A commentary on:
Guo et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020. e3319. 10.1002/dmrr.3319.
We are currently experiencing a catastrophic pandemic caused by Sars‐Coronavirus type 2 (Sars‐Cov‐2), which is undoubtedly more widespread and more lethal than other coronaviruses. Based on data from Wuhan in China, recently published in this journal, 1 we can already see that infected diabetics have a higher mortality, compared to non‐diabetic patients, which can be attributed to a greater susceptibility to infections due to chronic inflammation and immune dysfunction in these patients. 2 A scenario of hyperinflammation has been reported in critically ill patients with COVID‐19, caused by a cytokine storm, where IL‐6 is highly elevated, 3 which is and accompanied by lymphocytopenia, coagulopathy (characterized by increased D‐dimers) and hepatic overactivation (characterized by increased serum ferritin). 4
Hepatocytes respond to circulating cytokines (mainly IL‐6) by synthesizing and secreting specific proteins, described as acute‐phase proteins (APP). C‐reactive protein (CRP) is the prototype of these proteins and increases in inflammatory states, whether infectious or not. 5 However, this innate immune system protein is not very useful in differentiating bacterial from viral infections. Supporting a hyperinflammation scenario, mediated by IL‐6, a meta‐analysis highlighted a reduced lymphocyte/CRP ratio as a marker of severity in COVID19. 6 Ferritin is another APP, and is frequently used as clinical marker, particularly in very used and very appropriate for cases of viral infections, 7 including COVID‐19. In fact, Guo and co‐workers found elevated levels of serum ferritin in COVID‐17, but no difference was found between diabetic and non‐diabetic Sar‐Cov‐2 infected patients (P = .15, Table 2). However, when other comorbidities (such as hypertension and pulmonary disease) were excluded, both the CRP and ferritin markers were observed as significantly elevated (P < .01, Table 4) in the diabetic patients, compared to non‐diabetic ones.
Nevertheless, we want, herein, to call attention to another APP, serum amyloid A (SAA). SAA, like CRP, is increased in chronic inflammatory processes, such as diabetes and obesity. A meta‐analysis study indicated a strong correlation between elevated SAA and obesity, a major risk for diabetes mellitus type 2. 8 SAA is a pentraxin that activates the classic complement system via C1q and reinforces the production of the primary cytokines, IL‐β1 and TNF, contributing to the cytokine storm.
Interestingly, other important actions have been described for this protein; SAA is able to induce an atypical coagulation, which is dependent on fibrinogen, and mediate red blood cell (RBC) agglutination. 9 Moreover, when SAA is elevated it is found in apoB‐containing lipoproteins (LDL and HDL), potentially favouring vascular atherogenesis. 10 , 11
It may be hypothesized that an acute increase in SAA (compared to the already high levels found in diabetics) occurs in COVID‐19 (see Figure 1). This could contribute to the severity of the clinical condition by leading to coagulopathy (not always accompanied by a large increase in fibrinogen, another APP), a reduction in pulmonary and tissue gas exchange (due to RBC agglutination) and atherogenesis (accentuating cardiovascular dysfunction). Although SAA may be more useful than CRP as a marker of viral infections, 12 data on SAA have not been reported in COVID‐19. In a very recent paper, also from the Wuhan dataset, authors suggested SAA as a severity marker for COVID‐19. 13 However, there is no information available on comorbidities in these critically ill patients.
FIGURE 1.
