Abstract
Data describing the clinical progression of coronavirus disease 2019 (COVID‐19) in transplant recipients are limited. In New York City during the surge in COVID‐19 cases, a systematic approach to monitoring and triaging immunocompromised transplant patients was required in the context of strained healthcare resources, limited outpatient testing, and heightened hospital exposure risks. Public health guidance at the onset of the COVID‐19 outbreak recommended outpatient monitoring of mildly symptomatic patients without specific recommendations for special populations such as transplant recipients. We developed and implemented a systematic monitoring algorithm for kidney transplant recipients at our transplant center who reported mild symptoms suggestive of COVID‐19. We describe the outcomes of the first 44 patients monitored through this algorithm. A total of 44 kidney transplant recipients thought to be symptomatic for COVID‐19 disease were followed for a minimum of 14 days. The majority of mildly symptomatic patients (34/44) had clinical progression of disease and were referred to the emergency department where they all tested PCR positive and required hospitalization. More than half of these patients presented with hypoxia requiring supplemental oxygen, 39% were intubated within 48 hours, and 53% developed acute kidney injury but did not require dialysis. There were 6 deaths. During surge outbreaks, kidney transplant patients with even mild symptoms have a high likelihood of COVID‐19 disease and most will worsen requiring hospitalization for supportive measures. Earlier outpatient testing and hospitalization may improve COVID‐19 outcomes among transplant recipients.
Keywords: coronavirus, COVID‐19, kidney transplant, outbreak
Abbreviations
- CDC
Centers for Disease Control and Prevention
- COVID‐19
coronavirus disease 2019
- ED
emergency department
- EMR
electronic medical record
- NYS DOH
New York State Department of Health
1. INTRODUCTION
An outbreak of coronavirus disease 2019 (COVID‐19) caused by SARS‐CoV‐2 virus began in Wuhan Province, China, in late 2019 and subsequently evolved to a global pandemic. Clinical presentation of COVID‐19 can vary from mild respiratory symptoms to severe pneumonia with hypoxic respiratory failure. 1 , 2 Cities around the world including New York, identified as geographic “hot spots” during this pandemic, faced the need for an approach to the management and diagnosis of patients presenting with mild symptoms suggestive of COVID‐19 in the context of (a) limited availability of outpatient testing in the early weeks of the pandemic, (b) considerations of emergency department (ED) and hospital resource constraints during surge capacity, and (c) unclear efficacy of potential therapeutic agents some of which were only available to hospitalized patients. Little was known about the clinical presentation and progression of COVID‐19 in immunosuppressed patients at the start of New York City's outbreak. 3 Our transplant center developed an algorithm for the assessment and triage of outpatient transplant recipients reporting symptoms of possible COVID‐19. We report here our experience of outpatient management that was initially informed by guidance from the Centers for Disease Control and Prevention (CDC) and New York State Department of Health (NYS DOH) and subsequently modified by the outcomes in this report to inform best practices for high‐risk immunosuppressed transplant recipients suspected of having COVID‐19. 4 , 5
2. MATERIALS AND METHODS
Our multidisciplinary transplant team identified the need for a systematic approach to management of transplant recipients reporting symptoms consistent with possible COVID‐19. For our kidney transplant program, our multidisciplinary team included 2 transplant infectious disease physicians, 2 transplant nephrologists, an abdominal transplant nurse practitioner (NP), a transplant infectious disease NP, and our transplant clinic director to formulate and implement an outpatient management and triage algorithm. The algorithm was informed by publicly available guidelines from CDC and from the NYS DOH which did not include recommendations specifically for immunosuppressed populations.
A standardized intake assessment and documentation template were created and implemented for incoming calls from patients with symptoms or potential COVID‐19 exposures. Due to atypical presentations of other respiratory viral infections in immunosuppressed patients, our approach included a detailed assessment of patient‐reported symptoms followed by daily telephone monitoring for changes or progression of symptoms over 14 days. We utilized our existing 24‐hour on‐call center staffed by a registered nurse or nurse practitioner (RN/NP) for patient calls and a templated electronic medical record (EMR) note to ensure comprehensive and consistent capture of clinical data and follow‐up plans (Figure 1). Best practices for staff and patient communication were followed including use of language interpreter services when indicated.
