Abstract
Immunosuppression leaves transplanted patients at particular risk for severe acute respiratory syndrome 2 (SARS‐CoV‐2) infection. The specific features of coronavirus disease 2019 (COVID‐19) in immunosuppressed patients are largely unknown and therapeutic experience is lacking. Seven transplanted patients (two liver, three kidneys, one double lung, one heart) admitted to the Ludwig‐Maximilians‐University Munich because of COVID‐19 and tested positive for SARS‐CoV‐2 were included. The clinical course and the clinical findings were extracted from the medical record. The two liver transplant patients and the heart transplant patient had an uncomplicated course and were discharged after 14, 18, and 12 days, respectively. Two kidney transplant recipients were intubated within 48 hours. One kidney and the lung transplant recipients were required to intubate after 10 and 15 days, respectively. Immunosuppression was adapted in five patients, but continued in all patients. Compared to non‐transplanted patients at the ICU (n = 19) the inflammatory response was attenuated in transplanted patients, which was proven by decreased IL‐6 blood values. This analysis might provide evidence that continuous immunosuppression is safe and probably beneficial since there was no hyperinflammation evident. Although transplanted patients might be more susceptible to an infection with SARS‐CoV‐2, their clinical course seems to be similar to immunocompetent patients.
Keywords: clinical course, coronavirus, COVID‐19, SARS‐CoV‐2, transplantation
1. INTRODUCTION
On 7 January 2020, the new coronavirus severe acute respiratory syndrome 2 (SARS‐CoV‐2) was identified as the cause of coronavirus disease 2019 (COVID‐19). 1 , 2 Although the majority of infections are asymptomatic or mild, there are patients who have severe courses that require hospitalization. Of these, approximately a fifth get oxygen and 17% are in need of invasive ventilation. 2 The case fatality rate varies and can be as high as 28.3%. However, case fatality rate is strongly related to the age of the patients and concomitant comorbidities of the patients such as heart disease, diabetes mellitus. 1 , 2 , 3
Patients with a compromised immune system are more susceptible to viral infections than immunocompetent patients. 4 , 5 Thus, it can be assumed that transplant patients under immunosuppression and plagued with numerous comorbidities are at particular risk for an unfavorable course of COVID‐19. However, data on COVID‐19 in patients after solid organ transplantation are scarce. Profound information about the management of immunosuppression and the clinical course in solid organ recipients are still lacking. 6 , 7 , 8 , 9 , 10 Here, we present comprehensive data about the clinical courses of solid organ recipients suffering of COVID‐19. We aim to evaluate the clinical courses of these patients and provide a rationale for maintenance of immunosuppression.
2. PATIENTS AND METHODS
This is a retrospective cohort study carried out at the University Hospital of the Ludwig‐Maximilians‐University Munich, Germany. Every patient hospitalized due to COVID‐19 was screened for a history of solid organ transplantation. To securely diagnose a patient positive for SARS‐CoV‐2, a positive reverse real‐time polymerase chain reaction (PCR) assay result of a respiratory specimen was demanded. 1 , 2 In total, seven consecutive solid organ recipients (two liver, three kidneys, one double lung, and one heart) were identified and included in the present analysis. Inflammatory response and early outcomes were compared to a cohort of non‐transplanted, non‐immunosuppressed COVID‐19 patients treated at the same time on our ICU (n = 19). Patients qualified for inclusion to the control collective if they were immunocompetent and stayed at least 19 days on the ICU such as the shortest ICU treatment of a transplanted patient.
The clinical course and the clinical findings recorded during treatment were extracted from the electronic medical record. The data collection within the CORKUM (COVID‐19 Register des LMU Klinikums) network was approved by the local ethics committee. Results are displayed as means with standard deviation and time frames (age and time since transplantation) as medians with IQ range. Univariate analysis was carried out by using Chi‐squared test for categorical parameters (eg, age, temperature); p‐values lower than 0.05 were considered significant. Prism 8.0 for Mac (GraphPad Software, Inc, La Jolla, CA) was used for statistical analysis.
3. RESULTS
Here, we describe the clinical presentation of the first seven transplanted patients hospitalized for COVID‐19 in our institution (Table 1). We have analyzed the clinical courses of two liver transplant recipients, three kidney transplant recipients, one double lung transplant recipient, and one heart transplant recipient. The median age of four males and three females was 61.4 (18.2‐80.6) years. The median time after transplantation, on immunosuppression, respectively, was 5.6 (1.5‐15) years. Maintenance of immunosuppression is detailed in Table 2.
