To the Editor:
Coronavirus 2019 (COVID‐19), the disease caused by SARS‐CoV‐2, typically causes mild disease and rarely leads to hospitalization in children. 1 , 2 , 3 , 4 While multiple reports document comparatively severe disease from COVID‐19 among adult oncology patients as compared to those without cancer, 5 , 6 the impact of SARS‐CoV‐2 infection in children with cancer is poorly understood. Reports from China have described eight severe pediatric cases, including one in a child with cancer. 7 The United States has reported 15 pediatric patients requiring intensive care including three pediatric deaths, but it is unclear how COVID‐19 contributed to their deaths. 1 Clinicians from Italy described five cases of COVID‐19 among pediatric oncology patients, none of them requiring intensive care. 8 , 9
Our hospital has cared for 17 children with COVID‐19 including two with underlying malignancy. Both cancer patients, presented here, have had severe disease requiring intensive care.
A 16‐year‐old African American female with body mass index (BMI) of 46 kg/m2, with intermediate‐risk orbital alveolar rhabdomyosarcoma, underwent routine surveillance imaging during week 10 chemotherapy with vincristine and daily irinotecan. An [F18]‐fluorodeoxy‐D‐glucose‐positron emission tomography and computerized tomography (PET‐CT) scan demonstrated bilateral ground glass and consolidative opacities in her lower lung lobes “indistinguishable from COVID‐19 disease” (Figure 1). Upon further questioning, she reported mild exertional dyspnea and dysgeusia during the previous week. She was admitted for observation and tested positive for SARS‐CoV‐2 by reverse‐transcriptase PCR (RT‐PCR).
FIGURE 1.
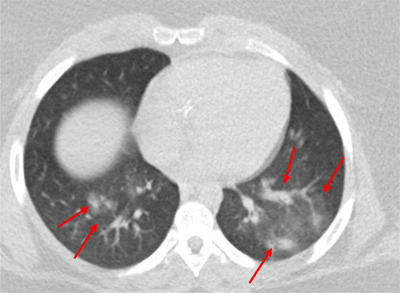
Chest CT imaging of patient 1 on hospital day 0 showed scattered ground glass and consolidative opacities (arrow) in bilateral lower lobes. CT, computerized tomography
Initially, she was clinically stable with ongoing vomiting and diarrhea. Fever developed on hospital day (HD) 3. On admission, she received prophylactic anticoagulation 10 and oxygen as needed for comfort. However, on HD 7, she clinically deteriorated with increased work of breathing, hypoxia, and fatigue necessitating transfer to the intensive care unit (ICU) for support with heated high‐flow nasal cannula (HFNC). Hydroxychloroquine (400 mg twice daily for two doses followed by 200 mg twice daily) was initiated on HD 6 as a bridge to compassionate use of remdesivir (100 mg daily for five doses; dosing limited by elevated alanine transferase), which started on HD 10. Hydroxychloroquine was subsequently discontinued. An increase in her corrected QT interval from 416 to 440 was noted on daily electrocardiogram while on hydroxychloroquine, but she did not experience any arrythmias.
Additionally, rising IL‐6 levels (15 pg/mL) and impending respiratory failure prompted use of tocilizumab (800 mg daily on HD 7 and 8). 11 , 12 Her fever curve and C‐reactive protein subsequently improved, but she remained febrile and required high levels of oxygen support until initiation of remdesivir (Figure 2A). She was weaned to room air and transferred out of the ICU on HD 14.
FIGURE 2.
