Abstract
Graft‐versus‐host disease (GVHD) is a common complication of hematopoietic stem cell transplant, which is known to be mediated by cytotoxic T‐cell effectors and dysregulated inflammatory cytokines. Similarly, the lung injury observed in severe COVID‐19 cases appears to be related to a massive production of pro‐inflammatory cytokines. The selective JAK1/2 inhibitor ruxolitinib has shown promising results in the context of GVHD, and different trials are currently underway in patients with severe COVID‐19; nevertheless, no clinical observation of safety or efficacy of treatment with ruxolitinib in this context has been published yet. We describe a first case of severe COVID‐19 developed after hematopoietic stem cell transplantation in a patient with a concomitant chronic GVHD (cGVHD), in which a treatment with ruxolitinib was administered with good tolerance and positive outcome.
Keywords: ARDS (acute respiratory distress syndrome), chronic graft‐versus‐host disease (cGVHD), COVID‐19, hematopoietic stem cell transplant (SCT), ruxolitinib
1. INTRODUCTION
Recipients of hematopoietic stem cell transplant are at particular risk of developing the pandemic infection by severe acute respiratory coronavirus 2 (SARS‐CoV‐2), named coronavirus disease 2019 (COVID‐19); in fact, the immunosuppression related to this procedure is particularly profound and lasting. Moreover, transplant‐related complications as graft‐versus‐host disease (GVHD) further impair B‐ and T‐cell function and antiviral immune response. 1 Currently, there is no approved treatment for COVID‐19, and several drugs have been tested with conflicting results. 2 , 3 , 4 We describe a first case of severe COVID‐19 developed after hematopoietic stem cell transplantation in a patient with a concomitant chronic GVHD (cGVHD), in which a treatment with ruxolitinib was administered with good tolerance and positive outcome.
2. METHODS
Patient's medical records were collected, including clinical features, laboratory tests, radiographic imaging, treatment received, and outcome. The presence of SARS‐CoV‐2 RNA in nasopharyngeal swab was assessed by the CDC 2019‐nCoV Real‐Time RT‐PCR Diagnostic Panel described in the CDC published protocols. The diagnostic panel contains primers and probes covering two regions of the SARS‐CoV‐2 N gene and the human RNase P gene used as control for sample integrity. 5
The institutional review board of the Ancona University Hospital approved the off‐label treatment, and an informed consent for data collection and off‐label treatment was obtained.
3. RESULTS
A 59‐year‐old man who had undergone an allogeneic hematopoietic stem cell transplantation from a matched sibling brother for high‐risk, triple‐negative (JAK2, MPL, CALR) myelofibrosis, presented at our outpatient clinic for a scheduled appointment at 1 year after transplant, on March 3, 2020. He had no complaints, and physical examination was normal. His medical history was remarkable for an insulin‐dependent type 2 diabetes and a latent tuberculosis infection. Blood count was normal; bone marrow biopsy showed a complete remission, with no evidence of fibrosis. Chimerism was 100% donor, and CD3+/CD4+ lymphocyte count was 278 per mm3. He has been receiving ruxolitinib 5 mg bid (off‐label use) for a steroid‐refractory, moderate cGVHD with involvement of skin and mouth, achieving a complete response at the time of office visit. 6 He was then discharged with a follow‐up appointment scheduled 3 months later, confirming ruxolitinib treatment up to the next visit.