TABLE 1.
Baseline characteristics of the analyzed patients on admission
| KiTx 1 | KiTx 2 | KiTx 3 | LiTx 1 | LiTx 2 | LuTx | HTx | |
|---|---|---|---|---|---|---|---|
| Age | 80 | 61 | 45 | 65 | 18 | 65 | 48 |
| Gender | Female | Male | Male | Female | Male | Male | Female |
| Years since transplantation | 7 | 1.5 | 5.6 | 5.6 | 15 | 7.9 | 5 |
| Comorbidities | aH, CHD, HD, obesity | aH, CHD, IDDM, obesity | aH | aH | aH, HD | aH, CHD, HD |
aH IDDM |
| Fever (>37.3°C) | Yes | Yes | Yes | Yes | No | Yes | No |
| Cough | Yes | Yes | Yes | Yes | No | Yes | No |
| Myalgia | Yes | Yes | Yes | Yes | No | Yes | No |
| GI‐symptoms | No | No | No | No | No | No | No |
| qSOFA score | 3 | 3 | 1 | 2 | 0 | 2 | 0 |
| CURB‐65 score | 2 | 2 | 0 | 3 | 0 | 2 | 0 |
| CCI | 9 | 7 | 3 | 8 | 8 | 7 | 3 |
| Time from onset to hospital admission, days | 5 | 3 | 4 | 1 | 1 | 6 | 0 |
Abbreviations: aH, arterial hypertension; CCI, Charlson Comorbidity Index; CHD, coronary heart disease; CURB‐65 (C, confusion; U, urea > 7 mmol/L; R, respiratory rate > 30/min; B, blood pressure systolic < 90 mm Hg or diastolic < 60 mm Hg, 65 = patient ≥ 65 years old); HD, hemodialysis; IDDM, insulin‐dependent diabetes mellitus; qSOFA, quick sequential organ failure assessment; Tx, transplantation.
TABLE 2.
Maintenance of immunosuppression on admission and respective modifications (MPA – mycophenolic acid)
| Patient | On admission | During hospitalization | ||
|---|---|---|---|---|
| KiTx 1 | MPA: | 720 mg/d | Prednisolone: | 2.5 mg/d |
| Prednisolone: | 2.5 mg/d | |||
| KiTx 2 | Tacrolimus: | 3 mg/d | Cyclosporine A | |
| Trough level: | 5.8 ng/mL | Target trough level: | 40‐60 ng/mL | |
| MPA: | 1440 mg/d | |||
| Prednisolone: | 2.5 mg/d | Prednisolone: | 2.5 mg/d | |
| KiTx 3 | CellCept: | 2000 mg/d | Cyclosporine A | |
| Target trough level: | 40‐60 mg/mL | |||
| Prednisolone: | 10 mg/d | Prednisolone: | 10 mg/d | |
| LiTx 1 | Everolimus: | 4 mg/d | Everolimus | |
| Trough level: | 4.7 ng/mL | Target trough level: | 3‐8 ng/mL | |
| CellCept: | 500 mg/d | |||
| LiTx 2 | CellCept: | 1000 mg/d | CellCept: | 1000 mg/d |
| LuTx 1 | Tacrolimus: | 2.5 mg/d | Tacrolimus | |
| Trough level: | 7.6 ng/mL | Target trough level: | 6‐8 ng/mL | |
| CellCept: | 1000 mg/d | |||
| Prednisolone: | 10 mg/d | Prednisolone: | 10 mg/d | |
| HTx | Tacrolimus: | 4 mg/d | Tacrolimus | |
| Trough level: | 5.1 ng/mL | Target trough level: | 5‐7 ng/mL | |
| Sirolimus: | 1 mg/d | Sirolimus | ||
| Trough level: | 2.3 ng/mL | Target trough level: | 2‐5 ng/mL | |
3.1. On admission
On admission all, but one asymptomatic liver and the heart transplant patient, presented with cough and fever (Table 3). High resolution thoracic CT scan showed in all cases the characteristic opaque dorsolateral infiltrates typical for COVID‐19. 11 A positive SARS‐CoV‐2 PCR of a respiratory specimen confirmed COVID‐19. All but one kidney transplant patient presented with adequate graft function. Nonetheless, all patients were on ongoing immunosuppressants.
TABLE 3.
