Introduction
Presently, the exact dermatologic manifestations of COVID‐19 infection, its characteristics, prevalence, temporal relationship to other clinical findings, and severity of underlying illness are still being evaluated. The first report on such skin lesions was published by Recalcarti et al. 1 who described skin rashes in 18 (20.4%) of 148 observed patients with COVID‐19. Eight patients developed cutaneous involvement at the onset and 10 patients after hospitalization. Cutaneous manifestations included erythematous rash in 14 patients, widespread urticaria in three patients, and chickenpox‐like vesicles in one patient. The trunk was the most involved region. Pruritus was negligible, and lesions typically healed in several days. No correlation with disease severity was clearly observed.
According to Su and Lee, differentiating the skin lesions related to COVID‐19 from other infectious exanthems and dermatoses can be challenging. 2 They excluded 60 of 148 patients from their study as they were taking new medications prior to developing symptoms of COVID‐19 infection, indicating that a drug allergic reaction could not be excluded as a cause of their dermatologic presentation. A total of 20.4% of patients included in the study were diagnosed with erythematous rash, diffuse urticaria, and chickenpox‐like vesicular rashes.
Guan W, Ni Z., Hu Yu et al. 3 reported a very low incidence of skin manifestations in Chinese patients with COVID‐19, where it was observed in 0.2% of the patients. This study does not provide a detailed description of the skin lesions nor the criteria for its diagnosis.
According to Estébanez et al., erythema and urticaria may be some of the first symptoms of severe COVID‐19 infection. The described 28‐year‐old woman, 13 days after laboratory diagnosis, developed pruritic lesions of the heels described as confluent “erythematous‐yellowish” papules; 3 days later, they appeared as pruritic, hardened, erythematous plaques. The clinical differential diagnosis included urticaria, urticarial vasculitis, idiopathic plantar hidradenitis, and neutrophilic dermatosis. No biopsy was obtained, and the author did not specify whether the skin lesions could have represented Cutaneous Adverse Drug Reactions (CADR). 4
Iviensan et al. 5 described the occurrence of livedo reticularis in two patients with COVID‐19 infection. In the first patient, livedo appeared on the 7th day of illness and regressed after 19 hours. The appearance of the rash was accompanied by weakness and hematuria. Hematuria resolved within 1 day. In the second patient, livedo appeared 10 days after laboratory confirmation of COVID‐19, and by that time, the patient had already recovered. In both cases, the rash was located unilaterally.
Joob and Wiwanitkit reported the appearance of a petechial rash and thrombocytopenia in a patient infected with COVID‐19 at the onset of the disease that preceded the development of respiratory symptoms. 6
Materials and methods
We have asked physicians of Moscow City Hospital #40, which has been exclusively dedicated to treating COVID‐19 infection, to screen their patients for any skin manifestations and to immediately consult us when such manifestations became apparent. After obtaining informed consent, we subsequently directly or indirectly visited the patients who were identified as having dermatologic conditions potentially related to COVID‐19 and, where possible, performed interviews with specific attention to the onset of skin eruptions and their temporal relationship to systemic symptoms and to initiation of new medications. We then excluded from the analysis the patients who (i) had longstanding skin manifestations that preceded the typical symptoms of COVID‐19 infection by at least 14 days, or (ii) had been initiated on any new medications within 14 days preceding the onset of skin eruptions. Out of 78 referred patients, we excluded 63 patients which left us with 12 patients whose skin manifestations were likely triggered by viral infection and selected three patients with overlapping dermatologic findings where CADR could not be fully excluded.
Case #1
A 92‐year‐old female patient with moderate COVID‐19 infection presented with papulonecrotic cutaneous vasculitis (Fig. 1a,b). Papular and papular‐pustular elements appeared upon admission to the hospital and coincided with the diagnosis of pneumonia. Further evolution of morphological elements led to the formation of papulonecrotic rash, including the development of hemorrhagic crusts.
Figure 1.
