As of May 11, 2020, coronavirus disease 2019 (Covid‐19) has been confirmed in 4 152 670 people worldwide, carrying a mortality of approximately 6.8%, 1 compared with a mortality rate of less than 1% from influenza. There is an urgent need for effective treatment. Current focus has been on the development of novel therapeutics, including antivirals as well as vaccines to provide primary protection. Accumulating evidence suggests that a subgroup of patients with severe Covid‐19 might have a cytokine storm syndrome. We recommend identification and treatment of such hyper inflammation using existing, approved therapies with proven safety profiles to address the immediate need to reduce the rising mortality.
In order to change the approach to patients with severe general conditions by physicians (including anesthesiologists, emergency room resuscitators, infectious disease doctors, cardiologists), a few concepts must be elaborated. The conventional treatment of patients with viral infection has been antipyretic and analgesics to treat the flu‐like symptoms and the use of antivirals, in those specific infections where specific antivirals have been identified. Virologists and pharmacologists succeeded in the development of antivirals mainly for herpesviruses 2 and HIV‐1, and more recently for HBV and HCV. 3 Acute viral infections, including seasonal influenza and measles, commonly resolve without treatment, although 1% to 2% of the cases may progress to severe respiratory and cardiac distress. So far, intubation and mechanical respiratory support have been available for acute respiratory distress syndrome (ARDS) patients waiting for a spontaneous recovery. Only for those with severe deterioration with no signs of improvement and often in the septic shock phase, were corticosteroids used as a last resort. But steroid efficacy is not consistent, ranging from highly effective 4 to a negative treatment, contributing to patient mortality, 5 so steroids are not recommended routinely for Covid‐19 cases.
A new era is emerging: patient treatment with drugs specifically targeted to precise biomolecular pathways. The cytokine storm‐related pneumonia observed in cancer patients treated with novel biotherapies (including CAR‐T cells) has opened the field to anti‐IL6R monoclonal antibodies (mAb) 6 and other molecules that act on the IL‐6/IL‐6R axis. 7 Cytokine storms have been reported also for acute syndrome associated to DNA viruses, in particular HHV‐8 or EBV virus‐associated hemophagocytic syndrome (VAHS). 8 In particular, the lung injury present in Covid‐19 represents a cytokine‐storm reaction akin to anaphylaxis that progresses to ARDS. We propose that clinicians in the front line coping with Covid‐19 should focus on this reaction and give it the urgency they would afford to traditional cases of anaphylaxis.
Physicians are more familiar with IgE‐mediated anaphylaxis, which represents the major mechanism underlying allergic anaphylaxis and is primarily mediated by histamine release (Figure 1). 9 The cytokine‐release reaction, mainly related to IL6 (besides TNF‐α and IL‐1β), represents a hypersensitivity reaction (HSR), triggered by chimeric, humanized, and human mAbs and chemotherapeutic agents, including oxaliplatin. HSR mediators (ie, IL‐6) activate monocytes, macrophages, mast cells, and other immune cells with the Fc gamma receptor (FcgR)—an essential player of many immune system effector functions, including the release of inflammatory mediators and antibody‐dependent cellular cytotoxicity. 9 Cytokine storm reactions are further characterized by activation of direct and indirect activation of the coagulation pathway. In particular the complement cascade generates anaphylatoxins, such as C3a and C5a, which bind to complement receptors resulting in the release of histamine, leukotrienes, and prostaglandins. 9 All such molecules contribute to the main symptoms such as flushing, hives, hypoxia, vasodilation, and hypotension. In patients infected with influenza A virus (eg, H5N1), the inflammatory cytokines such as IL‐1β, IL‐8, and IL‐6 play a major role in mediating and amplifying acute lung injury (ALI) and ARDS by stimulating C5a chemotaxis. The C5a induces innate immune cells including mast cells, neutrophils, and monocytes/macrophages to release proinflammatory cytokines such as IL‐12, TNF‐α, and macrophage inflammatory proteins‐1α. In addition, C5a also stimulates adaptive immune cells such as T and B cells to release cytokines such as TNF‐α, IL‐1β, IL‐6, and IL‐8. The clinical condition caused by many cytokines triggered by highly pathogenic viruses like H5N1, has been called a “cytokine storm”. Cytokines were rapidly induced at 24 hours post‐infection with H5N1. The pro‐inflammatory cytokines including IL‐1β and TNF‐α might contribute to the severity of disease by promoting maximal lung inflammation caused by H5N1 viral infection. 10 Cytokines have been also blamed for enhancing or modifying virus receptor exposure on endothelial cells lining the myocardial tissue, increasing susceptibility to H1N1 virus infection. 11 Compared to healthy volunteers, H7N9‐infected patients have significantly higher levels of cytokines such as IL‐6, IFN‐γ‐inducible protein 10 (IP‐10), IL‐10, IFN‐γ, and TNF‐α. A dangerous cytokine storm also occurs in SARS. 10 The representative SARS‐CoV ssRNAs had powerful immunostimulatory activities inducing releasing pro‐inflammatory cytokines TNF‐α, IL‐6, and IL‐12. Elevated levels of some pro‐inflammatory cytokines including monocyte chemoattractant protein‐1 (MCP‐1), transforming growth factor‐beta1 (TGF‐β1), TNF‐α, IL‐1, and IL‐6, produced by cells infected by SARS‐CoV, might cause ALI. In addition, one cytokine could induce other cytokines to further enhance the pro‐inflammatory response as was noted when elevated levels of TNF‐α induced other cytokines like IL‐6. Thus, the cytokine storm reaction plays an important role in ALI. Limited data are available on the interaction between IL‐6 and C5a. In a mouse sepsis model, IL‐6 inhibition reduced the expression of tissue C5aR. 12 More recently, studies of coronary artery disease (CAD) found that IL‐6 and complement may both contribute to the progression of cardiovascular diseases. In patients with non‐ST‐elevation myocardial infarction (NSTEMI), IL‐6 inhibition with the anti‐IL‐6R mAb (tocilizumab) reduced C5aR1 and C5aR2 in whole blood along with decrease of C‐reactive protein (CRP) and percutaneous coronary intervention (PCI)‐related troponin T (TnT) release. 13
FIGURE 1.
