figure 3). The ocular and genital mucosal surfaces were normal. The lesions occurred on the fifth day of COVID‐19 treatment, which included hydroxychloroquine (Day 1: 2 × 400 mg, Day 2‐4: 2 × 200 mg), azithromycin (Day 1: 1 × 500 mg, Day 2‐4: 1 × 250 mg), and oseltamivir (2 × 75 mg for 5 days). The symptoms related to COVID‐19 pneumonia started 10 days before the rash. The patient had no previous medical history of a similar eruption. There was also no history of herpetic infection. Complete blood count, biochemical parameters, and serological tests including Herpes simplex virus IgM and IgG, Ebstein‐Barr virus IgM and IgG, Cytomegalovirus IgM and IgG, HbsAg, Anti HCV vs Mycoplasma IgM vs IgG antibodies were within normal limits. Biopsy was not performed due to the risk of infection. The patient was clinically diagnosed with Erythema multiforme major (EMM) and all medications were stopped. The patient was then started on 40 mg/day oral methylprednisolone tapered by 5 mg every day. Topical anesthetic and antiseptic mouthwashes were also applied. On the eighth day of the treatment, pneumonia and the cutaneous lesions regressed considerably, and the patient was discharged without additional complications (Figures 4 and 5).
FIGURE 1.
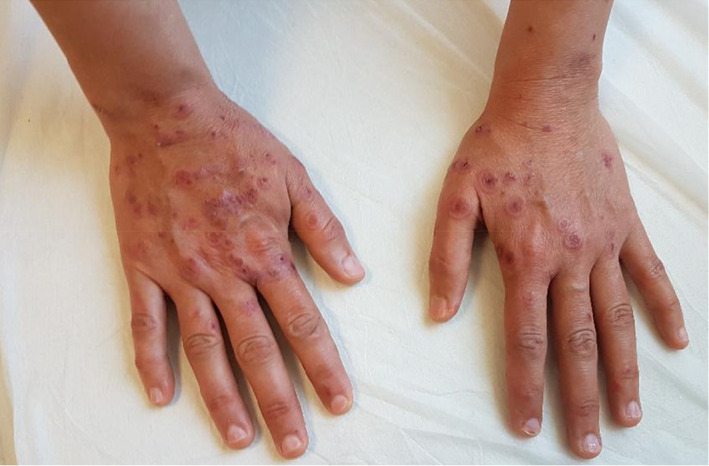
Erythematous targetoid lesions distributed over the dorsal sides of the hands
FIGURE 2.
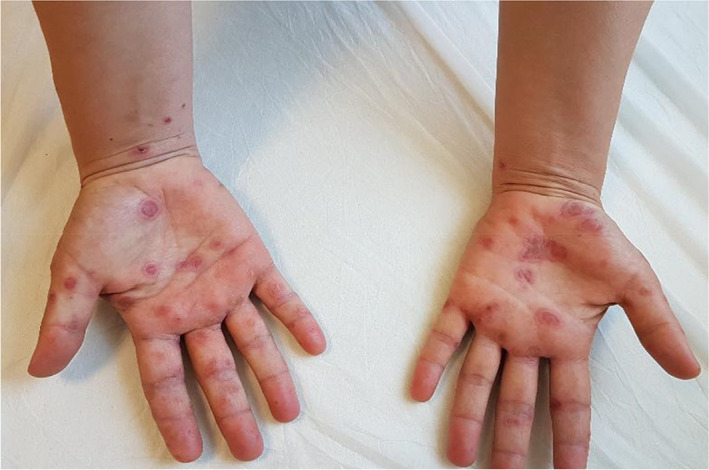
Erythematous targetoid lesions distributed over the palmar surfaces
FIGURE 3.
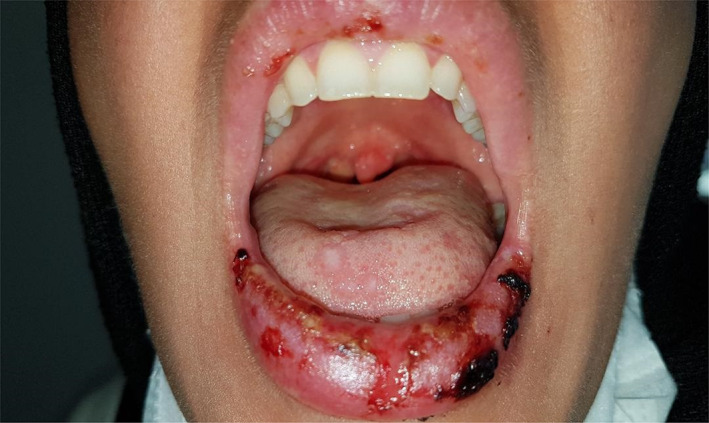
Ulcerated lesions involved the lower lip and tongue
FIGURE 4.