Two weeks later, he presented at the emergency room complaining of fatigue, dry cough, and mild dyspnea. Oxygen saturation was 97% while breathing in room air; body temperature was 36.8°C. Arterial blood gas analysis was normal. Blood count showed 5.15 × 109/L white blood cells, with slight lymphopenia (0.9 × 109/L); platelet count was 135 × 109/L; and hemoglobin was within normal range. C‐reactive protein was elevated (119 mg/L), while procalcitonin was normal. Coagulation parameters showed elevated D‐dimer (5700 ng/mL) and fibrinogen (480 mg/dL). A chest x‐ray evidenced a mild interstitial lung pattern. The nasopharyngeal swab resulted positive for SARS‐CoV‐2 RNA at nucleic acid amplification with real‐time reverse‐transcription polymerase chain reaction (rRT‐PCR); plasma cytomegalovirus (CMV) DNA was negative. He was then transferred to the nearest COVID‐19–dedicated hospital. At hospital admission, ruxolitinib was discontinued, assuming a possible deleterious immunosuppressive activity and delayed viral clearance in a patient with an active viral infection, as previously reported. 7 , 8 A treatment was started with intravenous piperacillin‐tazobactam, levofloxacin, low‐molecular‐weight heparin, lopinavir/ritonavir, and hydroxychloroquine. The ongoing therapy with isoniazid and valacyclovir was continued. Steroids were not administered. On day 5 after admission, the patient developed an acute respiratory distress syndrome (ARDS); arterial blood gas analysis showed severe hypoxia (PaO2/FiO2 ratio of 142), and high flow oxygen therapy with continuous positive airway pressure (CPAP) ventilation was initiated (Fi02 50%, PEEP 10 cmH20). A computed tomography of the chest showed a significant evolution of pulmonary infiltrates, with bilateral, peripheral ground‐glass opacities with diffuse consolidation (Figure 1). On day 8, treatment with lopinavir‐ritonavir was discontinued, as results of a negative trial had been published meanwhile. 9 On day 10, since the patient condition was not improving (PaO2/FiO2 ratio of 141), ruxolitinib was resumed (off‐label use) at the dose of 5 mg bid. In fact, on that day, accumulating evidence suggested that mitigation of the exaggerated inflammatory response associated with COVID‐19 might be beneficial in patients with severe symptoms. 10
FIGURE 1.
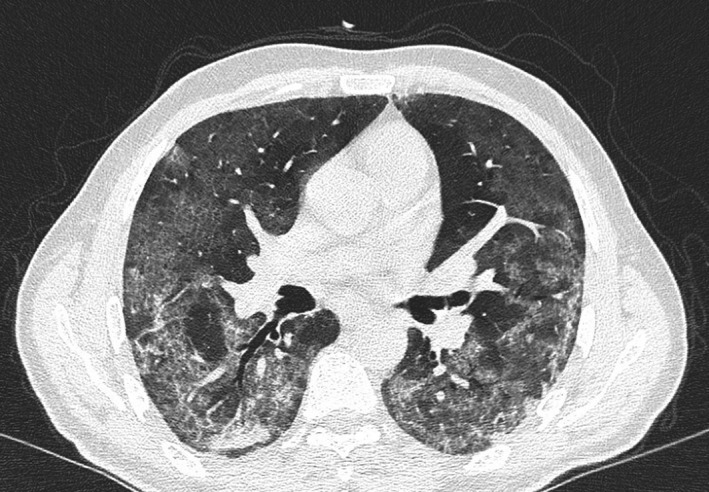
Computed tomography scan of the chest performed the day before initiation of treatment with ruxolitinib
In order to promptly detect common adverse reactions related to the drug, complete laboratory test was repeated twice weekly. Since no event was recorded, and platelet count remained stable, on day 24 (day 14 of ruxolitinib) the dose was escalated to 10 mg bid. Patient's symptoms improved dramatically, and PaO2/FiO2 ratio progressively increased (Figure 2). On day 32 after admission (day 22 of ruxolitinib), oxygen saturation and arterial blood gas analysis were within normal range in room air, and oxygen therapy was withdrawn. C‐reactive protein decreased to normal value; platelet and white blood cell count remained stable, and no significant alteration of other laboratory tests was evidenced. Blood cultures, sputum test, and QuantiFERON test resulted negative. Further, no CMV reactivation was detected. On day 40 (day 30 of ruxolitinib), the dosage was decreased to 5 mg bid, as previously prescribed for cGVHD. The patient was declared to be cured and was discharged from the hospital on day 45 after admission. Of note, nasopharyngeal swab was still positive for SARS‐CoV‐2 RNA at the time of discharge.
FIGURE 2.