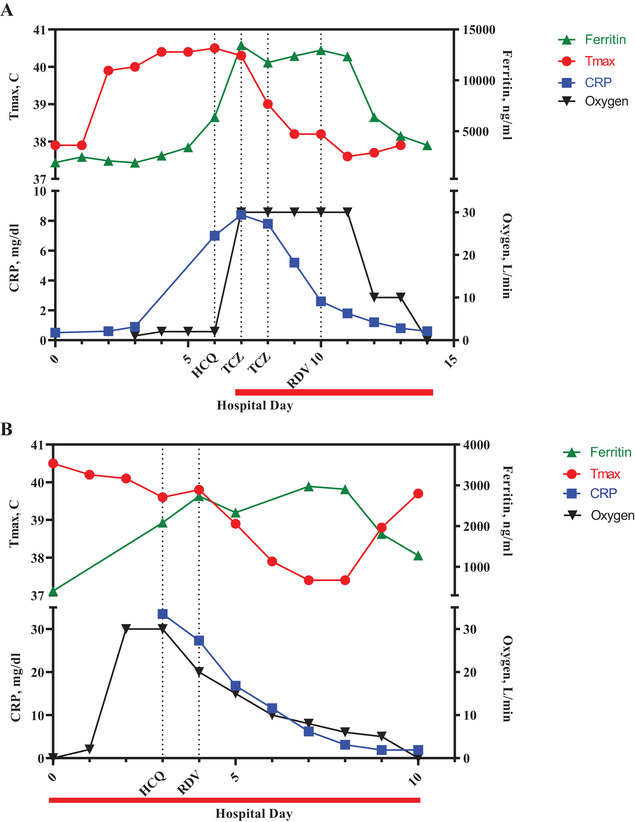
A, Trends of ferritin (green), maximum daily temperature (red), C‐reactive protein (blue), and supplemental oxygen (black) of patient 1. Hydroxychloroquine was started on hospital day 6 (HD 6), tocilizumab was given on HD 7 and 8, and remdesivir was started on HD 10. Patient 1 ICU course was from HD 7 to HD 14 (red bar). B, Trends of ferritin (green), maximum daily temperature (red), C‐reactive protein (blue), and supplemental oxygen (black) of patient 2. Hydroxychloroquine was started on HD 3 and remdesivir was started on HD 4. Patient 2 ICU course was from HD 0 to HD 10 (red bar). T max, maximum daily temperature in degrees Celsius; CRP, C‐reactive protein; HCQ, hydroxychloroquine; TCZ, tocilizumab; RDV, remdesivir
A Caucasian 16‐year‐old male with BMI of 29 kg/m2 presented acutely with fever and gingival bleeding 19 months following completion of chemotherapy without radiation for intermediate‐risk Hodgkin lymphoma. Peripheral blood analysis revealed severe hyperleukocytosis (white blood cell count 178 270 cells/µL), thrombocytopenia, and coagulopathy and confirmed a diagnosis of therapy‐related acute myeloid leukemia (tAML). Chest X‐ray demonstrated bibasilar infiltrates. RT‐PCR confirmed infection with SARS‐CoV‐2. He was admitted to the ICU and started on hydroxyurea, where he quickly became hypoxic, requiring a maximum of 30 L by HFNC despite limiting intravenous fluids. Hydroxychloroquine (400 mg once) was initiated on HD 3 while awaiting compassionate use remdesivir, which started 12 h later (200 mg for dose one followed by 100 mg daily for nine doses). No cardiac complications of hydroxychloroquine were noted.
To avoid inducing prolonged pancytopenia typically associated with tAML therapy in the setting of infection with SARS‐CoV‐2, low‐dose cytarabine was added to hydroxyurea on HD 3, leading to a rapid decrease in peripheral blood leukemia burden on HD 5. The patient also received prophylactic anticoagulation along with fresh‐frozen plasma to counteract coagulopathy. 10 Inflammatory markers, fever curve, and oxygen requirement improved following both leukemia‐directed and antiviral therapy (Figure 2B). Transfer from the ICU occurred on HD 10.
While Georgia only represents approximately 2.5% of nationwide COVID‐19 cases, 13 , 14 we have seen multiple cases of severe COVID‐19. The Aflac Cancer and Blood Disorders Center is a large pediatric oncology practice with over 450 newly diagnosed patients annually. In contrast with published reports, 4 the pediatric cancer patients with COVID‐19 described here exhibited severe respiratory distress and significant hyperinflammation requiring intensive care.
Early evidence from China points to older age and underlying medical conditions as risk factors for severe COVID‐19. 15 Obesity, an established risk factor for severe disease in influenza patients, 16 also carries an increased risk of hospitalization for COVID‐19. 17 In an analysis of patients under age 60 years, obese patients were 1.8 and 3.6 times more likely to require intensive care for COVID‐19 with BMI 30‐34 kg/m2 or ≥35 kg/m2, respectively. 18 Our patients’ obesity may have likewise contributed to their development of severe COVID‐19.
Due to the critical illness of both patients, we initiated SARS‐CoV‐2‐directed treatment despite limited evidence in adults and a paucity of evidence in children. Recent adult data on compassionate use remdesivir demonstrate improvement in 68% of patients, consistent with our findings. 19 These parallel experiences support the inclusion of adolescents on trials evaluating antiviral effectiveness, as is commonly done in early‐phase oncology developmental therapeutic trials.