Baseline characteristics and comorbidities of COVID‐19 patients analyzed
| Total TX | TX non‐ICU | TX‐ICU | non‐TX ICU | |
|---|---|---|---|---|
| n = 7 | n = 3 | n = 4 | n = 19 | |
| Median age, years | 61.4 (18.2‐80.6) | 48.9 (18.2‐65.6) | 63.3 (45.3‐80.6) | 64.4 (42‐75.1) |
| Sex | ||||
| Female | 3 (43%) | 2 (67%) | 1 (25%) | 2 (11%) |
| Male | 4 (57%) | 1 (33%) | 3 (75%) | 17 (89%) |
| Years since transplantation | 5.6 (1.5‐15) | 5.6 (5‐15) | 6.3 (1.5‐7.9) | n.a. |
| Comorbidity | ||||
| Hypertension | 7 (100%) | 3 (100%) | 4 (100%) | 13 (68.4%) |
| Diabetes | 2 (28.6%) | 1 (33.3%) | 1 (25%) | 4 (21.1%) |
| Coronary heart disease | 3 (42.9%) | 0 | 3 (75%) | 5 (26.3%) |
| COPD | 2 (28.6%) | 0 | 2 (50%) | 2 (10.5%) |
| Smoker | 1 (14.3%) | 0 | 1 (25%) | 3 (15.8%) |
| Obesity | 2 (28.6%) | 0 | 2 (50%) | 4 (21.1%) |
| Fever (>37.3°C) | 5 (71.4%) | 1 (33.3%) | 4 (100%) | 13 (57.9%) |
On admission, most transplanted patients showed mean elevated CRP 11.1 mg/dL (±4.8), IL‐6 59.1 pg/mL (±20.3), ferritin 1104 ng/mL (±428), and LDH 319 U/L (±15.8) values, with mostly normal WBC 7 G/L (±1.5). Patients of the non‐transplanted intensive care control collective had comparable comorbidities and showed the following mean blood values on admission: CRP 10.5 mg/dL (±10.5), IL‐6 622.4 pg/mL (±1722), ferritin 802 ng/mL (±525.4), LDH 444.4 U/L (±144.5), WBC 25 G/L (±7.6).
3.2. Clinical course
The clinical course differed significantly between the transplant patients. The two liver transplant recipients (LiTx 1 and 2) and the heart transplant (HTx) recipient could be managed on a regular ward. These patients had neither dyspnea nor oxygen demand. Initially, the heart transplant patient was admitted to an external clinic because of cholangitis and transferred to our institution due to additional SARS‐CoV‐2 infection. The liver transplant recipients as well as the heart transplant recipient could be discharged after 14 (LiTx 1), 18 (LiTx 2) and 12 (HTx) days, respectively.
Two kidney transplant recipients (KiTx 1 and 2) had to be intubated within 48 hours after admission. The third kidney transplant recipient (KiTx 3) and the lung transplant recipient (LuTx) were initially managed with 4‐6l oxygen flow. However, they showed a delayed progression requiring intubation ten (KiTx 3) and 15 (LuTx) days after admission, respectively. One of the kidney transplant patients (KiTx 3) was extubated after three days of mechanical ventilation. Additionally, this patient was discharged in good health 17 days after admission. Weaning was initiated in the remaining kidney transplant patients after 16 (KiTx 1) 19 (KiTx 2) days of mechanical ventilation. The pulmonary function of these patients improved and they are in need of mechanical ventilation less than 12 hours a day. Nonetheless, weaning is not completed. Thus, only the lung transplant recipient is on mechanical ventilation since 24 days. Only one kidney recipient (KiTx 2) had transient graft loss and required hemodialysis.
3.3. Treatment
All transplant patients stayed on immunosuppressants. The immunosuppressive medication of the heart and of one liver recipient (LiTx 2) remained unchanged. However, immunosuppression was adapted in the kidney and lung transplant patients. The antimetabolite was discontinued in the kidney recipients, the lung recipient, and in one liver recipient (LiTx 1). Cyclosporine A was additionally initiated in KiTx 2 and KiTx 3. Target trough levels of cyclosporine A (40‐60 ng/mL), tacrolimus (6‐8 ng/mL), everolimus (3‐8 ng/mL), and sirolimus (2‐5 ng/mL) were evaluated regularly and within range during hospital stay. The individual adaption of immunosuppression is detailed in Table 2.