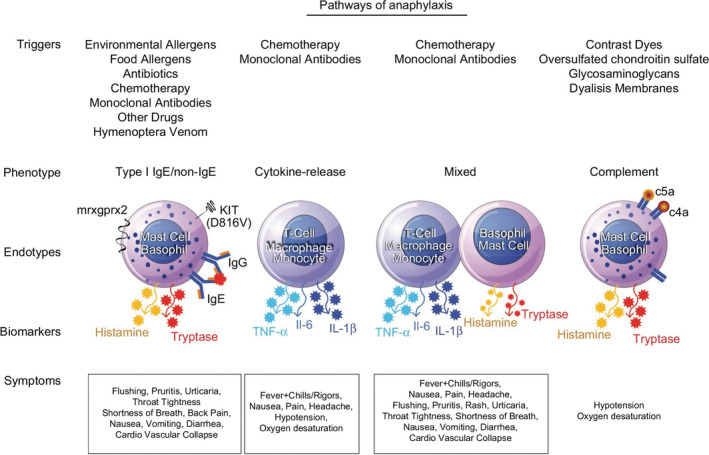
Types of anaphylaxis and the involved molecular pathways. Figure modified from Jimenez‐Rodriguez TW, Garcia‐Neuer M, Alenazy LA, Castells M. Anaphylaxis in the 21st century: phenotypes, endotypes, and biomarkers. J Asthma Allergy. 2018;11:121‐142. Published 2018 Jun 20. doi: 10.2147/JAA.S159411
In 2015, the authors of an article titled “The role of C5a in acute lung injury induced by highly pathogenic viral infections”, 10 were advocating the development of “a humanized anti‐human C5a antibody would be a potential therapeutic target for highly pathogenic viral infection‐induced acute lung injury”. Although an anti‐C5aR mAb is still in development (ie, the IPH5401 mAb, by Innate Pharma, Marseille, France), 14 given the available literature on the direct correlation between reduction of C5aR1/C5aR2 and IL6‐IL6R axis, it seems critical to use the available anti IL6R mAb. In particular, those formulations which have been already approved for clinical use with a different FDA/EMA indication could be subjected to smart repurposing. Many patients around the world may benefit if the scientific community maximizes the use of currently available and potentially life‐saving drugs when used as effective anti‐ARDS agents that also reduce cardiac stress that is present in the ongoing Covid‐19 epidemic. 15 This approach would include forming a virtual global team that include those with therapeutics experience administering mAbs to patients to reduce/interrupt cytokine storm (primarily oncologists) with clinicians on the front lines of Covid‐19 care (including intensivists, infectious diseases, and emergency physicians). Key research to be conducted during this pandemic should include controlled clinical trials of new mAbs and investigations of biomarkers that may have predictive value and guide the use of mAbs (like tocilizumab) to optimize selection of patients, monitor treatment, and enhance patient outcomes.
