Abstract
Background
Initial evidence from China suggests that most vulnerable subjects to COVID‐19 infection suffer from pre‐existing illness, including metabolic abnormalities. The pandemic characteristics and high‐lethality rate of COVID‐19 infection have raised concerns about interactions between virus pathobiology and components of the metabolic syndrome.
Methods
We harmonized the information from the recent existing literature on COVID‐19 acute pandemic and mechanisms of damage in non‐alcoholic fatty liver disease (NAFLD), as an example of chronic (non‐communicable) metabolic pandemic.
Results
COVID‐19‐infected patients are more fragile with underlying metabolic illness, including hypertension, cardiovascular disease, type 2 diabetes, chronic lung diseases (e.g. asthma, chronic obstructive pulmonary disease and emphysema) and metabolic syndrome. During metabolic abnormalities, expansion of metabolically active fat ('overfat condition') parallels chronic inflammatory changes, development of insulin resistance and accumulation of fat in configuring NAFLD. The deleterious interplay of inflammatory pathways chronically active in NAFLD and acutely in COVID‐19‐infected patients, can explain liver damage in a subgroup of patients and might condition a worse outcome in metabolically compromised NAFLD patients. In a subgroup of patients with NAFLD, the underlying liver fibrosis might represent an additional and independent risk factor for severe COVID‐19 illness, irrespective of metabolic comorbidities.
Conclusions
NAFLD can play a role in the outcome of COVID‐19 illness due to frequent association with comorbidities. Initial evidences suggest that increased liver fibrosis in NAFLD might affect COVID‐19 outcome. In addition, long‐term monitoring of post‐COVID‐19 NAFLD patients is advisable, to document further deterioration of liver damage. Further studies are required in this field.
Keywords: fatty liver, mitochondria, nitrosative stress, oxidative stress, SARS‐CoV‐2
1. INTRODUCTION
The global acute pandemic of severe acute respiratory syndrome (SARS) caused by the coronavirus SARS‐CoV‐2 (COVID‐19, Sarbecovirus subgenus, Betacoronavirus genus, Coronaviridae family) has suddenly become a major threat to public health. 1 , 2 Since late 2019, more than 3.6 million confirmed cases, more than 250,000 deaths in 213 countries at a world level (at May 5, 2020), and a huge burden of care have been recorded. 3
Although many subjects remain asymptomatic, 4 the most frequent and critical clinical presentation of COVID‐19 is the respiratory involvement, ranging from mild respiratory symptoms to severe pneumonia. However, the infection by SARS‐CoV‐2 virus represents a systemic disease, 5 which can lead to myocardial injury, 6 , 7 heart failure, 6 vascular inflammation, myocarditis, cardiac arrhythmias, 7 hypoxic encephalopathy, 8 multi‐organ failure and ultimately death. 9
In the first phase of the COVID‐19 disease, the pathogenic properties depend on binding of spike viral proteins to angiotensin I converting enzyme 2 (ACE2) receptors, 10 , 11 , 12 which allow the virus to enter the target cells. 13 Receptors are expressed in the epithelia of the upper respiratory tract (nasopharynx) as major site of replication and, in the human lung, in alveolar epithelial cells (type II) and ciliated cells. 11 , 14 , 15 ACE2 receptor expression also occur in vascular endothelium, in the brush border of intestinal enterocytes 11 , 16 and in cholangiocytes. 11 , 17 Thus, the symptomatic involvement of the gastrointestinal tract is possible with COVID‐19. 18 , 19 , 20 , 21 A recent USA report describes a clinically evident gastrointestinal involvement in 61% of COVID‐19‐positive subjects. 22 The presence of ACE2 receptors in the glandular cells of gastric, duodenal and distal enterocytes may result in malabsorption, unbalanced intestinal secretion and activation of the enteric nervous system, leading to gastrointestinal symptoms. 23 , 24
The liver can also become a target of COVID‐19 infection, although major liver damage is uncommon. 25 , 26 , 27 , 28 SARS‐Cov‐2 might affect the liver by direct (i.e. viral translocation from the gut to the liver) or indirect mechanisms (ie systemic inflammation, liver ischaemia and hypoxia, effects on pre‐existing liver diseases, drug‐related liver injury) and represents a new challenge for hepatologists. 28 Notably, non‐alcoholic fatty liver disease (NAFLD) is a chronic dysmetabolic pandemic which has become the most common liver disease in the world, with a prevalence rate of 30% in the Western population. 29 , 30 Moreover, NAFLD does not stands on its own but it is usually associated as 'fellow traveller' with a constellation of risk factors, metabolic syndrome and illness (Figure 1). 31 Along with this view, the acronym NAFLD has been recently re‐visited by coining the acronym MAFLD ('metabolic dysfunction‐associated fatty liver disease'). 32 NAFLD/MAFLD can therefore affect the final outcome in COVID‐19‐infected patients. 33 , 34 , 35 , 36 In addition, the liver itself has increased susceptibility to drugs in conditions of chronic injury. 37 , 38 , 39 In this context, the presence of inflammatory pathways (in particular those involving cytokines) present either in NAFLD 40 , 41 , 42 and COVID‐19‐infected patients 43 , 44 , 45 , 46 could increase liver inflammation or be a marker of metabolic risk factors further aggravating the clinical outcome.
FIGURE 1.