Transplant patients admitted to ICU received azithromycin and, apart from one kidney transplant patient (KiTx 1), hydroxychloroquine adapted to glomerular filtration rate as specific COVID‐19 treatment as suggested by Gautret et al. 12 No specific antiviral therapy or high‐dose cortisone therapy was given. To avoid opportunistic infections all patients were placed on empiric antimicrobial treatment with piperacillin/tazobactam. The heart transplant patient was treated with metronidazole and cefuroxime for cholangitis. The immunocompetent control collective at the ICU also received empiric antimicrobial treatment with piperacillin/tazobactam in 17 patients (89.5%) and 18 patients (94.7%) received azithromycin. Additionally, seven patients got antiviral treatment (36.8%) and three patients (15.8%) were treated with tocilizumab.
3.4. Transplant‐specific observations
In transplanted/immunosuppressed patients, inflammatory response on admission was generally lower as compared to non‐transplant patients. Moreover, during hospitalization, IL‐6 levels stayed significantly lower in transplanted patients as compared to non‐transplanted patients. A similar observation was made for LDH, but not for CRP and ferritin. The inflammation markers of the transplanted patients not requiring intensive care treatment remained low throughout their stay (Figure 1). Apart from the heart transplant patient, who suffered cholangitis, liver enzymes, coagulation function, and blood count were not impaired during hospital stay (Figure S1).
FIGURE 1.
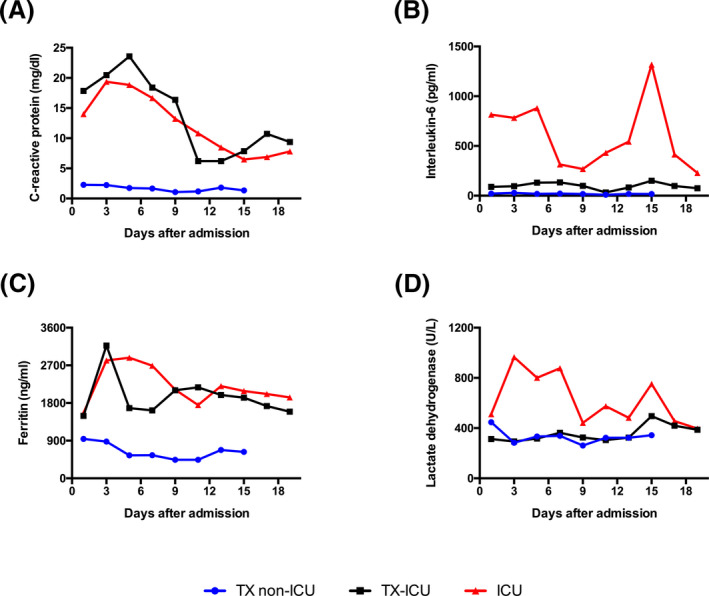
Time course of the inflammation markers (A) CRP, (B) IL‐6, (C) Ferritin, and (D) lactate dehydrogenase. Comparison of intensive care (n = 4) and non‐intensive care transplant patients (n = 3) with a non‐transplant (n = 19) COVID‐19 "control" cohort that was in intensive care at the same time. The average values are given. Due to the small number of cases and the uncontrolled comparison with non‐transplanted patients, only a trend but no significance can be given. Accordingly, we have omitted the error bars for the sake of clarity
There was no death of a transplanted patient within our institution.
4. DISCUSSION
The SARS‐CoV‐2 pandemic is spreading rapidly across the globe without any specific therapy or vaccination being available. 13 , 14 Risk factors for developing acute respiratory distress syndrome (ARDS), ICU admission and death have been published. 1 , 2 , 15 Nonetheless, these studies have been conducted in immunocompetent patients. Transplanted patients belong to a particularly vulnerable patient group, as they require life‐long immunosuppression increasing their susceptibility for viral infections. 4 , 5
It can be assumed that the clinical courses under immunosuppression differ from those of non‐immunosuppressed patients. The first assumption would be that immunosuppression is generally unfavorable for the course of the disease, but in the case of COVID‐19 it could be different. One of the problems of COVID‐19, apart from the direct damage caused by the virus, is a potentially excessive immune response, which in turn can lead to further damage to the lung parenchyma. 16 , 17 Here, immunosuppressive drugs could even be advantageous by preventing what is often called a cytokine storm. Indeed, we observed significantly lower IL‐6 levels in our patients than in the non‐immunosuppressed reference cohort. Although no statistical significant difference was seen, a clear trend was obvious. The early inflammatory response in transplanted patients seems to be attenuated. In this context, it could be shown in our clinic that the level of IL‐6 increase has a prognostic value for COVID‐19 progression (in review) and pharmacological inhibition of IL‐6 was proposed as a potential treatment strategy of COVID‐19. 18 , 19 Since the follow‐up period was relatively short, the results of the present study have to be interpreted with care. Nonetheless, no transplanted patient died due to SARS‐CoV‐2 infection. This finding is in contrast to recently published reports with mortality rates of solid organ recipients of up to 28%. 7 , 9 , 20 , 21 , 22 In this respect, Akalin et al even reported a mortality rate of intubated organ recipients of 64% (7/11). 9
