Abstract
We report a case of COVID‐19 in kidney transplant patient in Thailand. A 58‐year‐old 2 years post–kidney transplant recipient, with maintenance immunosuppression of tacrolimus, mycophenolate mofetil (MMF), and prednisolone, presented with acute diarrhea which followed by fever on day 12. Symptoms of pneumonia together with lymphopenia from complete blood count were developed on day 7 after onset of fever with the x‐ray finding of bilateral multifocal patchy infiltration. COVID‐19 infection has been confirmed by reverse real‐time polymerase chain reaction (PCR) in nasal swab as well as found in stool. Darunavir together with ritonavir, hydroxychloroquine, azithromycin, and favipiravir was initiated on the first day of admission at primary hospital. Patient has been transferred to our hospital on day 2 of admission in which tacrolimus together with MMF was discontinued. High‐flow nasal cannula oxygen therapy was required on days 4‐5 of hospitalization. Tocilizumab was administered after rising of serum IL‐6 level. Symptoms of pneumonia were improved in which no oxygen treatment required from day 10 of hospitalization. Drug interaction between tacrolimus and anti‐viral treatment leads to severely high level of tacrolimus which caused reversible acute kidney injury (AKI) after supportive treatment.
Keywords: COVID‐19, favipiravir, kidney transplant recipient, tocilizumab
1. BACKGROUND
Among pandemic of novel coronavirus disease 2019 (COVID‐19), nowadays, global number of patients of more than 3.6 million confirmed cases had raised mortality of 6.1%. 1 The symptom severity was varied from mild to severe diseases; some of them progressed to acute respiratory distress syndrome. Characteristics of patient with severe disease were lymphocytopenia, older age, and current smoking. 2 In addition, meta‐analysis of fifteen studies was shown the most severe disease likely to have underlying diseases with hypertension, diabetes, respiratory disease, and cardiovascular disease. 3 There are conflicting evidences concerning the severity of COVID‐19 in kidney transplant recipient. 4 , 5 , 6 Immunosuppressive drug may alter clinical presentation and severity of COVID‐19. 7 Herein, we reported favorable outcome of severe COVID‐19 pneumonia in kidney transplant recipient.
2. CASE REPORT
A 58‐year‐old man, taxi driver, who underwent first kidney transplantation from his wife 2 years ago with stable serum creatinine around 1.4 mg/dL, was referred from primary hospital with symptom of acute fever, nausea, and watery diarrhea followed by progressive dyspnea within 2 days. He also has underlying of hypertension, dyslipidemia, and post‐transplant diabetes mellitus (PTDM). The diagnosis of COVID‐19 was confirmed by reverse real‐time polymerase chain reaction (PCR) from nasal swab.
This patient received his first kidney transplantation form his wife 2 years ago at King Chulalongkorn Memorial Hospital (KCMH) with 6 HLA mismatches and no anti‐HLA detected. The induction therapy consisted of anti‐IL‐2 receptor antibody (basiliximab) and methylprednisolone followed by maintenance therapy of tacrolimus, mycophenolate mofetil, and prednisolone. He experienced CMV viremia with complete course of ganciclovir subsequence with valganciclovir treatment with result of viral suppression within first 3 months after kidney transplantation. The coadministration medications with immunosuppressive drugs were metoprolol, manidipine, losartan, simvastatin, glipizide, co‐trimoxazole, and acyclovir.
On March 13, 2020, he developed his first clinical presentation that was episodic watery diarrhea for 12 days and then followed by fever, myalgia, and dry cough. On day 6 of fever, he had shortness of breath which leads him to primary hospital the next day. Physical examination revealed body temperature of 39.2 degrees Celsius, blood pressure 118/65 mm Hg, pulse rate 92 beats per minute, respiratory rate 24 times per minutes, and oxygen saturation at room air of 94%. Respiratory examination revealed fine crepitation in both lung fields. Since taxi driver has been considered as high‐risk occupation, he underwent nasal swab for SARS‐CoV‐2 by real‐time reverse real‐time PCR which revealed positive for COVID‐19. Stool testing for SARS‐CoV‐2 by real‐time reverse real‐time PCR also revealed positive. Chest radiography was reported bilateral multifocal patchy infiltration (Figure 1). He has been diagnosed as having COVID‐19 pneumonia. Azithromycin together with hydroxychloroquine, darunavir, ritonavir, and favipiravir has been initiated (Figure 2). Tacrolimus dosage was decreased for 50%, and MMF was discontinued. Prednisolone has been continued with dose of 2.5 mg/d and prompted increase if there was sign and symptom of adrenal insufficiency. Ceftriaxone has also been initiated to prophylaxis for concomitant bacterial infection.