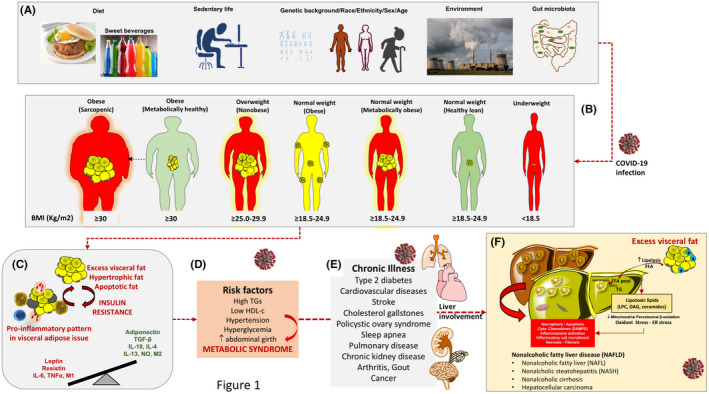
Sequences of pathophysiological mechanisms predisposing to metabolic illness and liver steatosis. Rationale to explain multi‐organ and liver damage during COVID‐19 infection. (A) Initial role of wrong lifestyles (hypercaloric, unbalanced, fructose‐ and refined carbohydrate‐enriched diet, sedentary behaviour), on a genetic/racial, ethnical and environmental background. Changes in intestinal microbiota can also govern additional metabolic changes due to biotransformation of foods, local inflammatory changes, increased intestinal permeability 112 to bacterial products (ie lypopolisaccharides). (B) Expansion of visceral fat may occur in different phenotypes, independently of simple body weight (encompassing the term 'adiposity' or 'overfat'). The three subtypes at risk include normal weight but metabolically obese subjects (characterized by high visceral adiposity, ie about % overfat, normal lean mass, propensity to develop metabolic abnormalities), 87 , 113 overweight individuals and obese sarcopaenic subjects (high visceral adiposity, decreased lean mass, likely several metabolic abnormalities). The subtype «normal weight obese» has increased (>30%) fat mass (not necessarily visceral adiposity), a normal lean mass, without metabolic abnormalities. Overfat conditions (in red) are predisposing to chronic metabolic inflammation, compromised immunity, increased risk of chronic disease and infections (including viral infections). Underweight, underfat individuals also share the same risk for chronic inflammation, compromised immunity, increased risk of chronic disease and infections. (C) The metabolically active vicious circle originates from the excess visceral fat with production of inflammatory molecules. In lean individuals or metabolically healthy subjects, anti‐inflammatory cytokines (transforming growth factor beta (TGF‐β), interleukin 10 (IL‐10), IL‐4, IL‐13, nitric oxide (NO)) activate M2 macrophage‐ and inhibit neutrophil‐mediated inflammation. T lymphocytes, neutrophils, B1 and B2 cells, NK cells and innate lymphoid cells also populate the fat tissue. 95 Hypertrophic or apoptotic adipocytes (in grey) in obese individuals can secrete pro‐inflammatory molecules (leptin, resistin, IL‐6 and tumour necrosis factor‐α) that activate a pro‐inflammatory M1 macrophage. 114 The pro‐inflammatory metabolic status is a factor promoting insulin resistance, as well as defective immune response (poor T cell and macrophage function). (D) Further progression of the chronic pro‐inflammatory status and insulin resistance paves the way to several metabolic risk factors contributing to the metabolic syndrome. (E) Chronic illness can follow with established risk factors. (F) Non‐alcoholic fatty liver disease (NAFLD) and the spectrum of liver abnormalities are the consequence of the accumulated metabolic abnormalities. Excess lipolysis during insulin resistance will increase the influx of free fatty acids (FFA), synthesis of triglycerides, enrichment of FFA pool with lipotoxic products (lysophosphatidylcholine (LPC); diacylglycerol (DAG); ceramides). Products mediate endoplasmic reticulum (ER) stress, oxidant stress and activation of the inflammasome (multiprotein cytoplasmic complex that responds to damage‐associated molecular patterns (DAMPs), as part of the innate immunity response). 38 , 39 , 90 , 115 Abbreviations: BMI, body mass index
Because of the pandemic characteristics and high‐lethality rate of SARS‐CoV‐2 infection, precise knowledge of the virus behaviour and of risk factors predisposing to COVID‐19 onset and progression has a key role in the near future to anticipate virus‐related events worldwide. In the analysis of Wang et al, hypertension, diabetes, chronic obstructive pulmonary disease (COPD), cardiovascular disease and cerebrovascular disease (OR 2.29‐5.97) were independent risk factors associated with COVID‐19‐infected patients. 47 Furthermore, a recent analysis of 1999 hospitalised COVID‐19‐infected patients in New York showed that BMI > 40 kg/m2 is one of the strongest predictor of hospitalisation (OR 6.2) and is exceeded only by age ≥ 75 years (OR 66.8) and age 65‐74 years (OR 10.9). 48 Finally, a study on 202 consecutive patients with confirmed COVID‐19 identified NAFLD as independently associated with COVID‐19 progression. 49
We discuss here the ongoing interaction of two different pandemic conditions: the recent, acute COVID‐19 outbreak and the chronic NAFLD as part of an even wider set of metabolic disorders. During COVID‐19 infection, the underlying NAFLD could pave the way to more severe hepatic and metabolically associated complications and become another prognostic marker of viral disease.
2. COVID‐19 AND NAFLD
In the liver, ACE2 receptors are mainly expressed in cholangiocytes (60% of cells) and in endothelial cells, rather than in hepatocytes (only 3% of cells) or Kupffer cells (where ACE2 receptors are absent). 17 , 50 , 51 Major factors involved in SARS‐CoV‐2 infection and liver damage are depicted in Figure 2.