FIGURE 1.

Templated assessment form for patient‐reported symptoms and daily telephone monitoring
Patients’ self‐reporting symptoms suspicious for COVID‐19 were assessed for the need for immediate triage to the ED by discussion of the intake symptoms and epidemiologic information with a transplant nephrologist. Symptoms were assessed to be mild if there was cough, fever, fatigue, myalgias, headache, sore throat or gastrointestinal symptoms in the absence of shortness of breath, chest pain, or home pulse oximeter reading <92 percent. Patients with mild symptoms deemed safe to be monitored as outpatients were called daily for 14 days to assess for progression of symptoms and to guide further triage. The decreased number of non‐urgent outpatient visits to our center allowed for the re‐deployment of providers to the COVID‐19 outpatient management center.
Patients with progressive symptoms were referred to the ED. Progressive symptoms were defined but not limited to fever for >2 days, shortness of breath, chest pain, decreased oral intake, and worsening diarrhea.
Four weeks after implementation of this outpatient strategy, we reviewed all patients to assess the number of cases requiring inpatient management and the number of cases confirmed with COVID‐19 by diagnostic testing.
Approval for this study was obtained from the NYU Grossman School of Medicine Institutional Review Board. The data reported here were extracted from retrospective review of patient electronic medical records.
3. RESULTS
3.1. Description of the patient cohort
From 3/15/2020 to 04/12/2020, a total of 44 kidney transplant recipients contacted the clinic reporting mild symptoms possibly related to COVID‐19 and entered our outpatient monitoring system (Figure 2). All 44 patients in this cohort have completed 14 days of monitoring or remain hospitalized. One of 44 patients (2.3%) tested positive for SARS‐CoV‐2 PCR at an outpatient community facility and never required hospitalization. Nine of 44 patients (20.4%) did not require hospitalization because symptoms resolved. Eight of these 9 patients were never tested for SARS‐CoV‐2 as access to outpatient testing was not available for clinically stable patients early in the outbreak. One of the 9 patients whose symptoms resolved was subsequently admitted for acute renal failure and during that admission was found negative for SARS‐CoV‐2 on PCR testing.
FIGURE 2.
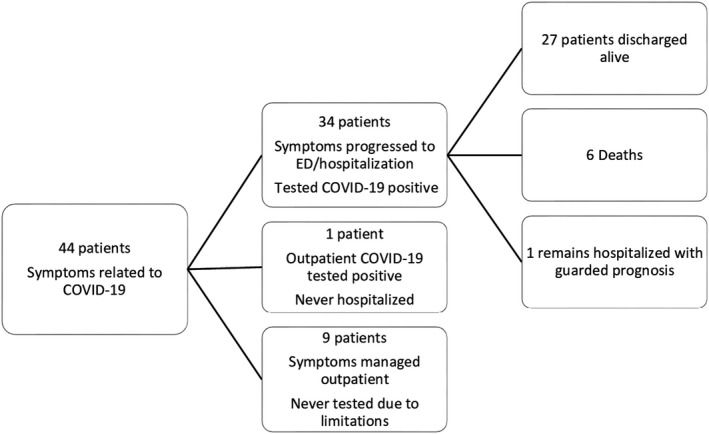
Patients managed through outpatient monitoring program
Thirty‐four of 44 (77.3%) patients had progression of symptoms requiring evaluation in the ED. All 34 patients tested positive for COVID‐19 by nasopharyngeal swab SARS‐CoV‐2 PCR on presentation. Nine of 34 patients were discharged from the ED/short stay unit and 7 of these 9 were readmitted to the hospital with progressive illness within a median of 5 days (IQR 4‐7.5) (Figure 3). Five of these 7 patients who returned to the hospital were severely hypoxic on readmission requiring supplemental high flow oxygen or intubation. Three of these 5 patients required intubation with 1 clinically recovered and 2 deceased.
FIGURE 3.
