To the Editor:
Pediatric cases represent 1‐5% of coronavirus disease 2019 (COVID‐19) cases worldwide. 1 This is linked to the fact that infected children present asymptomatic or pauci‐symptomatic forms and are therefore not tested, or children are less infected perhaps due to the lower expression level of angiotensin‐converting enzyme 2 (ACE2) in their nasal mucosa. 2
As of now, the global register of pediatric oncology patients of St Jude Children's Research Hospital and the International Society of Paediatric Oncology (SIOP) lists 167 cases of COVID‐19 in 24 countries. 3 So far no concerning reports have emerged from countries that have been facing the COVID‐19 epidemic such as China, Spain, Italy, and US. 4 , 5 , 6 , 7 Only France reported five patients with severe disease among 33 infected nationally. 8
In this report, we describe the clinical course of a pediatric patient infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) 3 months after hematopoietic stem cell transplant (HSCT).
The patient was a 17‐year‐old girl who underwent HSCT in Strasbourg University Hospitals, France on January 7, 2020 for acute myeloid leukemia subtype M5, according to the French‐British‐American classification, diagnosed on October 1, 2019. She was treated initially on the MyeChild protocol (high‐risk group) and had mild dilated cardiomyopathy with left ventricle ejection fraction around 50% treated by angiotensin‐converting‐enzyme (ACE) inhibitors following severe sepsis in the context of aplasia. She had HSCT after a consolidation course (first complete remission) with a related donor. The conditioning regimen was fludarabine/busulfan; cyclosporine was introduced for graft‐versus‐host disease (GVHD) prevention. One notable event during HSCT was cutaneous and digestive GVHD grade III treated by corticosteroids. Bone marrow aspirations (1, 2, and 3 months after HSCT) showed cytological remission and complete donor chimerism.
On March 24, 2020 (day 0), the patient was tested for SARS‐CoV‐2 due to multiple suspect cases in the family: one of her brother's classmates had tested positive, then the brother and parents showed fever, anosmia, and chest pain, and the patient's uncle developed a severe form of the disease. The patient presented only with rhinitis without fever or respiratory signs. Real‐time reverse transcriptase polymerase chain reaction (RT‐PCR) testing for SARS‐CoV‐2 was positive on nasopharyngeal swab specimens. No other viral reactivation or infection (cytomegalovirus, Epstein‐Barr virus, adenovirus) was detected.
At that time, the patient was still treated with prednisolone (0.4 mg/kg/day) for her digestive GVHD, cyclosporine (4 mg/kg/day), ACE inhibitors (0.12 mg/kg/day), and preventive antiinfectious treatment by sulfamethoxazole‐trimethoprime, posaconazole, phenoxymethylpenicillin, and valacyclovir. Complete blood count showed average hematological reconstitution with 3.5 × 109/L white blood cells including 2.2 × 109/L neutrophils, and preexisting lymphopenia (0.43 × 109/L), hemoglobin 96 g/L, and platelets 53 × 109/L (Table S1). The patient received intravenous immunoglobulin (Ig) (PRIVIGEN®) on March 10 and April 9 (day 16) for immune deficiency secondary to HSCT. Immunophenotyping (Table S1) showed that she was cytopenic in every lineage (B, T, and NK). Cytokines (interleukin [IL]‐6, IL‐8, IL‐10) were within normal range. Chest computed tomography (CT) on March 31 revealed scattered ground glass opacities in the right lower lobe compatible with COVID‐19, and 1 month later a fibrosis aspect (Figure 1) reported as a usual evolution of COVID‐19. 9
FIGURE 1.