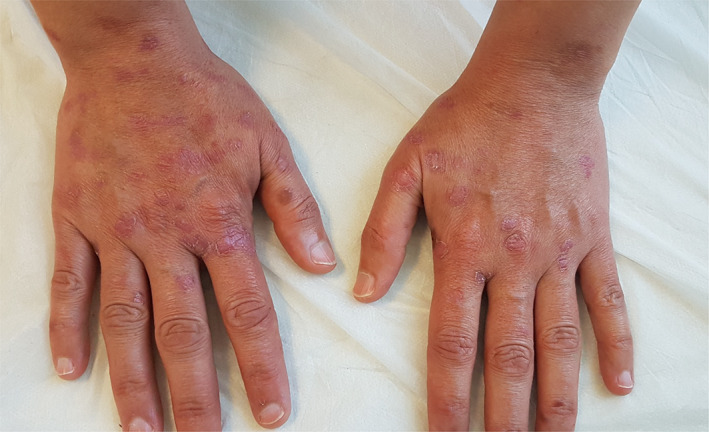
Considerable improvement in cutaneous lesions following the administration of oral methylprednisolone
FIGURE 5.
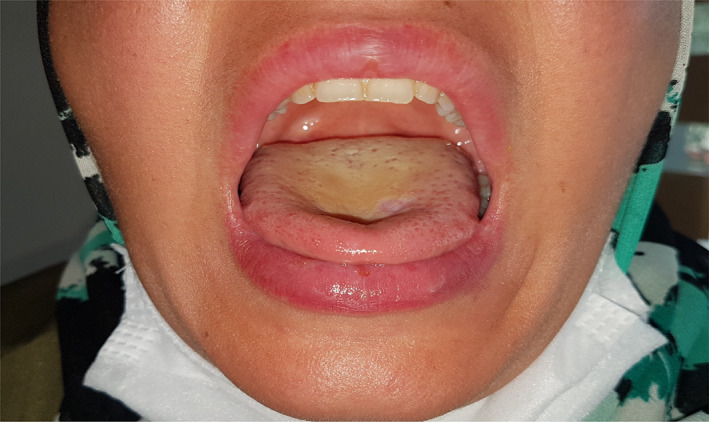
Resolution of the mucosal lesions following the treatment
Erythema multiforme (EM) is an acute, immune‐mediated disease characterized by target‐like cutaneous lesions. EMM is the term describing EM with severe mucosal involvement while EM minor refers to EM without or with only mild mucosal disease. Many factors, including infections, medications, malignancy, autoimmune disorders, radiation, and sarcoidosis have been associated with EM. More than 50% of EMM cases are associated with medications. The patient in this manuscript has given written informed consent to the publication of her case details. 4
There are only a few case reports describing EM in patients with COVID‐19. Robustelli Test et al 5 reported a case of acute generalized exanthematous pustulosis accompanying by EM‐like lesions in a 70‐year‐old patient with COVID‐19 under hydroxychloroquine treatment. Jimenez‐Cauhe et al 6 observed EM‐like eruption in four patients with COVID‐19. All patients were managed with systemic corticosteroids with resolution of the cutaneous lesions within 2 to 3 weeks. They concluded that EM‐like exanthem might be a peculiar pattern of exanthem associated with COVID‐19. Janah et al 2 identified atypical palmar EM lesions in two patients with COVID‐19. They suggested that the eruption might be associated with SARS‐CoV‐2 rather than hydroxychloroquine or other infectious agents.
In our patient, the lesions might also be related to COVID‐19, however, it is not possible to rule out the involvement of the hydroxychloroquine and/or oseltamivir which have been reported to cause many severe cutaneous reactions including erythema multiforme, Steven‐Johnson syndrome, and toxic epidermal necrolysis. 7 , 8 In this context, we hypothesized that the drugs administered to our patient might potentiate the cutaneous reaction induced by SARS‐CoV‐2.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1. WHO Health Organization . Naming the coronavirus disease (COVID‐19) and the virus that causes it. World Health Organization; 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it.
- 2. Janah H, Zinebi A, Elbenaye J. Atypical erythema multiforme palmar plaques lesions due to Sars‐Cov‐2. J Eur Acad Dermatol Venereol. 2020. [published online ahead of print, 2020 May 9]. 10.1111/jdv.16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020. [published online ahead of print, 2020 Apr 29]. 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Warnock JK, Morris DW. Adverse cutaneous reactions to antidepressants. Am J Clin Dermatol. 2002;3(5):329‐339. [DOI] [PubMed] [Google Scholar]
- 5. Robustelli Test E, Vezzoli P, Carugno A, et al. Acute generalized exanthematous pustulosis with erythema multiforme‐like lesions in a COVID‐19 woman. J Eur Acad Dermatol Venereol. 2020. [published online ahead of print, 2020 May 9]. 10.1111/jdv.16613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jimenez‐Cauhe J, Ortega‐Quijano D, Carretero‐Barrio I, et al. Erythema multiforme‐like eruption in patients with COVID‐19 infection: clinical and histological findings. Clin Exp Dermatol. 2020. [published online ahead of print, 2020 May 9]. 10.1111/ced.14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abou Assalie N, Durcan R, Durcan L, Petri MA. Hydroxychloroquine‐induced erythema multiforme. J Clin Rheumatol. 2017;23(2):127‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zuo W, Wen LP, Li J, Mei D, Fu Q, Zhang B. Oseltamivir induced Stevens‐Johnson syndrome/toxic epidermal necrolysis‐case report. Medicine (Baltimore). 2019;98(19):e15553. [DOI] [PMC free article] [PubMed] [Google Scholar]