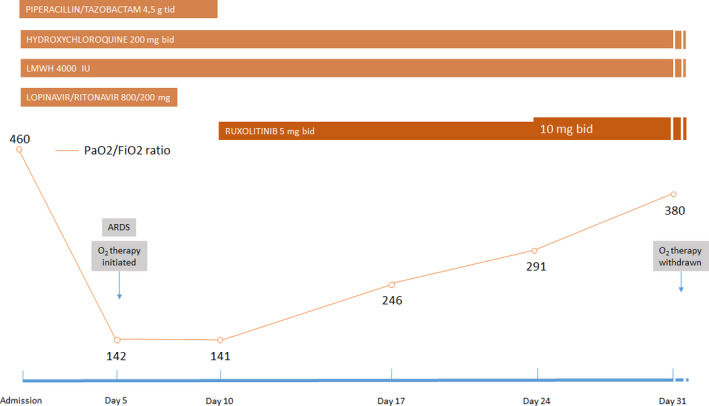
Trend of PaO2/FiO2 ratio and treatment received during hospital stay. Abbreviations: ARDS, acute respiratory distress syndrome; LMWH, low‐molecular‐weight heparin
4. DISCUSSION
To our knowledge, this represents the first case of severe COVID‐19 successfully treated in a recipient of hematopoietic stem cell transplantation with concomitant cGVHD. A major pathogenetic mechanism of the lung injury observed in severe COVID‐19 cases appears to be related to a massive production of pro‐inflammatory cytokines, and different approaches targeting this aspect are currently under investigation. 11 , 12 In fact, the immunologic and inflammatory cascade which follows SARS‐CoV‐2 infection is complex and not completely understood. Dysregulation of both innate and adaptive immunity leads to an exaggerated macrophage activation and T‐cell proliferation, with a consequent abnormal production of pro‐inflammatory mediators (ie, IFN‐g, IL‐2, IL‐6, IL‐12, TNF‐a). Interestingly, a macrophage activation syndrome (MAS) resembling the one causing secondary hemophagocytic lymphohistiocytosis (sHLH) has been described in some patients with COVID‐19. 13 Further, T‐cell response plays a key role in viral clearance as well as in hyperinflammation. Of note, strikingly high proportion of T helper 17 cells (Th17) have been found in peripheral blood of patients with severe COVID‐19, further supporting a TH17 type cytokine production in this disease. 14 The Janus kinase (JAK) and signal transducers and activators of transcription (STAT) pathways play a major role both in innate and adaptive immune response, as well as in tissue inflammation. Different trials testing the selective JAK1/2 inhibitor ruxolitinib in patients with severe COVID‐19 are currently underway worldwide (NCT04338958, NCT04331665, NCT04359290); nevertheless, no clinical observation of safety or efficacy of treatment with ruxolitinib in this context has been published yet. Preclinical studies demonstrated that ruxolitinib could potently reduce pro‐inflammatory cytokine production, including interferon‐g (IFN‐g), interleukin‐2 (IL‐2), IL‐6, IL‐12, and IL‐23; further, it has been shown to inhibit T‐cell expansion, and differentiation into T helper 17 subsets, in addition to increasing FOXP3+ regulatory T (Treg) cells in mice models of GVHD. 15 , 16 A recently published prospective randomized study demonstrated superiority of ruxolitinib over investigator's choice therapy in acute GVHD. 17 In our patient, the concomitant cGVHD could have contributed to the development of an abnormal inflammatory process following SARS‐CoV‐2 infection. In such context, JAK2 inhibition might exert a pleiotropic activity, contributing to the immune homeostasis and regulating the cross‐talk between the innate and adaptive immune system, thus preventing tissue damage. Of note, steroids were avoided throughout the duration of the hospital stay. In fact, the use of steroids in patients with severe COVID‐19 remains controversial, due to concerns about delayed viral clearance. Ruxolitinib exerts a more refined immunomodulatory activity; nevertheless, in the setting of a severe viral infection as COVID‐19, the right timing of treatment initiation is matter of debate. In fact, due to the powerful inhibition of T‐cell activity and cytokine production, ruxolitinib administration in an early phase of the disease could impair T‐cell expansion and viral clearance. On the contrary, the immunomodulatory activity of the drug might be better exploited when an immune response has been built (ie, 7‐10 days after the appearance of symptoms) and the viral load has already decreased. At that point, patients with signs of hyperinflammation are more likely to benefit from the drug. Most importantly, in our patient with a latent tuberculosis infection, administration of ruxolitinib resulted safe, and no sign of tuberculosis reactivation or other infectious complications were observed. 7 , 18
5. CONCLUSIONS
Our report suggests that in this patient with severe COVID‐19 developed after a hematopoietic stem cell transplant, treatment with ruxolitinib was feasible and well tolerated. Moreover, we observed a dramatic clinical improvement after ruxolitinib administration, albeit we cannot exclude that other factors could have contributed. The present report requires further confirmation, and results from prospective trials testing ruxolitinib in COVID‐19 patients are eagerly awaited.