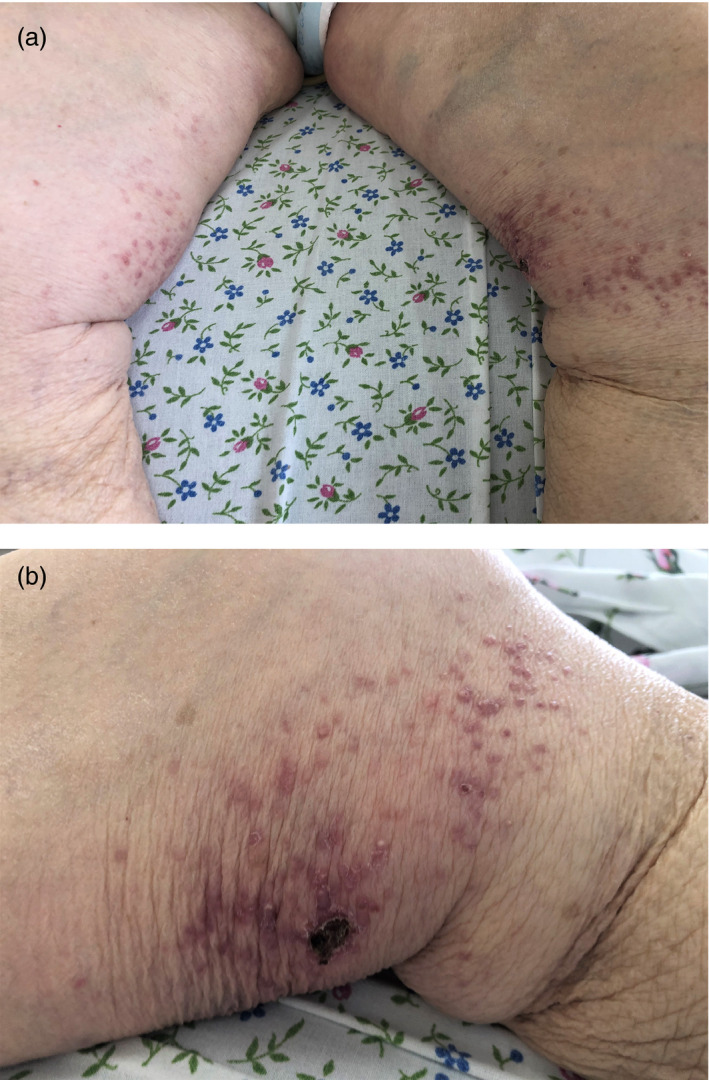
(a and b) Papulonecrotic skin vasculitis in a 92‐year‐old female patient
Case #2
A 74‐year‐old male patient with COVID‐19 pneumonia was diagnosed with polymorphic cutaneous vasculitis (Fig. 2), which was present on admission. Its progression coincided with an increase in the severity of illness. The skin manifestations started to subside as the patient started to improve.
Figure 2.
Polymorphic cutaneous vasculitis in a 74‐year‐old male patient with COVID‐19 pneumonia
Case #3
A 59‐year‐old male patient presented with fever of 39°C and a rash of the lower extremities in combination with hemorrhagic elements which were consistent with polymorphic cutaneous vasculitis (Fig. 3a,b). The patient rapidly deteriorated and was admitted to the intensive care unit and placed on mechanical ventilation. CT of the chest showed ARDS with 75% involvement of the lung parenchyma.
Figure 3.