FIGURE 2.
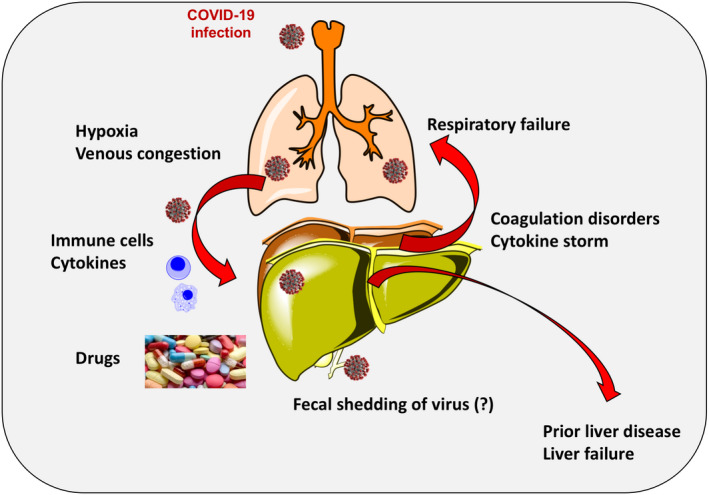
Major factors involved in COVID‐19 infection and liver damage. Factors include lung involvement leading to hypoxia and venous congestion with liver stasis, role of immune cells and cytokines, drug‐induced liver damage and addition of coagulation disorders and cytokine storm. A prior liver disease might exaggerate the damage from ongoing COVID‐19 infection. Non‐alcoholic fatty liver disease might represent per se a condition of intrinsic frailty (due to ongoing lipotoxicity, chronic inflammatory status, insulin resistance, oxidant stress, immune response), or be a marker of additional coexisting metabolic disorders which will aggravate the clinical course of COVID‐19
In Chinese patients, the prevalence of acute liver injury during COVID‐19 disease was 15.4%. 52 However, an involvement of the liver has been reported in about 60% of cases, 53 and the risk of liver dysfunction seems to increase in older age. 54
Ji et al 49 reported on 202 COVID‐19‐infected patients and NAFLD status. Liver abnormalities were 50% on admission and 75% during hospitalization, manifesting as hepatocellular pattern (only 3% with ductular or mixed pattern); 33% of the patients had persistent abnormal liver function from admission to last follow‐up. COVID‐19 progression was associated with male sex, age > 60 years, higher BMI, underlying comorbidity and NAFLD. In this study, univariate and multivariate logistic regression analyses indicated NAFLD as an independent risk factor for COVID‐19 progression (OR 6.4; 95% CI 1.5‐31.2). NAFLD was also associated with higher likelihood of abnormal liver function from admission to discharge, and longer viral shedding time.
The risk of severe COVID‐19 presentation increases by the coexistence of obesity and NAFLD, 55 pointing to a specific and additional role for pathogenic mechanisms involved in NAFLD onset and progression.
NAFLD has also been previously linked with increased risk of recurrent bacterial infections, 56 and with increased 30‐day all‐cause mortality in patients with community‐acquired pneumonia. 57
A meta‐analysis examined 313 severe group cases and 1167 non‐severe group cases with respect to liver disease in patients with COVID‐19. Patients with previous liver disease were not at increased risk of disease progression (OR: 0.67, 95% CI: 0.30‐1.49, P = .326). 47 Limitations in this survey, however, include the poor number of available cases, different severity definitions, underlying coexisting illness and unspecified liver diseases. On the other hand, in a series of 310 patients with COVID‐19 and NAFLD, the presence of intermediate or high FIB‐4 scores greatly and independently increased the risk of a severe progression of the COVID‐19 disease. 58 Patients with NAFLD show a different risk since they are exposed to a significant metabolic risk. Several mechanisms of damage could link COVID‐19 to liver and require attention (Figure 2).