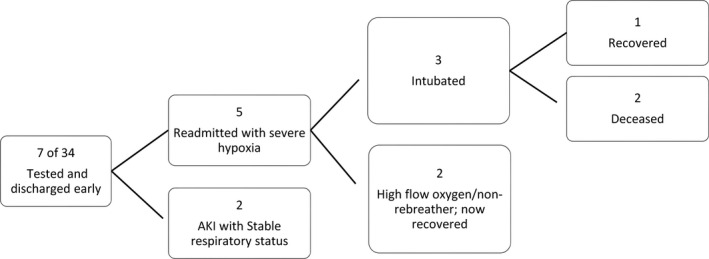
Readmissions for patients with COVID‐19 after short stay/ED visit
3.2. Demographics
Baseline patient characteristics of the 34 patients hospitalized with progressive COVID‐19 symptoms are shown in Table 1. Median age of this cohort was 59 (IQR 52.5‐63.8) years. The majority of patients (64.7%) were male, and the median BMI was 27.4. Time since organ transplantation ranged from 34 days to 11.6 years with 14 of 34 (41%) patients having been transplanted within the past 1 year.
Table 1.
Baseline characteristics of 34 kidney transplant recipients with progression of mild symptoms from COVID‐19 to hospitalization
| n (%) | |
|---|---|
| Age, years (median, IQR) | 59 (52.5‐63.8) |
| Male sex | 22 (64.7%) |
| Race | |
| African American | 15 (44.1%) |
| Hispanic | 8 (23.5%) |
| Asian | 2 (6.7%) |
| White | 7 (20.6%) |
| Other | 2 (6.7%) |
| Body mass index, kg/m2 (median, IQR) | 27.4 (24‐31.5) |
| Kidney transplant within the prior 12 mo | 14 (41%) |
| Time since transplant, days (median, IQR) | 444 (237 −651) |
| Transplant type | |
| Deceased donor organ | 27 (79.4%) |
| Living donor | 7 (20.6%) |
| Maintenance immunosuppression | |
| Mycophenolate mofetil | 33 (97.1%) |
| Tacrolimus | 29 (85.3%) |
| Cyclosporine | 1 (2.9%) |
| Belatacept | 6 (17.6%) |
| Everolimus | 1 (2.9%) |
| Prednisone (5 mg per day) | 34 (100%) |
| Duration of outpatient monitoring prior to hospitalization, days (median, IQR) | 5 (1‐7) |
| Duration from symptom onset to hospitalization, days (median, IQR) | 8 (4.5‐10) |
3.3. Clinical course of admitted patients
The majority (56%) of patients had cough or fever as an initial symptom of COVID‐19, while approximately 15% of patients reported diarrhea. Thirty‐two of the 34 (94.1%) patients were lymphopenic on presentation with a median absolute lymphocyte count of 400 cells/μL (IQR 300‐600). Almost all (32/34) had bilateral airspace opacities on admission chest radiograph. Median C‐reactive protein on presentation was 101.45 mg/L (range: 1.6‐389; reference normal range 0‐5 mg/L). Eighteen of the 34 (52.9%) hospitalized patients had hypoxia on presentation requiring supplemental oxygen and 38.9% required intubation within 48 hours of admission. In addition, 18 of 34 (52.9%) of patients developed acute kidney injury during their hospitalization but none required dialysis. Thirty‐three of 34 (97%) patients were treated with off‐label use of hydroxychloroquine for 5 days and 27 of 34 received azithromycin for 5 days with monitoring of QTc interval on EKG. Nine (26.5%) patients were either enrolled in a placebo‐controlled trial of a cytokine inhibiting agent (clazakizumab) or treated with the off‐label use of the anti‐IL‐6 receptor monoclonal antibody tocilizumab.
3.4. Outpatient immunosuppression management
The maintenance immunosuppression regimen for nearly all patients included an antimetabolite agent (97.1%), a calcineurin inhibitor (88.2%), and low‐dose prednisone (100%). Antimetabolite agents were discontinued in 26 of 33 (78.8%) patients, decreased by 50%‐75% in 6 of 33 (18.2%) patients and continued without change in 1 of 33 (3%) patients. The six patients on belatacept had their next dose held or delayed. Calcineurin inhibitors were continued in all patients.