While inflammatory markers were elevated in both patients, patient 1 experienced a more severe cytokine storm despite ongoing chemotherapy, prompting use of tocilizumab. Her respiratory status subsequently stabilized and her fever and inflammatory markers declined consistent with suppression of IL‐6, 20 followed by further improvement with remdesivir. It is unclear which intervention was most successful, but clinical improvement in COVID‐19 disease following tocilizumab is consistent with published adult data. 21
Pediatric oncology specialists have published recommendations for providing care to children undergoing cancer treatment during the COVID‐19 pandemic. 8 , 22 They urge continuation of standard aggressive chemotherapy given the curable nature of most pediatric cancers and current evidence, suggesting milder COVID‐19 disease courses in children. Adult oncologists recognize the need for a more nuanced approach balancing the likelihood of oncologic cure, expected oncologic disease course, and risk of severe COVID‐19. 23 For patient 2, we encountered difficulty determining whether hyperleukocytosis or COVID‐19 contributed more to his respiratory impairment; however, given his critical illness, we ultimately modified his induction chemotherapy in line with the adult approach.
Published guidelines recommend minimizing exposure of COVID‐19 to pediatric oncology patients. 8 , 22 Although we applaud the efforts of our colleagues 8 to create COVID‐19‐free facilities to protect our vulnerable patient population from this disease, we question the practical implementation of this approach given the prevalence of asymptomatic COVID‐19 24 and overlapping symptoms of COVID‐19 with chemotherapy side effects such as anemia, nausea, and dysgeusia. 25 The minimal initial symptoms in patient 1 were attributed to her daily outpatient chemotherapy, leading to multiple personnel exposures in our clinic. Following this patient encounter, our hospital has implemented universal masking and eye protection in line with updated guidelines. 14 Alternatively, testing for SARS‐CoV‐2 prior to scheduled chemotherapy could prevent exposures but is challenging given limited testing availability in our country.
Pediatric oncologists should recognize the unappreciated risk of severe COVID‐19 in our patient population, particularly among obese teenagers. Based on our observations, we recommend monitoring inflammatory markers, initiation of prophylactic anticoagulation on admission, and consideration of remdesivir and/or tocilizumab should a patient with pediatric cancer develop severe COVID‐19.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ACKNOWLEDGMENTS
We wish to thank Bradley Rostad, Daniel Wechsler, Marianne Yee, William Stokes, Kathryn Sutton, Michael Briones as well as the ICU and infectious disease physicians involved in these children's care.
REFERENCES
- 1. CDC COVID‐19 Response Team . Coronavirus disease 2019 in children ‐ United States, February 12‐April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz AT, Zeichner SL. COVID‐19 in children: initial characterization of the pediatric disease. Pediatrics. 2020;. 145(5):e20200834. [DOI] [PubMed] [Google Scholar]
- 3. Liu W, Zhang Q, Chen J, et al. Detection of COVID‐19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382(14):1370‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020. 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 7. Sun D, Li H, Lu XX, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020. 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouffet E, Challinor J, Sullivan M, Biondi A, Rodriguez‐Galindo C, Pritchard‐Jones K. Early advice on managing children with cancer during the COVID‐19 pandemic and a call for sharing experiences. Pediatr Blood Cancer. 2020. 10.1002/pbc.28327 [DOI] [PubMed] [Google Scholar]
- 9. Balduzzi A, Brivio E, Rovelli A, et al. Lessons after the early management of the COVID‐19 outbreak in a pediatric transplant and hemato‐oncology center embedded within a COVID‐19 dedicated hospital in Lombardia, Italy. Estote parati. Bone Marrow Transplant. 2020. 10.1038/s41409-020-0895-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(5):1023‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci USA 2020. 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Georgia Department of Public Health . https://dph.georgia.gov/covid-19-daily-status-report. Accessed April 13, 2020.
- 14. Centers for Disease Control and Prevention . https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed April 15, 2020.
- 15. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luzi L, Radaelli MG. Influenza and obesity: its odd relationship and the lessons for COVID‐19 pandemic. Acta Diabetol. 2020. 10.1007/s00592-020-01522-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019—COVID‐NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid‐19 hospital admission. Clin Infect Dis. 2020. 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID‐19. N Engl J Med. 2020. 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin‐6 (IL‐6) and soluble IL‐6 receptor after administration of an anti–IL‐6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112(10):3959‐3964. [DOI] [PubMed] [Google Scholar]
- 21. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020. 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kotecha RS. Challenges posed by COVID‐19 to children with cancer. Lancet Oncol. 2020;21(5):e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schrag D, Hershman DL, Basch E. Oncology practice during the COVID‐19 pandemic. JAMA. 2020. 10.1001/jama.2020.6236. [DOI] [PubMed] [Google Scholar]
- 24. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA. 2020;323(14):1406‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hovan AJ, Williams PM, Stevenson‐Moore P, et al. A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer. 2010;18(8):1081‐1087. [DOI] [PubMed] [Google Scholar]