A direct cytopathic viral damage is a possibility. SARS‐CoV‐2 in gut lumen could translocate to the liver via portal flow and induce a direct damage due to active viral replication in hepatic cells through ACE2 receptors. 59 This effect is not necessarily linked to increased liver SARS‐CoV‐2 uptake, since NAFLD/MAFLD is not associated with changes in expression of liver genes implicated in SARS‐CoV‐2 infection. A study did not find significant differences in human liver biopsies comparing gene expression of four proteins: angiotensin‐converting enzyme 2, cellular protease Transmembrane Protease Serine 2, phosphatidylinositol 3‐phosphate 5‐kinase, and cathepsin L protein (genes ACE2, TMPRSS2, PIKfyve and CTSL, respectively). 60 Thus, a role for the hepatic innate immunity populations in increasing the likelihood of symptomatic COVID‐19 infections (see below) is possible. 61
Hepatocellular hypoxia in chronic liver diseases in COVID‐19‐infected patients might lead to increased expression of ACE2 receptors, 51 and hypoxia‐inducible factors (HIFs), a family of transcription factors activated by hypoxia. Such changes might further aggravate metabolic diseases such as NAFLD, 62 aggravating NAFLD progression. 54 , 63 From a clinical point of view, specific abnormalities of bile duct chemistry are rare in COVID‐19‐infected patients 9 and, thus, the ACE2‐mediated liver injury could be mainly secondary to the localization of these receptors in the endothelial cells 17 and NAFLD progression might include exaggerated production of ROS and NO derivatives, 64 inflammatory pathways leading to cellular crosstalk with Kupffer cells 65 and HIF‐2α upregulation, 66 through suppression of fatty acid β‐oxidation and induction of lipogenesis in the liver via PPARα. 63 This hypothesis is partly supported by liver histology from patients deceased due to severe COVID‐19, reporting moderate microvesicular steatosis and mild lobular and portal activity, possibly due to a direct effect of SARS‐CoV‐2 infection or to drug‐induced liver injury (DILI). 67
Dysregulated systemic and hepatic innate immunity. 44 , 68 ACE2 receptors in enterocytes 69 would predispose to viral translocation to the liver with potentials for viral circulation via the reticular system. 70 The innate immune cellular cluster in the liver would be activated with inflammatory and changes due to cytokine production (Figure 3). Patients with severe COVID‐19 infection display elevation of inflammatory biomarkers such as C‐reactive protein (CRP), serum ferritin, LDH, D‐dimer and interleukin (IL‐6, IL‐2). 71 IL‐6, in particular, appears as a key factor in the onset and progression of the 'cytokine storm' described in COVID‐19‐infected patients, 54 and increased IL‐6 levels have been reported in subjects with NAFLD. 40 , 72 IL‐6 plays an important role in the 'cytokine storm' of COVID‐19‐infected patients. 54 Increased IL‐6 levels occur in NAFLD 40 , 72 and could represent a marker or mediator of related atherosclerosis 72 and comorbidities often found in COVID‐19‐infected patients. The cytokine MCP‐1 is often increased in COVID‐19‐infected patients 45 and acts as a further hit for steatohepatitis. 73
Drug‐induced liver injury (DILI): initial clinical guidelines recommended antiviral agents for COVID‐19, with some of them, including lopinavir/ritonavir, remdesivir, chloroquine, tocilizumab, and uminefovir, Chinese traditional medicine, being potentially hepatotoxic in some patients (and a few have subsequently already been proven to be ineffective). The presence of underlying metabolic abnormalities and NAFLD might facilitate DILI. 49 , 74
Reactivation of pre‐existing liver disease: patients with pre‐existing chronic liver disease may be more susceptible to liver damage from SARS‐CoV‐2. 75 Biological drugs like tocilizumab and baricitinib might also cause HBV reactivation and thus lead to liver function deterioration. On the other hand, it is still unknown whether SARS‐CoV‐2 infection exacerbates cholestasis in those with underlying cholestatic liver diseases. Such pathways might aggravate NAFLD.
Hepatic lipid metabolism. Lipid production and lipid breakdown in the liver provide lipid species which negatively regulate the underlying status of chronic metabolic inflammation. Basic and clinical research suggest that the complex network of factors acting within the liver can drive innate immune activation. This pathway directly triggers and amplifies hepatic inflammation and affects the development of hepatic fibrosis in NAFLD/NASH. 76
FIGURE 3.
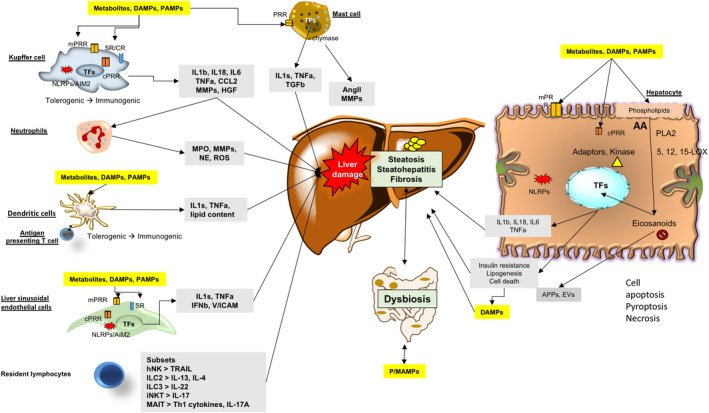
Population of innate immune cells playing a role in progression of NAFLD. Immune cells include mast cells (MC), Kupffer cells (KC), neutrophils, dendritic cells (DC), liver sinusoidal endothelial cells (LSEC), resident innate‐like lymphocytes (ILC) and hepatocytes. Kupffer cells, neutrophils, dendritic cells, liver sinusoidal endothelial cells and hepatocytes detect the presence of gut‐derived P/MAMPs (microbe‐associated molecular pattern molecules), endogenous DAMPs (damage‐associated molecular pattern Molecules), PAMPs (pathogen‐associated molecular pattern molecules) and excessive metabolites via PRRs (pattern recognition receptor), leading to the increased release of pro‐inflammatory cytokines and chemokines. In liver sinusoidal endothelial cells, stressor‐induced upregulation of expression of adhesion molecules plus chemokines, stimulate recruitment of neutrophils and monocytes to the liver. Activated neutrophils initiate liver damage mainly by releasing enzymes and ROS (reactive oxygen species). Activated dendritic cells also present antigens to T cells with initiation of adaptive responses. Kupffer cells and hepatocytes regulate release and endocytosis of APPs (acute‐phase protein), thus extending their innate immune function to extrahepatic organs. Kupffer cells, mast cells and hepatocytes increase expression of other factors MMPs (matrix metalloprotease), Ang II (angiotensin II), TGF (transforming growth factor) and HGF (hepatic growth factor) to stimulate HSC (hepatic stellate cell) activation and liver fibrosis. Innate immune signals also mediate metabolic changes (e.g. lipogenesis and insulin resistance) and cell apoptosis, pyroptosis or necrosis in hepatocytes. KCs and LESCs express high levels of SR (scavenger receptor), which clears circulating molecules and organisms. SR plays a key role in the innate immune response. Innate‐like lymphocytes, including NKs (natural killer cell), ILCs (innate lymphoid cell), iNKTs (invariant natural killer T cell) and MAITs (mucosal‐associated invariant T cell), also generate multiple cytokines and influence their local microenvironment of the liver. ILC are fundamental cell that transit from an immune‐tolerant state (a condition in which they produce interleukin (IL‐10), transforming growth factor (TGF‐β), etc) to an immune‐active phenotype (producing IL‐1s, TNF‐α, etc). ILC form the first line of defence against invading organisms and environmental challenges through pattern recognition receptor (PRR) ligation and activation of complement receptors (CRs) or scavenger receptors (SRs). Together, these events result in liver steatosis, inflammation and fibrosis and lead to NASH and advanced complications. Abbreviations: CCL2, C‐C motif chemokine 2; TF, transcriptional factor; Th1, T helper 1 (Adapted from Cai et al 68 , 116 and Jenne & Kubes 117 )
Although there is no direct evidence that, in the acute phase of the disease, a major liver damage occurs more frequently in COVID‐19‐infected patients with pre‐existing NAFLD, the common pathogenic mechanisms involved in COVID‐19 and NAFLD could generate, in COVID‐19‐infected patients, an increased risk of NAFLD progression to steatohepatitis in the long term. 77 Thus, in these patients, a close follow‐up aimed at explore the long‐term outcomes of liver injury is needed.