Of course, the analyzed cohort is too small to draw clear conclusions at this point, but the observation per se is new and offers a potential therapeutic perspective. Another open question is whether different immune suppressants have different effects on the course of the disease. For the calcineurin inhibitor cyclosporine A, an antiviral efficacy for coronaviruses such as SARS‐CoV and Middle East respiratory syndrome coronavirus (MERS‐CoV) could be shown, 23 , 24 , 25 which shows 79% and 50% homology with the current SARS‐CoV‐2 virus, respectively. 26 Nonetheless, only little is known about the clinical course in transplanted patients with MERS‐CoV or SARS‐CoV infection. 27 , 28 For mTOR‐inhibitors, an antiviral activity on the cytomegalovirus is known and mTOR‐inhibitors have been used as a senolytic substance like chloroquine and azithromycin as an orphan treatment option. 29 , 30
A further question we asked ourselves is to what extent the course of the disease is influenced by the viral infection itself or by the immune response. It also seems unclear how the balance between immune defense and anti‐inflammation is to be maintained and whether an imbalance between the two could explain the biphasic and/or unfavorable courses observed. Immunosuppression in this context may provoke virus persistence. In this context, we found that all our immunosuppressed patients requiring mechanical ventilation showed prolonged virus persistence compared to clearance times reported by Liu et al and Yuan et al. 31 , 32
It is interesting to speculate whether different transplants are varying with regard to their vulnerability to SARS‐CoV‐2 infection. In our cohort, we noticed that the liver transplant and heart transplant patients took a relatively mild course. This could be due to the fact that successful liver and heart transplants have relatively little additional comorbidities. In contrast, kidney transplanted patients have more serious, mainly cardiovascular concomitant diseases due to the long dialysis treatment. 33 , 34 From theoretical considerations, as well as in our patients, it is to be assumed that lung transplantation is particularly prone to severe disease. In addition to the high level of immunosuppression required, the lung is the direct target of the virus and the transplant alters the lung physiology. 35
What conclusions can be drawn from the experiences with this transplant collective? The first and perhaps the most important result of the present study is that there is no consistent pattern for COVID‐19 illness under immunosuppression. There are very mild courses of the disease under immunosuppression, but also severe ones, but overall the situation is not as bleak as first case reports suggest. 7 , 20 , 36 It seems advisable to maintain an adequate immunosuppression to avoid acute rejection and subsequent organ failure; there is currently no data basis for changing the immunosuppression at the time of infection. Our experience has been encouraging that with the appropriate allocation of intensive care resources, it is possible to successfully manage even severe courses of COVID‐19 in transplanted patients.
CONFLICT OF INTERESTS
None.
AUTHOR CONTRIBUTIONS
Florian Bösch and Nikolaus Börner each made substantial contributions to the study concept and Markus O. Guba provided overall leadership and guidance to the investigation. Florian Bösch, Nikolaus Börner, Stephan Kemmner, Christopher Lampert, Sven Jacob and Dionysios Koliogiannis took responsibility for the integrity of the data and the accuracy of the data analysis. Florian Bösch, Nikolaus Börner, Stephan Kemmner, Christopher Lampert, Sven Jacob and Dionysios Koliogianni: collected, analyzed and interpreted the data. Stephan Kemmner, Manfred Stangl, Sebastian Michel, Nikolaus Kneidinger, Christian Schneider, Michael Fischereder, Michael Irlbeck and Gerald Denk: provided clinical care to the patients, assisted with clinical descriptions, and data. Florian Bösch, Nikolaus Börner and Markus O. Guba: Drafted and revised the manuscript. Jens Werner, Martin K. Angele and Markus O. Guba made substantial revisions to the manuscript and provided substantial contributions to the manuscript. All authors reviewed, revised, and approved the final manuscript.