3.5. Overall outcomes
For the 34 patients with progression from mild symptoms to hospitalization for COVID‐19, the median time to ED referral for further evaluation was 5 days (IQR 1‐7) after contacting our program, the median duration of symptoms prior to hospitalization was 8 days (QR 4.5‐10), and the median length of hospitalization was 9.5 days (IQR 5.25‐15.75). One patient remains hospitalized in guarded condition. To date, there have been 6 deaths among the 35 patients with confirmed COVID‐19 who required hospitalization due to progressive symptoms. Five of the 6 deceased patients were intubated, while one was treated with positive pressure ventilation because he declined intubation. Of the 5 patients who required intubation and subsequently died, 2 had tested positive in the ED with stable oxygenation 4 days prior to readmission. The median time from symptom onset to death was 23 days (range 21‐58) with median hospital days of 16 (IQR 14.3‐19.3). No patients died outside of the hospital.
Twenty‐six of the 34 hospitalized patients have returned home, and one patient was discharged to a rehabilitation unit. Patients successfully discharged had a median of 8 days of hospitalization (IQR 4.5‐13.5) with a median of 8 days of symptoms prior to hospitalization (IQR 5‐11). Twenty‐four discharged patients have reported complete resolution of their symptoms with median duration of symptoms 22.5 days (IQR 18.8‐30). Of the four patients who remain symptomatic, 3 are improving at home and 1 remains hospitalized. These four patients have had duration of symptoms for a median of 51 days (IQR 46‐52).
4. DISCUSSION
Transplant recipients are typically well connected to routine medical care and, due to intensive peri‐transplant patient education, generally understand they are at heightened risk for infection secondary to immunosuppression. Since January 2017, 576 patients have undergone kidney transplantation at our center and have access to our on‐call service. Our on‐call staffing provided a reasonable foundation for systematic outpatient monitoring of mildly symptomatic transplant recipients during the COVID‐19 outbreak as recommended by CDC general guidelines. Due to uncertainty about the clinical manifestation and outcomes of COVID‐19 disease in immunosuppressed patients, we chose a cautionary approach with a rigorous 14‐day telephone monitoring schedule for kidney transplant recipients who self‐reported symptoms suggestive of COVID‐19.
Importantly, despite a resource‐intensive outpatient monitoring strategy, we found that the vast majority of transplant recipients who self‐reported symptoms had clinical worsening while being monitored at home and progressed to hospitalization with confirmation of COVID‐19 diagnosis. Further, 34 of 35 (97.1%) patients diagnosed with COVID‐19 were hospitalized. This observation suggests greater disease burden compared to a report of early outcomes by Husain et al of kidney transplant outpatients in New York City where approximately half of 22 patients with confirmed COVID‐19 required hospitalization and the majority had symptom resolution as outpatients. 6 Further data from transplant centers reporting outcomes of outpatient management are needed to better understand the safety of outpatient monitoring, particularly where outpatient diagnostic testing is limited.
There are several risks to progression of COVID‐19 in outpatient transplant recipients and delayed access to potential directed therapies. First, more than half of patients in our cohort were hypoxic by the time of hospital presentation. The long‐term effects of more severe COVID‐19 pneumonia and associated lung injury are unknown but could be potentially mitigated by earlier diagnosis and inpatient management. Secondly, though few patients initially reported diarrhea, by day of admission 11 of 34 (32%) of patients had developed progressive diarrhea and inability to tolerate food/liquids. Progression of respiratory and gastrointestinal manifestations of COVID‐19 disease in the outpatient setting also poses greater exposure risk to household contacts. Earlier testing of symptomatic outpatients can guide contact tracing and provide additional benefits for infection control during an outbreak. Finally, more than half of the subsequently hospitalized kidney transplant recipients in our cohort developed acute kidney injury, which can impact long‐term allograft function. Earlier diagnosis of COVID‐19 followed by inpatient supportive care may reduce the incidence of acute kidney injury. The risk of allograft rejection due to reduction of immunosuppression in the setting of severe infection remains unclear at this early stage of the COVID‐19 pandemic. Simply reducing immunosuppression alone in the outpatient setting due to suspected infection without confirmation of COVID‐19 or initiation of COVID‐19 directed therapies may carry a higher risk of rejection and acute kidney injury.