3. NAFLD, VIRUS AND METABOLIC ALTERATIONS
Studies from China confirm that most vulnerable subjects to COVID‐19 infection suffer from pre‐existing illness that includes hypertension, cardiovascular disease, diabetes, chronic lung disease (e.g. asthma, chronic obstructive pulmonary disease, and emphysema), cancer and chronic inflammation. 9 , 34 , 78 , 79
Several of such conditions, alone or in combination, predispose or are associated with metabolic changes of the liver, namely NAFLD. Although there is a hope for more specific therapies in COVID‐19 infection, including vaccines, 80 a rational approach against future outbreaks must include preventive measures such as lifestyle changes to decrease the burden of chronic metabolic disorders, adiposity and associated pro‐inflammatory status while preserving an healthy immune response. 81 , 82
This conclusion is supported by emerging relationships between COVID‐19 outcomes and frequent metabolic abnormalities which coexist with NAFLD.
Diabetes mellitus has been described as an additional risk to the progression of COVID‐19, 34 , 47 probably also due to the presence of an 'overfat' condition (see below), low‐grade chronic inflammation, insulin resistance, obesity 38 , 83 , 84 , 85 and a dysregulation of ACE2. 61 Of note, the ACE2 is also expressed in the endocrine pancreas. Thereby, COVID‐19 might facilitate a status of insulin resistance and impaired insulin secretion. 86
Independently from diabetes, the presence of an 'overfat' condition (i.e., excess body fat that impairs health 87 ) has developed as a pandemic worldwide and can occur in obesity, overweight and even normal weight subjects with excess fat involving the liver as well in terms of steatosis (Figure 1). Several abnormalities can cluster together with overfat, that is overweight, obesity, chronic 'metabolic' inflammation and insulin resistance, eventually configuring the metabolic syndrome (MetS). 9 , 88 , 89
Excess body fat can impair immunity, as confirmed by the higher incidence of both autoimmune and immune diseases. 90 A defective immune response (mainly of T lymphocytes and macrophages) with underlying adiposity will compromise the immune system to increase the risk of infections, and chronic respiratory diseases. 91 , 92 Notably, the overfat condition appears to be a risk factor in infectious viral diseases. 93 , 94 In particular, overfat might negatively affect immune function and host defence mechanisms, 95 while the response to viral and bacterial hits becomes defective in overfat hosts. 93 , 95 , 96 , 97
Lastly, illness, such as asthma, chronic obstructive pulmonary disease, emphysema and cancer, can be further associated with the overfat condition 9 , 78 , 79 ). Thus, whether the overfat condition represents an additional negative factor during COVID‐19 infection requires attention.
MetS, on the other hand, is a frequent and important underlying condition in patients developing infections, and MetS components (combined with liver steatosis) might further deteriorate with infections. 9 , 34 , 78 , 79 Major contributing risk factors for MetS include overweight and obesity. 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 In this case, individuals have increased morbidity in response to COVID‐19 infection. 110 , 111
4. CONCLUSIONS AND FUTURE PERSPECTIVES
The pandemic characteristics and high‐lethality rate of SARS‐CoV‐2 infection have raised concerns about mechanisms of injury in patients at risk. Initial evidence from China indicated that the subjects most vulnerable to COVID‐19 suffer from pre‐existing illness. COVID‐19 acute pandemic often develops in patients with major metabolic abnormalities, including fatty liver disease, which is part of a chronic pandemic together with body fat accumulation. During metabolic abnormalities, the expansion of metabolically active fat ('overfat condition') parallels chronic inflammatory changes, 9 , 34 , 78 , 79 the development of insulin resistance and, in the liver, the accumulation of fat and, possibly, an underlying fibrosis. In this context, the deleterious interplay of the complex inflammatory pathways chronically present in NAFLD can be acutely boosted in the setting of COVID‐19, magnifying liver injury and deteriorating outcome in metabolically compromised populations. Thus, NAFLD should be considered as prognostic indicator during COVID‐19 and, on the other hand, close long‐term monitoring of patients with NAFLD who experienced COVID‐19 might be needed.