CONFLICT OF INTEREST
Olivieri A received honoraria as consultant from Novartis in 2017 and 2018. The other authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
FS and AO wrote the manuscript draft; IS, GM, M.M, and IF revised the manuscript; and MG, MLM, and PB were involved in patient treatment and collected data from medical records. All the authors approved the final version of the manuscript.
ACKNOWLEDGEMENTS
The authors would like to thank all the medical staff involved in treating this patient.
Saraceni F, Scortechini I, Mancini G, et al. Severe COVID‐19 in a patient with chronic graft‐versus‐host disease after hematopoietic stem cell transplant successfully treated with ruxolitinib. Transpl Infect Dis. 2021;23:e13401. 10.1111/tid.13401
REFERENCES
- 1. Capitini CM, Nasholm NM, Duncan BB, Guimond M, Fry TJ. Graft‐versus‐host disease impairs vaccine responses through decreased CD4+ and CD8+ T cell proliferation and increased perforin‐mediated CD8+ T cell apoptosis. J Immunol. 2013;190(3):1351‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat Commun. 2020;11(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of Hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020. 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. https://www.fda.gov/media/134922/download. Accessed June 24, 2020.
- 6. Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid‐refractory graft‐versus‐host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062‐2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sant'Antonio E, Bonifacio M, Breccia M, Rumi E. A journey through infectious risk associated with ruxolitinib. Br J Haematol. 2019;187(3):286‐295. [DOI] [PubMed] [Google Scholar]
- 8. Lussana F, Cattaneo M, Rambaldi A, Squizzato A. Ruxolitinib‐associated infections: a systematic review and meta‐analysis. Am J Hematol. 2018;93(3):339‐347. [DOI] [PubMed] [Google Scholar]
- 9. Cao B, Wang Y, Wen D, et al. A trial of Lopinavir‐Ritonavir in adults hospitalized with severe covid‐19. N Engl J Med. 2020;382(19):1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stebbing J, Phelan A, Griffin I, et al. COVID‐19: combining antiviral and anti‐inflammatory treatments. Lancet Infect Dis. 2020;20(4):400‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019‐nCoV acute respiratory disease. Lancet. 2020;395:e30‐e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spoerl S, Mathew NR, Bscheider M, et al. Activity of therapeutic JAK 1/2 blockade in graft‐versus‐host disease. Blood. 2014;123(24):3832‐3842. [DOI] [PubMed] [Google Scholar]
- 16. Carniti C, Gimondi S, Vendramin A, et al. Pharmacologic inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin Cancer Res. 2015;21:3740‐3749. [DOI] [PubMed] [Google Scholar]
- 17. Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for glucocorticoid‐refractory acute graft‐versus‐host disease. N Engl J Med. 2020;382(19):1800‐1810. [DOI] [PubMed] [Google Scholar]
- 18. Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety and survival with ruxolitinib in patients with myelofibrosis: results of a median 2‐year follow‐up of COMFORT‐I. Haematologica. 2013;98(12):1865‐1871. [DOI] [PMC free article] [PubMed] [Google Scholar]