FIGURE 1.
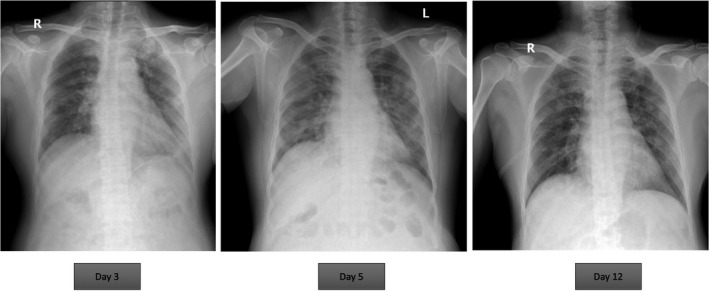
Chest radiography of the patient
FIGURE 2.
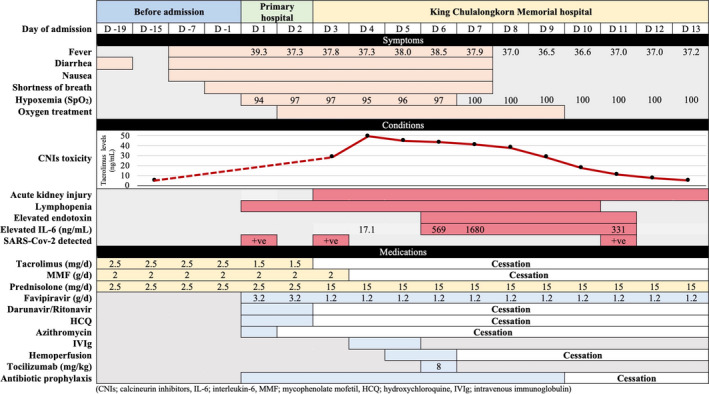
Clinical course, conditions, and treatment of the patient
On day 2 of admission, he required oxygen therapy to maintain adequate oxygenation. He has been transferred to our hospital which is an organ transplant center. The initial laboratory results showed lymphopenia of 452 cells/µL, rising of Cr from 1.4 at baseline to 2.2 mg/dL, serum Na of 128 mEq/L, and IL‐6 level of 17.1 pg/mL (reference level < 7 pg/mL). Tacrolimus trough level revealed 28.9 ng/mL which leads to discontinuation of tacrolimus, darunavir, ritonavir, and azithromycin. On days 4‐5 of admission (day 11‐12 of fever), lymphocyte count was decreased to 250 cells/µL, PaO2/FiO2 ratio was lowered to 226, and the chest radiography revealed increased bilateral infiltration which required high‐flow nasal cannula oxygen therapy. The intravenous immunoglobulin (IVIg) 2 g/kg/d was administered for 2 consecutive days. He also underwent polymyxin B hemoperfusion which was indicated by increased level of endotoxin tested by EEA™. On day 6, the IL‐6 level increased to 569 pg/mL, and single dose of 8 mg/kg of tocilizumab was administered.
The clinical of patient was improved which no longer oxygen therapy required on day 4 following initiation of tocilizumab. The infiltration was significantly decreased on chest radiography. However, despite early discontinuation of tacrolimus together with darunavir and ritonavir, the trough level of tacrolimus was peaked on day 4 of admission which was 49.4 ng/mL. Tacrolimus has been withdrawn for 10 days until the trough level was lowered to therapeutic level which was the same period as his serum Cr returned to baseline. After symptoms were improved, the MMF was reintroduced after lymphopenia was recovered and COVID‐19 was undetected.