Finally, a further challenge in the diagnosis and treatment of patients with NAFLD is to reduce the vulnerability from non‐communicable diseases, increasing the individual resilience to future outbreaks.
CONFLICT OF INTEREST
None declared.
Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID‐19 and non‐alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. 10.1111/eci.13338
Funding information
The present paper originates in the context of the projects FOIE GRAS (#722619) and mtFOIE GRAS (#734719), which have received funding from the European Union's Horizon 2020 Research and Innovation framework, under the Marie Skłodowska‐Curie Grant Agreement.
REFERENCES
- 1. World Health Organization . Coronavirus disease 2019 (COVID 19). Situation Report ‐ 72. Geneva: World Health Organization; 2020. [Google Scholar]
- 2. European Centre for Disease Prevention and Control . COVID‐19 pandemic, 2020. https://www.ecdc.europa.eu/en/covid‐19‐pandemic. Accessed July 2, 2020.
- 3. Verelst F, Kuylen E, Beutels P. Indications for healthcare surge capacity in European countries facing an exponential increase in coronavirus disease (COVID‐19) cases, March 2020. Euro Surveill. 2020;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albano D, Bertagna F, Bertoli M, et al. Incidental findings suggestive of Covid‐19 in asymptomatic patients undergoing nuclear medicine procedures in a high prevalence region. J Nucl Med. 2020;61(5):632‐636. [DOI] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus‐Infected Pneumonia in Wuhan, China. JAMA, 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020. [DOI] [PubMed] [Google Scholar]
- 8. Chen T, Wu DI, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single‐cell RNA‐seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019‐nCoV infection. Front Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffmann M, Kleine‐Weber H, Krüger N, Mueller MA, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019‐nCoV) uses the SARS‐coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. BioRxiv. 2020. [Google Scholar]
- 14. Jia HP, Look DC, Shi L, et al. ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614‐14621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single‐cell RNA expression profiling of ACE2, the receptor of SARS‐CoV‐2. bioRxiv. 2020;2020.01.26.919985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The human protein atlas . ACE2 protein expression summary. https://www.proteinatlas.org/ENSG00000130234‐ACE2. 2020. Accessed July 2, 2020.
- 17. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gu J, Han B, Wang J. COVID‐19: Gastrointestinal manifestations and potential fecal‐oral transmission. Gastroenterology. 2020;158(6):1518‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao QY, Chen YX, Fang JY. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cholankeril G, Podboy A, Aivaliotis VI, et al. High prevalence of concurrent gastrointestinal manifestations in patients with SARS‐CoV‐2: early experience from California. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smyk W, Janik MK, Portincasa P, et al. focus on the lungs but do not forget the gastrointestinal tract. Eur J Clin Invest. 2020;19:e13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nobel YR, Phipps M, Zucker J, et al. Gastrointestinal symptoms and COVID‐19: case‐control study from the United States. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang H, Kang Z, Gong H, et al. The digestive system is a potential route of 2019‐nCov infection: a bioinformatics analysis based on single‐cell transcriptomes. BioRxiv. 2020. [Google Scholar]
- 24. Liang W, Feng Z, Rao S, et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020. [DOI] [PubMed] [Google Scholar]
- 25. Bangash MN, Patel J, Parekh D. COVID‐19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li J, Fan JG. Characteristics and mechanism of liver injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rismanbaf A, Zarei S. Liver and Kidney Injuries in COVID‐19 and their effects on drug therapy; a letter to editor. Arch Acad Emerg Med. 2020;8:e17. [PMC free article] [PubMed] [Google Scholar]
- 28. Méndez‐Sánchez N, Valencia‐Rodríguez A, Qi X, et al. What has the COVID‐19 pandemic taught us so far? Addressing the problem from a hepatologist's perspective. J Clin Transl Hepatol. 2020;8:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non‐alcoholic fatty liver disease. Dig Dis. 2010;28:155‐161. [DOI] [PubMed] [Google Scholar]
- 30. European Association for the Study of the L, European Association for the Study of D and European Association for the Study of O . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol. 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 31. Alberti K, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640‐1645. [DOI] [PubMed] [Google Scholar]
- 32. Eslam M, Sanyal AJ, George J, et al. International consensus panel MAFLD: a consensus‐driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999‐2014.e1. [DOI] [PubMed] [Google Scholar]
- 33. Maddaloni E, Buzzetti R. Covid‐19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020;e33213321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020;e3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Novel Coronavirus Pneumonia Emergency Response Epidemiology T . [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145‐151.32064853 [Google Scholar]
- 36. Kalligeros M, Shehadeh F, Mylona EK, et al. Association of obesity with disease severity among patients with COVID‐19. Obesity (Silver Spring). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Molina‐Molina E, Krawczyk M, Stachowska E, Lammert F, Portincasa P. Non‐alcoholic fatty liver disease in non‐obese individuals: prevalence, pathogenesis and treatment. Clin Res Hepatol Gastroenterol. 2019;43:638‐645. [DOI] [PubMed] [Google Scholar]