Supporting information
Figure S1
Bösch F, Börner N, Kemmner S, et al. Attenuated early inflammatory response in solid organ recipients with COVID‐19. Clin Transplant. 2020;34:e14027. 10.1111/ctr.14027
Florian Bösch and Nikolaus Börner contributed equally to this manuscript.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang X, Fang J, Zhu Y, et al. Clinical characteristics of non‐critically ill patients with novel coronavirus infection (COVID‐19) in a Fangcang Hospital. Clin Microbiol Infect. 2020;26(8):1063‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dendle C, Mulley WR, Holdsworth S. Can immune biomarkers predict infections in solid organ transplant recipients? A review of current evidence. Transplant Rev (Orlando). 2019;33:87‐98. [DOI] [PubMed] [Google Scholar]
- 5. Razonable RR, Eid AJ. Viral infections in transplant recipients. Minerva Med. 2009;100:479‐501. [PubMed] [Google Scholar]
- 6. Bin L, Yangzhong W, Yuanyuan Z, Huibo S, Fanjun Z, Zhishui C. Successful treatment of severe COVID‐19 pneumonia in a liver transplant recipient. Am J Transplant. 2020;20:1891‐1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandez‐Ruiz M, Andres A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020;20(7):1849‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guillen E, Pineiro GJ, Revuelta I, et al. Case report of COVID‐19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020;20(7):1875‐1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020;382(25):2475‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong SY, Brubaker AL, Wang AX, Taiwo AA, Melcher ML. What solid organ transplant healthcare providers should know about renin‐angiotensin‐aldosterone system inhibitors and COVID‐19. Clin Transplant. 2020;34(7):e13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology. 2019;2020:200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):e105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenbaum L. Facing Covid‐19 in Italy – ethics, logistics, and therapeutics on the epidemic's front line. N Engl J Med. 2020;382(20):1873‐1875. [DOI] [PubMed] [Google Scholar]
- 15. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin‐6 (IL‐6) blockade for coronavirus disease 2019 (COVID‐19)‐induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:e102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi Y, Wang Y, Shao C, et al. COVID‐19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID‐19 in long‐term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5(6):532‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tschopp J, L'Huillier AG, Mombelli M, et al. First experience of SARS‐CoV‐2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020;20(10):2876‐2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carbajo‐Lozoya J, Muller MA, Kallies S, Thiel V, Drosten C, von Brunn A. Replication of human coronaviruses SARS‐CoV, HCoV‐NL63 and HCoV‐229E is inhibited by the drug FK506. Virus Res. 2012;165:112‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Wilde AH, Zevenhoven‐Dobbe JC, van der Meer Y, et al. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol. 2011;92:2542‐2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li HS, Kuok DIT, Cheung MC, et al. Effect of interferon alpha and cyclosporine treatment separately and in combination on Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) replication in a human in‐vitro and ex‐vivo culture model. Antiviral Res. 2018;155:89‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. AlGhamdi M, Mushtaq F, Awn N, Shalhoub S. MERS CoV infection in two renal transplant recipients: case report. Am J Transplant. 2015;15:1101‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe Acute Respiratory Syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant. 2003;3:977‐981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sargiacomo C, Sotgia F, Lisanti MP. COVID‐19 and chronological aging: senolytics and other anti‐aging drugs for the treatment or prevention of corona virus infection? Aging (Albany NY). 2020;12(8):e103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolf S, Lauseker M, Schiergens T, et al. Infections after kidney transplantation: a comparison of mTOR‐Is and CNIs as basic immunosuppressants. A systematic review and meta‐analysis. Transpl Infect Dis. 2020;22(3):e13267. [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Yan LM, Wan L, et al. Viral dynamics in mild and severe cases of COVID‐19. Lancet Infect Dis. 2020;20(6):656‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yuan J, Zou R, Zeng L, et al. The correlation between viral clearance and biochemical outcomes of 94 COVID‐19 infected discharged patients. Inflamm Res. 2020;69(6):599‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goralczyk AD, Bari N, Abu‐Ajaj W, et al. Calcineurin inhibitor sparing with mycophenolate mofetil in liver transplantion: a systematic review of randomized controlled trials. Am J Transplant. 2012;12:2601‐2607. [DOI] [PubMed] [Google Scholar]
- 34. Webster A, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2005;(4):CD003961. [DOI] [PubMed] [Google Scholar]
- 35. Ahya VN, Diamond JM. Lung transplantation. Med Clin North Am. 2019;103:425‐433. [DOI] [PubMed] [Google Scholar]
- 36. Huang JF, Zheng KI, George J, et al. Fatal outcome in a liver transplant recipient with COVID‐19. Am J Transplant. 2020;20(7):1907‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