3. DISCUSSION
The recent data hypothesized that COVID‐19 leads to activation immune system, including T cells, which causes severe lung tissue injury with evidence of CD4 T cells in post‐mortem biopsy of ARDS patient and subsequently shows lymphocytopenia in severe disease. 8 However, kidney transplant recipients who require maintenance immunosuppressive drugs to prevent allograft rejection may have atypical presentation of COVID‐19 such as gastrointestinal disturbance (diarrhea, nausea) before the onset of fever and upper respiratory tract symptoms. A study from Guillen et al was also reported COVID‐19 in kidney transplantation patient presented with first symptom of gastrointestinal viral disease 2 days before onset of fever. 7 There is evidence of SARS‐CoV‐2 in feces which indicated small bowel infection resulting in acute diarrhea and can be transmitted by fecal‐oral root. 9 However, many case reports have found that the presentation of COVID‐19 in kidney transplant recipients was similar to general population, and further exploration of characteristics of gastrointestinal disease might be required.
Diabetes type 2 and hypertension of COVID‐19 patient were the risk factors of severe disease according to current meta‐analysis in general population. 3 This case also had such risk factors. In addition, laboratory investigations showed lymphocytopenia and hyponatremia with improvement after treatment of COVID‐19 pneumonia which has been revealed in case reports. 7
Significant drug interaction between protease inhibitors (PIs) such as darunavir/ritonavir and tacrolimus must be concerned. Extremely prolonged elimination half‐life of tacrolimus with PIs in the present study was 10 days as reported in previous study of HIV‐infected kidney transplantation patient. 10 Acute kidney injury of this patient can be explained by significantly high level of tacrolimus. However, acute tubular necrosis from SARS‐CoV‐2 cannot be excluded. Dosage reduction or withdrawal of tacrolimus in the setting of COVID‐19 infection must be promptly initiated.
Favipiravir, an antiviral drug approved in Japan which inhibits RNA dependent RNA polymerase of viruses, has been chosen as the main antiviral agent in this patient. 11 Effective activity of favipiravir to COVID‐19 is limited in current reports including kidney transplant recipients. The recent open‐label control study from China reported favipiravir had shorter viral clearance time more than lopinavir/ritonavir with fewer side effects such as diarrhea and transaminitis in non‐transplant COVID‐19. 12 A randomized‐controlled study of favipiravir vs. arbidol revealed shorter latencies to relief fever and cough in favipiravir group. 13 However, the rate of oxygen therapy and non‐invasive mechanical ventilation was not different. Since favipiravir is mainly metabolized in liver by aldehyde oxidase, drug‐drug interaction with tacrolimus is less than protease inhibitors. The Ministry of Public Health of Thailand recommends favipiravir in patient with COVID‐19 in the following conditions: (a) mild symptoms with progressive infiltration from chest radiography, (b) mild symptoms with risk factors of such as age > 60 years, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), cardiovascular disease, cerebrovascular disease, uncontrolled diabetes mellitus, body mass index ≥ 35 kg/m2, cirrhosis, immunocompromise, and lymphopenia, and (c) symptoms of pneumonia or decreased SpO2. In this case, we introduced favipiravir and stopped another antiviral treatment according to severe drug‐drug interaction from PIs and tacrolimus. Adjunction with the other managements, the patient's clinical courses were improved without any side effect of favipiravir.
There is no specific treatment strategy for kidney transplant patients infected with COVID‐19. Treatment is similar to those patients without kidney transplants. However, lowering net state of immunosuppression should be introduced, particularly in early phase of infection in which virus is detectable. Anti‐proliferative medications, such as MMF, should be withdrawn, and calcineurin inhibitor, such as tacrolimus, should be decreased by 50%. Complete withdrawal of immunosuppressive drug has been reported, especially in severe disease. 6 Despite of mild disease, reduced dose of immunosuppressive strategies was appropriate. 4 Decision of antiviral therapy depends on the severity of COVID‐19. However, cytokine storm phase, represented by marked elevation of inflammatory marker such as hsCRP, ferritin, and especially elevation of IL‐6, was associated with severe disease of COVID‐19. 14 Anti‐IL6 receptor antibody should be introduced in patient with early symptom of pneumonia together with high level of IL‐6. 15 In this case, the improvement of symptoms and chest radiography has been found after the initiation of tocilizumab (Figure 2).