- 39. Molina‐Molina E, Lunardi Baccetto R, Wang DQ, de Bari O, Krawczyk M, Portincasa P. Exercising the hepatobiliary‐gut axis. The impact of physical activity performance. Eur J Clin Invest. 2018;48:e12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fricker ZP, Pedley A, Massaro JM, et al. Liver fat is associated with markers of inflammation and oxidative stress in analysis of data from the Framingham heart study. Clin Gastroenterol Hepatol. 2019;17(6):1157‐1164.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Feldman A, Eder SK, Felder TK, et al. Clinical and metabolic characterization of obese subjects without non‐alcoholic fatty liver: a targeted metabolomics approach. Diabetes Metab. 2019;45:132‐139. [DOI] [PubMed] [Google Scholar]
- 42. Jarrar MH, Baranova A, Collantes R, et al. Adipokines and cytokines in non‐alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412‐421. [DOI] [PubMed] [Google Scholar]
- 43. Pedersen SF, Ho YC. SARS‐CoV‐2: a storm is raging. J Clin Invest. 2020;130:2202‐2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58(7):1021‐1028. [DOI] [PubMed] [Google Scholar]
- 47. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID‐ 19: evidence from meta‐analysis. Aging (Albany NY). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID‐19 disease in New York City. medRxiv. 2020. [Google Scholar]
- 49. Ji D, Qin E, Xu J, et al. Non‐alcoholic fatty liver diseases in patients with COVID‐19: A retrospective study. J Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics. Tissue‐based map of the human proteome. Science. 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 51. Paizis G, Tikellis C, Cooper ME, et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Feng G, Zheng KI, Yan Q‐Q, et al. COVID‐19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8:18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zheng KI, Gao F, Wang XB, et al. Obesity as a risk factor for greater severity of COVID‐19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nseir W, Taha H, Khateeb J, Grosovski M, Assy N. Fatty liver is associated with recurrent bacterial infections independent of metabolic syndrome. Dig Dis Sci. 2011;56:3328‐3334. [DOI] [PubMed] [Google Scholar]
- 57. Nseir WB, Mograbi JM, Amara AE, Abu Elheja OH, Mahamid MN. Non‐alcoholic fatty liver disease and 30‐day all‐cause mortality in adult patients with community‐acquired pneumonia. QJM. 2019;112:95‐99. [DOI] [PubMed] [Google Scholar]
- 58. Targher G, Mantovani A, Byrne CD, et al. Risk of severe illness from COVID‐19 in patients with metabolic dysfunction‐associated fatty liver disease and increased fibrosis scores. Gut. 2020. [DOI] [PubMed] [Google Scholar]
- 59. Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. bioRxiv. 2020. [Google Scholar]
- 60. Biquard L, Valla D, Rautou PE. No evidence for an increased liver uptake of SARS‐CoV‐2 in metabolic associated fatty liver disease. J Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ji D, Xu J, Qin E, et al. Reply to: 'No evidence for an increased liver uptake of SARS‐CoV‐2 in metabolic associated fatty liver disease'. J Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gonzalez FJ, Xie C, Jiang C. The role of hypoxia‐inducible factors in metabolic diseases. Nat Rev Endocrinol. 2018;15:21‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen J, Chen J, Fu H, et al. Hypoxia exacerbates nonalcoholic fatty liver disease via the HIF‐2alpha/PPARalpha pathway. Am J Physiol Endocrinol Metab. 2019;317:E710‐E722. [DOI] [PubMed] [Google Scholar]
- 64. Dar WA, Sullivan E, Bynon JS, Eltzschig H, Ju C. Ischaemia reperfusion injury in liver transplantation: cellular and molecular mechanisms. Liver Int. 2019;39:788‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hernández A, Geng Y, Sepúlveda R, et al. Chemical hypoxia induces pro‐inflammatory signals in fat‐laden hepatocytes and contributes to cellular crosstalk with Kupffer cells through extracellular vesicles. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165753. [DOI] [PubMed] [Google Scholar]
- 66. Chen J, Chen J, Huang J, et al. HIF‐2alpha upregulation mediated by hypoxia promotes NAFLD‐HCC progression by activating lipid synthesis via the PI3K‐AKT‐mTOR pathway. Aging (Albany NY). 2019;11:10839‐10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cai J, Zhang XJ, Li H. The role of innate immune cells in nonalcoholic steatohepatitis. Hepatology. 2019;70:1026‐1037. [DOI] [PubMed] [Google Scholar]
- 69. Lin LU, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS‐CoV‐2 infection. Gut. 2020;69(6):997‐1001. [DOI] [PubMed] [Google Scholar]
- 70. Ji D, Xu J, Qin E, et al. Reply to: ‘No evidence for an increased liver uptake of SARS‐CoV‐2 in metabolic associated fatty liver disease’. J Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jing L, Sumeng L, Jia L. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. medRxiv, 2020;33:26.5‐54.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Simon TG, Trejo MEP, McClelland R, et al. Circulating Interleukin‐6 is a biomarker for coronary atherosclerosis in nonalcoholic fatty liver disease: results from the multi‐ethnic study of atherosclerosis. Int J Cardiol. 2018;259:198‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gao B, Tsukamoto H. Inflammation in alcoholic and nonalcoholic fatty liver disease: friend or foe? Gastroenterology. 2016;150:1704‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Boeckmans J, Rodrigues RM, Demuyser T, Pierard D, Vanhaecke T, Rogiers V. COVID‐19 and drug‐induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94(4):1367‐1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Centers for Disease Control and Prevention . People who are at higher risk for severe illness. 2020.