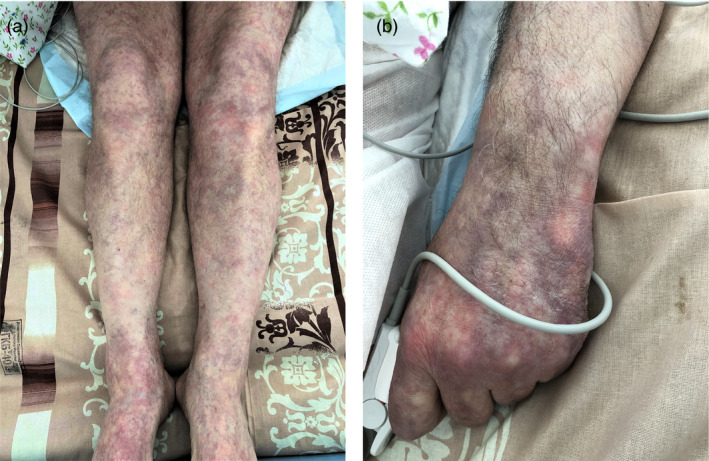
(a and b) Polymorphic cutaneous vasculitis in a 59‐year‐old male patient with severe COVID‐19 disease
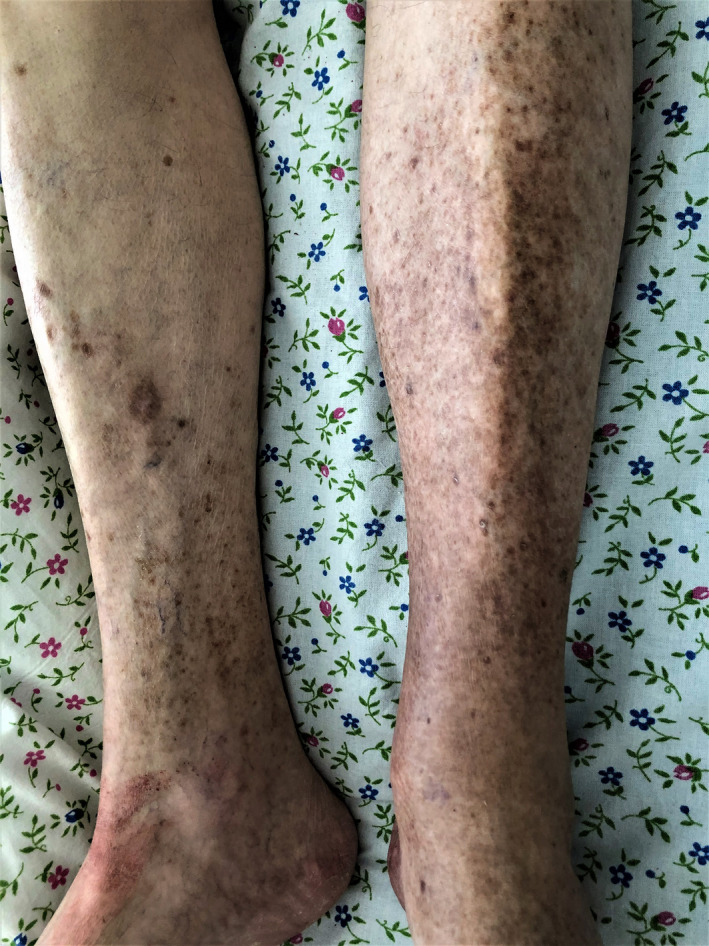
Case #4
An 80‐year‐old male patient was noted to have a dense petechial and ecchymotic rash on the abdomen, hands, and lower extremities (Fig. 4a–c). Skin lesions developed in the first week of COVID‐19 infection while receiving concomitant therapy with hydroxychloroquine. Administration of systemic glucocorticosteroids led to a rapid regression of the skin lesions. Despite the use of concomitant medication, given the compelling clinical characteristics, we suspect that the patient's hemorrhagic vasculitis was likely triggered by infection.
Figure 4.
Hemorrhagic vasculitis in an 80‐year‐old male patient on the skin of the abdomen (a), hands (b), and lower extremities (c)
Case #5
A 63‐year‐old male patient with a COVID‐19 infection developed painful hyperemic pernio‐like lesions which formed over the first MTP joints (Fig. 5). Of note, the patient had no history of gout, and his uric acid was within normal limits.
Figure 5.