Based on our experience to date, we strongly advocate that even mildly symptomatic transplant recipients should undergo rapid outpatient SARS‐CoV‐2 PCR testing with early hospital admission. Moreover, data have recently emerged describing the increased number of deaths in transplant recipients with COVID‐19 which is 10X that of the general population and 3X when compared to the general population age 70 years and older 7 , 8 suggesting that a one‐size‐fits‐all approach to outpatient COVID‐19 management is insufficient for transplant recipients. The decision to admit or discharge an immunocompromised patient with COVID‐19 from the ED should be individualized to the host, taking into account not only severity of symptoms but inflammatory markers such as C‐reactive protein and ferritin levels, barriers to close outpatient follow‐up and risk of clinical deterioration outside of the hospital. Patients discharged early in their disease course have a high probability of mortality as evidenced by disease progression in the 7 patients who required readmission. Early discharge in patients with ongoing symptoms must be done with great caution and we recommend it be done in consultation with the primary transplant team.
A strength of our report is a focus on outcomes of mildly symptomatic outpatients who were subsequently confirmed to have COVID‐19. A challenge during the New York City surge was the inability to test outpatients for other respiratory viruses which can cause similar symptoms. Our report also notably includes completion of a minimum of 14 days of monitoring for all patients described providing longer follow‐up than early reports. Limitations of our study include reporting bias as some patients with mild symptoms may not have contacted our call center, potentially overestimating the percentage of mildly symptomatic patients with progressive infection. However, the majority of our transplant recipients are well connected to care and all are aware of our 24‐hour call center. Additionally, all patients with active accounts received a MyChart COVID‐19 prevention message from our transplant center which included the importance of reporting symptoms suggestive of COVID‐19 and all patients transplanted within the past 5 years received phone calls with prevention education messages. We also believe that the heightened public awareness of this novel and potentially fatal coronavirus at the onset of the COVID‐19 outbreak in the United States would have contributed more likely to over‐reporting, rather than under‐reporting of symptoms by patients. To this point, 24 patients without symptoms called our transplant center during the study period reporting concerns of possible exposures to persons with confirmed or suspected COVID‐19 infection. Potential barriers to self‐reporting of symptoms by telephone include limited assistance from family members for making telephone calls due to self‐isolation and language barriers. As noted, eight patients with mild symptoms never underwent SARS‐CoV‐2 PCR testing and may or may not have had COVID‐19.
Our experience suggests that transplant recipients with even mild symptoms are likely to have a high risk of progressive COVID‐19 and its associated morbidity and mortality. Our approach to monitoring outpatients during the COVID‐19 pandemic surge in New York City benefited from a large integrated and multidisciplinary transplant provider team including transplant infectious diseases physicians. Yet, despite these resources, which may not be available at all transplant clinics, based on the progression of symptoms in our patient cohort we deem a symptom‐based outpatient monitoring approach to be inadequate for our high‐risk population. Our practice was modified to include SARS‐CoV‐2 PCR testing for symptomatic transplant recipients at our institution as a result of early findings from this algorithm. Prompt outpatient testing can allow earlier access to COVID‐19 directed therapies and supportive care which are likely to improve associated patient outcomes. Allocation of testing resources should prioritize higher risk populations such as immunocompromised transplant recipients.
AUTHORS CONTRIBUTION
All authors contributed to the conception and implementation of the outpatient management algorithm described in this work and participated in drafting, revising, and approving the final version of this manuscript.
ACKNOWLEDGEMENTS
We would like to acknowledge our kidney and liver transplant coordinators and nurse practitioners for their contributions to the implementation of our outpatient monitoring algorithm.
Mehta SA, Leonard J, Labella P, et al. Outpatient management of kidney transplant recipients with suspected COVID-19—Single-center experience during the New York City surge. Transpl Infect Dis. 2020;22:e13383. 10.1111/tid.13383
REFERENCES
- 1. Zhong Z, Zhang Q, Xia H, et al. Clinical characteristics and immunosuppressants management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020.1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID‐19) 2020. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐guidance‐management‐patients.html. Accessed April 10, 2020.
- 5. Update on COVID‐19 for New York State Healthcare Providers . https://coronavirus.health.ny.gov/system/files/documents/2020/03/covid19_hcp_webinar_31320.pdf. Accessed April 10, 2020.
- 6. Husain SA, Dube G, Morris H, et al. Early outcomes of outpatient management of kidney transplant recipients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15:892–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and Kidney Transplantation. N Engl J Med. 2020;382(25):2475‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