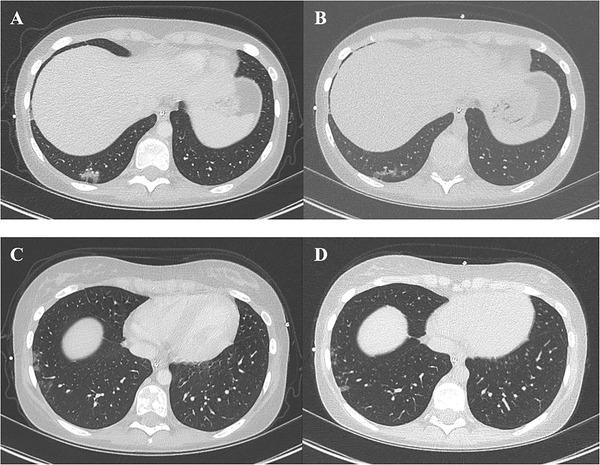
Chest computed tomography (CT) scan on day 7 shows scattered ground glass opacities in the right lower lobe close to the pleura (A and C), and the reevaluation of chest CT on day 36 shows a slight resorption of ground glass opacities (B and D)
The SARS‐CoV‐2 RT‐PCR (nasopharyngeal swab) was still positive 21 and 42 days after the initial positive test, while negative on day 56. Serology tests were performed on days 7, 14, and 56 after the first positive RT‐PCR, with two different techniques: immunochromatographic lateral flow assay (Biosynex COVID‐19 BSS®), and enzyme‐linked immunosorbent assay (ELISA) to detect IgA and IgG (Euroimmun IgA, IgG). She developed neither IgA, IgM nor IgG on day 7. IgA remained negative on days 14 and 56. IgM antibody was detected on day 14 only and remained weakly positive on day 56, and IgG antibody was positive on day 56 only.
Forty patients followed in our pediatric onco‐hematology department (Strasbourg University Hospital) have been tested by SARS‐CoV‐2 RT‐PCR since March 1, 2020, with six being positive. All of them were asymptomatic or presented with mild disease, and none of them needed treatment or hospitalization. Despite being at high risk for a severe form of COVID‐19 due to the postallograft immunosuppression, mild dilated cardiomyopathy, GVHD, corticosteroid therapy, and severe lymphopenia and despite the pulmonary images on chest CT, the patient we report only presented rhinitis.
There are now several studies describing the kinetics of anti‐SARS‐CoV‐2 IgM and IgG. Most report that IgM antibody is detectable 5‐14 days after first symptoms. 10 , 11 However, symptom severity may also affect the rate of seropositivity. A delayed or absent humoral response against SARS‐CoV‐2 has been reported in some patients 12 and may result in negative serology results. 13 Surprisingly, despite her immunosuppressed condition and her mild symptoms, she developed an immune response with IgM produced on day 14, which is consistent with the literature for immunocompetent patients. 14 IgG only appeared on day 56.
Furthermore, the virus has been reported to remain detectable for up to 3 weeks, 15 but our patient still had a weak positive RT‐PCR after 42 days.
Very little data are available on children with cancer, but it seems that in this fragile population COVID‐19 is largely pauci‐symptomatic. In any event, clinical management in this population is a challenge because oncologic treatment is essential and cannot be postponed.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supporting information
Table S1
REFERENCES
- 1. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin‐converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427‐2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St Jude Global. Global Registry of COVID‐19 in Pediatric Cancer. http://global.stjude.org/en-us/global-covid-19-observatory-and-resource-center-for-childhood-cancer/registry.html
- 4. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Rojas T, Pérez‐Martínez A, Cela E, et al. COVID‐19 infection in children and adolescents with cancer in Madrid. Pediatr Blood Cancer. 2020;67(7):e28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Balduzzi A, Brivio E, Rovelli A, et al. Lessons after the early management of the COVID‐19 outbreak in a pediatric transplant and hemato‐oncology center embedded within a COVID‐19 dedicated hospital in Lombardia, Italy. Estote parati. Bone Marrow Transplant. 2020:1‐6. 10.1038/s41409-020-0895-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boulad F, Kamboj M, Bouvier N, Mauguen A, Kung AL. COVID‐19 in children with cancer in New York city. JAMA Oncol. 2020. 10.1001/jamaoncol.2020.2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. André N, Rouger‐Gaudichon J, Brethon B, et al. COVID‐19 in pediatric oncology from French pediatric oncology and hematology centers: high risk of severe forms? Pediatr Blood Cancer. 2020;67(7):e28392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):P425‐P434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID‐19). Clin Infect Dis. 2020. 10.1093/cid/ciaa310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao J, Liao X, Wang H, et al. Early virus clearance and delayed antibody response in a case of COVID‐19 with a history of co‐infection with HIV‐1 and HCV. Clin Infect Dis. 2020. 10.1093/cid/ciaa408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yongchen Z, Shen H, Wang X, et al. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):833‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID‐19: current state of the science. Immunity. 2020;52(6):910‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. To KK‐W, Tsang OT‐Y, Leung W‐S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