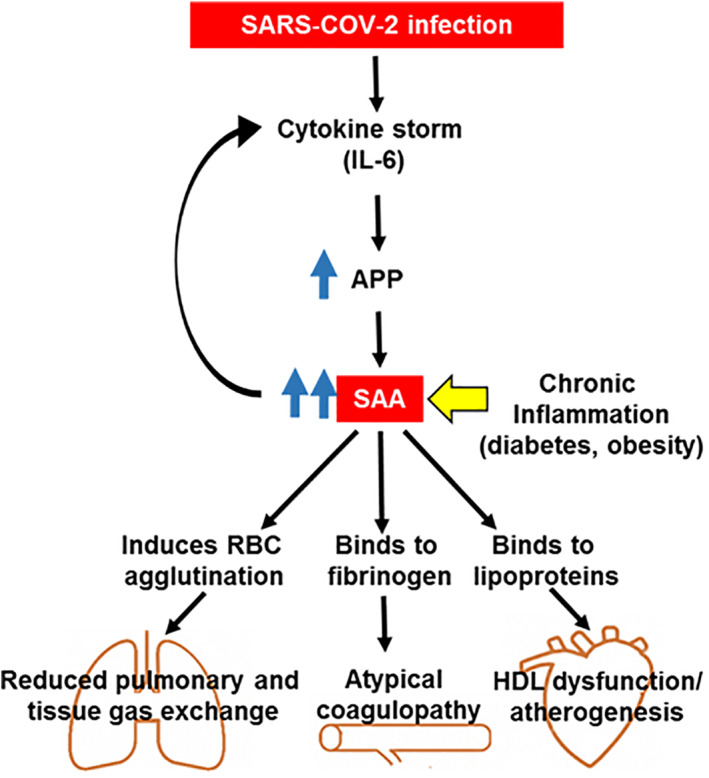
Serum amyloid A (SAA) may play a key role in the pathogenesis of COVID‐19 in diabetic patients. Interleukin‐6 (IL‐6) induces the synthesis and release of acute‐phase proteins (APP) in hepatocytes. Serum amyloid A (SAA), which is already elevated in chronic inflammatory conditions (eg, obesity and diabetes mellitus), is one of the APP generated. This APP reinforces primary cytokine production, contributing to a cytokine storm. SAA directly binds to fibrinogen leading to an atypical coagulopathy. Moreover, it binds to apoB‐containing lipoproteins, leading to HDL dysfunction, and induces red blood cell (RBC) agglutination. Taken together, these changes contribute to embolic and multiinfarct events in COVID‐19
Guo and colleagues' data on the severity of COVID‐19 in diabetic patients are very clear. 1 This commentary is to point out the need to investigate levels of SAA, particularly in obese and diabetic patients. This protein may play a key role in the pathogenesis of COVID‐19, in addition to having a potential prognostic role. It would be of importance to include SAA measurement in ongoing protocols and further reports.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge to public funding agencies in Brazil: National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES), Research Support Foundation of Rio Grande do Sul (FAPERGS) and National Institute of Science and Technology for Excitotoxicity and Neuroprotection (INCTEN).
REFERENCES
- 1. Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes/Metabolism Research and Reviews. 2020;e3319. 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hussain A, Bhowmik B. Do Vale Moreira NC. COVID‐19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore BJB, June CH. Cytokine release syndrome in severe COVID‐19. Science. 2020;368:473‐474. [DOI] [PubMed] [Google Scholar]
- 4. Henderson LA, Canna SW, Schulert GS, et al. On the alert for cytokine storm: immunopathology in COVID‐19. Arthritis Rheumatol. 2020;72:1059‐1063. 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandes BS, Steiner J, Molendijk ML, et al. C‐reactive protein concentrations across the mood spectrum in bipolar disorder: a systemic review and meta‐analysis. Lancet Psychiat. 2016;3:1147‐1156. [DOI] [PubMed] [Google Scholar]
- 6. Lagunas‐Rangel FA. Neutrophil‐to‐lymphocyte ratio and lymphocyte‐to‐C‐reactive protein ratio in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. J Med Virol. 2020. 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slaats J, Ten Oever J, van de Veerdonk FL, Netea MG. IL‐1β/IL‐6/CRP and IL‐18/ferritin: distinct inflammatory programs in infections. PLoS Pathog. 2016;12:e1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao Y, He X, Shi X, et al. Association between serum amyloid a and obesity: a meta‐analysis and systematic review. Inflamm Res. 2010;59:323‐334. [DOI] [PubMed] [Google Scholar]
- 9. Page MJ, Thomson GJA, Nunes JM, et al. Serum amyloid a binds to fibrin(ogen), promoting fibrin amyloid formation. Sci Rep. 2019;9:3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han CY, Tang C, Guevara ME, et al. Serum amyloid a impairs the antiinflammatory properties of HDL. J Clin Invest. 2016;126:266‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson PG, Thompson JC, Shridas P, et al. Serum amyloid a is an exchangeable Apolipoprotein. Arterioscler Thromb Vasc Biol. 2018;38:1890‐1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Y, Zhang J, Sheng H, Li H, Wang R. Acute phase reactant serum amyloid a in inflammation and other diseases. Adv Clin Chem. 2019;90:25‐80. [DOI] [PubMed] [Google Scholar]
- 13. Li H, Xiang X, Ren H, et al. Serum amyloid a is a biomarker of severe coronavirus disease and poor prognosis. J Infect. 2020;80:646‐655. 10.1016/j.jinf.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]