Severe COVID pneumonia reported by Zhou et al found that sepsis is one of the common complications and 50% of patients had gram‐negative septicemia with high mortality rate of 28%. 16 Our patient underwent polymyxin B hemoperfusion which indicated by increased level of endotoxin. And ceftriaxone also administered to prevent bacterial infection.
From our experience, management of COVID‐19 pneumonia in kidney transplant patients should be consisted of early detection, early effective antiviral treatment, decreased immunosuppressant including avoiding drug interaction, and anti‐IL‐6 receptor antibody when symptoms of pneumonia progress and increase level of IL‐6.
4. CONCLUSION
This is a case of COVID‐19 infection in kidney transplant patient in Thailand. This patient had presentation with gastrointestinal disturbance and followed by severe pneumonia. Antiviral therapy, particularly favipiravir together with decreased immunosuppression and anti‐IL‐6 receptor antibody, provides favorable outcomes. Decision on timing for anti‐IL6 receptor antibody initiation can be guided by IL‐6 level monitoring.
AUTHOR CONTRIBUTIONS
NT: Participated in research design, performance of the research, acquisition of data, writing, drafting, and revising the manuscript. TT: Participated in performance of the research, acquisition of data, writing, drafting, and revising of the manuscript. ST: Participated in performance of the research and drafting. KT: Participated in acquisition of data and drafting. PT: Participated in acquisition of data. WC: Participated in performance of the research. SU: Participated in performance of the research. YA: Participated in research design. TS: Participated in performance of the research. NS: Participated in performance of the research. KJ: Participated in performance of the research. LP: Participated in performance of the research. OP: Participated in performance of the research.
Thammathiwat T, Tungsanga S, Tiankanon K, et al. A case of successful treatment of severe COVID‐19 pneumonia with favipiravir and tocilizumab in post–kidney transplant recipient. Transpl Infect Dis. 2021;23:e13388. 10.1111/tid.13388
REFERENCES
- 1. Coronavirus disease (COVID‐2019) situation reports [Internet]. 2020. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports
- 2. Guan W‐J, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsushita K, Ding N, Kou M, et al. The relationship of COVID‐19 severity with cardiovascular disease and its traditional risk factors: a systematic review and meta‐analysis. medRxiv. 2020. 10.1101/2020.04.05.20054155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang H, Chen Y, Yuan Q, et al. Identification of kidney transplant recipients with coronavirus disease 2019. Eur Urol. 2020;77(6):742‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID‐19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang J, Lin H, Wu Y, et al. COVID‐19 in post‐transplantation patients‐ report of two cases. Am J Transplant. 2020:1–3. 10.1111/ajt.15896 [DOI] [Google Scholar]
- 7. Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID‐19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020:1‐4. 10.1111/ajt.15874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antonio R, Silvia M. Immunosuppression drug‐related and clinical manifestation of coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant. 2020. 10.1111/ajt.15905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li X‐Y, Dai W‐J, Wu S‐N, Yang X‐Z, Wang H‐G. The occurrence of diarrhea in COVID‐19 patients. Clin Res Hepatol Gastroenterol. 2020;44(3):284‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mertz D, Battegay M, Marzolini C, Mayr M. Drug‐drug interaction in a kidney transplant recipient receiving HIV salvage therapy and tacrolimus. Am J Kidney Dis. 2009;54(1):e1‐e4. [DOI] [PubMed] [Google Scholar]
- 11. Du YX, Chen XP. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019‐nCoV infection. Clin Pharmacol Ther. 2020. 10.1002/cpt.1844 [DOI] [PubMed] [Google Scholar]
- 12. Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID‐ 19: an open‐label control study. Engineering. 2020. 10.1016/j.eng.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen C, Zhang Y, Huang J, et al. Favipiravir versus Arbidol for COVID‐ 19: a randomized clinical trial. medRxiv. 2020;2020.03.17.20037432. [Google Scholar]
- 14. Ulhaq ZS, Soraya GV. Interleukin‐6 as a potential biomarker of COVID‐19 progression. Méd Mal Infect. 2020;50(4):382‐383. 10.1016/j.medmal.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020;92(7):814‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054. [DOI] [PMC free article] [PubMed] [Google Scholar]