- 76. Bai L, Li H. Innate immune regulatory networks in hepatic lipid metabolism. J Mol Med (Berl). 2019;97:593‐604. [DOI] [PubMed] [Google Scholar]
- 77. Prins GH, Olinga P. Potential implications of COVID‐19 in non‐alcoholic fatty liver disease. Liver Int. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363‐374. [DOI] [PubMed] [Google Scholar]
- 82. Jones DS. History in a crisis ‐ lessons for Covid‐19. N Engl J Med. 2020;382:1681‐1683. [DOI] [PubMed] [Google Scholar]
- 83. Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792‐798. [DOI] [PubMed] [Google Scholar]
- 84. Ma J, Hwang S‐J, Pedley A, et al. Bi‐directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66:390‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263‐2273. [DOI] [PubMed] [Google Scholar]
- 86. Bindom SM, Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol. 2009;302:193‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ruderman NB, Schneider SH, Berchtold P. The, "metabolically‐obese," normal‐weight individual. Am J Clin Nutr. 1981;34:1617‐1621. [DOI] [PubMed] [Google Scholar]
- 88. Maffetone PB, Rivera‐Dominguez I, Laursen PB. Overfat and underfat: new terms and definitions long overdue. Front Public Health. 2016;4:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Maffetone PB, Rivera‐Dominguez I, Laursen PB. Overfat adults and children in developed countries: the public health importance of identifying excess body fat. Front Public Health. 2017;5:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Maffetone PB, Laursen PB. The prevalence of overfat adults and children in the US. Front Public Health. 2017;5:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rodriguez A, Socias L, Guerrero JE, et al. [Pandemic influenza A in the ICU: experience in Spain and Latin America. GETGAG/SEMICYUC/(Spanish Working Group on Severe Pandemic Influenza A/SEMICYUC)]. Med Intensiva. 2010;34:87‐94. [DOI] [PubMed] [Google Scholar]
- 92. Poulain M, Doucet M, Major GC, et al. The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. CMAJ. 2006;174:1293‐1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Van Kerkhove MD, Vandemaele KAH, Shinde V, et al. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 2011;8:e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Moser J‐A, Galindo‐Fraga A, Ortiz‐Hernández AA, et al. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respir Viruses. 2019;13:3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Frasca D, McElhaney J. Influence of obesity on pneumococcus infection risk in the elderly. Front Endocrinol (Lausanne). 2019;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community‐acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082‐3088. [DOI] [PubMed] [Google Scholar]
- 97. Paulsen J, Askim A, Mohus RM, et al. Associations of obesity and lifestyle with the risk and mortality of bloodstream infection in a general population: a 15‐year follow‐up of 64 027 individuals in the HUNT Study. Int J Epidemiol. 2017;46:1573‐1581. [DOI] [PubMed] [Google Scholar]
- 98. D'Adamo E, Marcovecchio ML, Giannini C, et al. The possible role of liver steatosis in defining metabolic syndrome in prepubertal children. Metabolism. 2010;59:671‐676. [DOI] [PubMed] [Google Scholar]
- 99. Grundy SM. Cholesterol gallstones: a fellow traveler with metabolic syndrome? Am J Clin Nutr. 2004;80:1‐2. [DOI] [PubMed] [Google Scholar]
- 100. Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881‐887. [DOI] [PubMed] [Google Scholar]
- 101. Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta‐analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403‐414. [DOI] [PubMed] [Google Scholar]
- 102. Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629‐636. [DOI] [PubMed] [Google Scholar]
- 103. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;56:1113‐1132. [DOI] [PubMed] [Google Scholar]
- 104. Grundy SM. Overnutrition, ectopic lipid and the metabolic syndrome. J Investig Med. 2016;64:1082‐1086. [DOI] [PubMed] [Google Scholar]
- 105. Esser N, Legrand‐Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141‐150. [DOI] [PubMed] [Google Scholar]
- 106. Esser N, Paquot N, Scheen AJ. Anti‐inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs. 2015;24:283‐307. [DOI] [PubMed] [Google Scholar]
- 107. Stenholm S, Koster A, Alley DE, et al. Adipocytokines and the metabolic syndrome among older persons with and without obesity: the InCHIANTI study. Clin Endocrinol (Oxf). 2010;73:55‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kim LJ, Nalls MA, Eiriksdottir G, et al. Associations of visceral and liver fat with the metabolic syndrome across the spectrum of obesity: the AGES‐Reykjavik study. Obesity (Silver Spring). 2011;19:1265‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Koster A, Stenholm S, Alley DE, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring). 2010;18:2354‐2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28(7):1195‐1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kass DA, Duggal P, Cingolani O. Obesity could shift severe COVID‐19 disease to younger ages. The Lancet. 2020;395(10236):1544‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Di Palo DM, Garruti G, Di Ciaula A, et al. Increased colonic permeability and lifestyles as contributing factors to obesity and liver steatosis. Nutrients. 2020;12:E564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ruderman N, Chisholm D, Pi‐Sunyer X, Schneider S. The metabolically obese, normal‐weight individual revisited. Diabetes. 1998;47:699‐713. [DOI] [PubMed] [Google Scholar]
- 114. Jager J, Aparicio‐Vergara M, Aouadi M. Liver innate immune cells and insulin resistance: the multiple facets of Kupffer cells. J Intern Med. 2016;280:209‐220. [DOI] [PubMed] [Google Scholar]
- 115. Vecchié A, Dallegri F, Carbone F, et al. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur J Intern Med. 2018;48:6‐17. [DOI] [PubMed] [Google Scholar]
- 116. Cai J, Xu M, Zhang X, Li H. Innate immune signaling in nonalcoholic fatty liver disease and cardiovascular diseases. Annu Rev Pathol. 2019;14:153‐184. [DOI] [PubMed] [Google Scholar]
- 117. Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996‐1006. [DOI] [PubMed] [Google Scholar]