Clinical Syndromes
- ALI
‐ Acute Lung Injury
- ARDS
‐ Acute Respiratory Distress Syndrome
- CAD
‐ Coronary Artery Disease
- HSR
‐ HyperSensitivity Reaction
- NSTEMI
‐ non‐ST‐Elevation Myocardial Infarction
- VAHS
‐ Virus‐Associated Hemophagocytic Syndrome
Cytokine and Chemokine List
- IL‐1
‐ Interleukin‐1
- IL‐1β
‐ Interleukin 1 beta (leukocytic pyrogen)
- IL‐6
‐ Interleukin 6
- IL‐6R
‐ Interleukin 6 Receptor
- IL‐8
‐ Interleukin 8 (or chemokine [C‐X‐C motif] ligand 8, CXCL8)
- IL‐10
‐ Interleukin 10 (or human cytokine synthesis inhibitory factor ‐CSIF)
- IL‐12
‐ Interleukin 12
- IFN‐γ
‐ Interferon gamma
- IP‐10
‐ Interferon gamma inducible protein 10 kD (or CXCL10)
- TNF‐α
‐ Tumor necrosis factor‐α
- TGF‐β1
‐ Transforming growth factor beta 1
- MIP‐1α
‐ Macrophage inflammatory protein 1α (CCL3)
- MCP‐1
‐ Monocyte chemoattractant protein‐1 (CCL2)
Virus List
- EBV
‐ Epstein‐Barr virus
- H1N1
‐ Influenza A virus subtype H1N1 (1918 and 2009 flu pandemic)
- H5N1
‐ Influenza A virus subtype H5N1 (Avian)
- H7N9
‐ Influenza A virus subtype H7N9 (Avian)
- HBV
‐ Hepatitis B Virus
- HCV
‐ Hepatitis C Virus
- HHV‐8
‐ Human Herpes virus 8 (Kaposi's sarcoma‐associated herpesvirus ‐ KSHV)
- HIV‐1
‐ Human Immunodeficiency virus 1
- SARS‐CoV
‐ Severe Acute Respiratory Syndrome‐associated coronavirus (CoV‐Urbani 2003)
- SARS‐CoV‐2
‐ Severe Acute Respiratory Syndrome‐associated coronavirus‐2 (Cov‐2019)
Others
- C3a
‐ Complement molecule C3a
- C5a
‐ Complement molecule C5a
- C5aR1
‐ C5a Receptor 1 (or C5aR, CD88)
- C5aR2
‐ C5a Receptor 2 (or C5L2, GPR77)
- CAR‐T
‐ Chimeric antigen receptor T cells
- FcgR
‐ Fc gamma Receptor
- EMA
‐ European Medical Agency
- FDA
‐ U.S. Food and Drug Administration
1. CONFLICT OF INTEREST
The authors have no competing interest.
AUTHORS CONTRIBUTIONS
Franco M. Buonaguro: article decision, planning and writing; Paolo Ascierto: concept discussion; Luigi Buonaguro: literature search and reviewing; Maria Lina Tornesello: literature search and reviewing; Gene D. Morse: concept discussion and manuscript revision; Igor Puzanov: article discussion; Christian Brechot: article reviewing; Robert C. Gallo: concept discussion and article reviewing.
REFERENCES
- 1.Coronavirus COVID‐19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) Last Updated at March 19, 2020, Available from https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- 2. Field HJ, Vere Hodge RA. Recent developments in anti‐herpesvirus drugs. Br Med Bull. 2013;106:213‐249. 10.1093/bmb/ldt011. [DOI] [PubMed] [Google Scholar]
- 3. Holmes JA, Rutledge SM, Chung RT. Direct‐acting antiviral treatment for hepatitis C. Lancet. 2019;393(10179):1392‐1394. 10.1016/S0140-6736(18)32326-2. [DOI] [PubMed] [Google Scholar]
- 4. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020. 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ni Y‐N, Chen G, Sun J, Liang B‐M, Liang Z‐A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta‐analysis. Crit Care. 2019;23:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shimabukuro‐Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pennisi M, Jain T, Santomasso BD, et al. Comparing CAR T‐cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 2020;4(4):676‐686. 10.1182/bloodadvances.2019000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li CF, Ye H, Liu H, Du MQ, Chuang SS. Fatal HHV‐8‐associated hemophagocytic syndrome in an HIV‐negative immunocompetent patient with plasmablastic variant of multicentric Castleman disease (plasmablastic microlymphoma). Am J Surg Pathol. 2006;30(1):123‐127. [DOI] [PubMed] [Google Scholar]
- 9. Jimenez‐Rodriguez TW, Garcia‐Neuer M, Alenazy LA, Castells M. Anaphylaxis in the 21st century: phenotypes, endotypes, and biomarkers. J Asthma Allergy. 2018;11:121‐142. 10.2147/JAA.S159411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang R, Xiao H, Guo R, Li Y, Shen B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect. 2015;4(5):e28. 10.1038/emi.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Puzelli S, Buonaguro FM, Facchini M, et al. The surveillance group for pandemic H1N1 2009 influenza virus in Italy and the Campania H1N1 task force. Cardiac Tamponade and heart failure due to myopericarditis as a presentation of infection with the pandemic H1N1 2009 influenza A virus. J Clin Microbiol. 2010;48(6):2298‐2300. 10.1128/JCM.00418-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riedemann NC, Neff TA, Guo R‐F, et al. Protective effects of IL‐6 blockade in sepsis are linked to reduced C5a receptor expression. J Immunol. 2003;170:503‐507. 10.4049/jimmunol.170.1.503. [DOI] [PubMed] [Google Scholar]
- 13. Orrem HL, Nilsson PH, Pischke SE, et al. IL‐6 receptor inhibition by Tocilizumab attenuated expression of C5a receptor 1 and 2 in non‐ST‐elevation myocardial infarction. Front Immunol. 2018;9:2035. 10.3389/fimmu.2018.02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. https://www.innate-pharma.com/en/pipeline/iph5401-first-class-anti-c5ar-mab
- 15. Zhou F, Yu T, Ronghui D, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