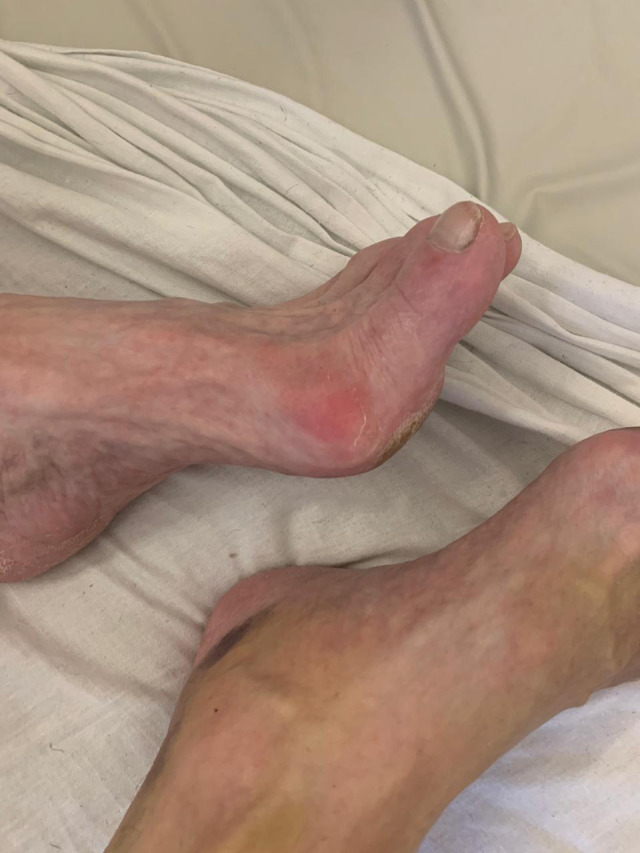
Acrodermatitis (pernio‐like lesions) in a 63‐year‐old male patient
Case #6
A 58‐year‐old male patient with severe COVID‐19 infection requiring mechanical ventilation developed a skin rash during early treatment that correlated with the severity of the underlying disease. Spotted elements of bright pink color were densely scattered at the ankles and on the dorsal surfaces of the feet and toes (Fig. 6). The rash developed before any new medications were initiated.
Figure 6.
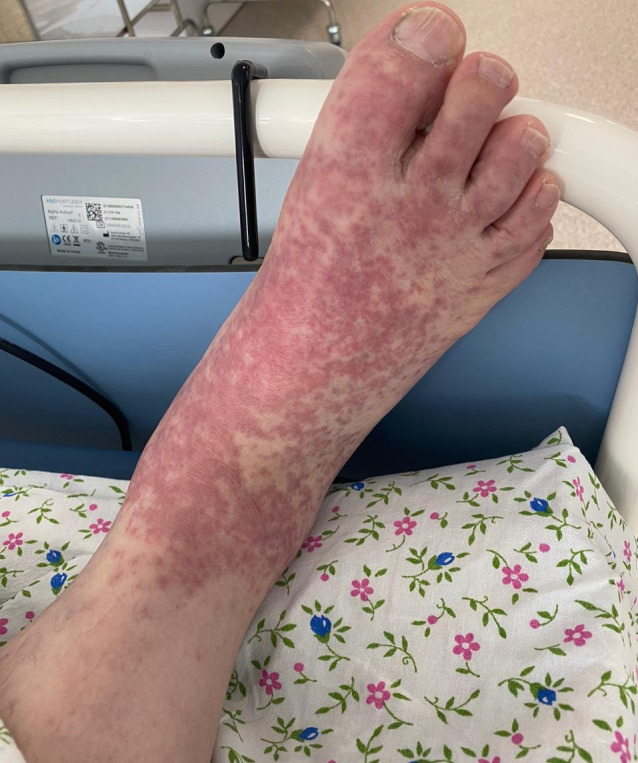
Erythematous rash on the feet of a 58‐year‐old male patient
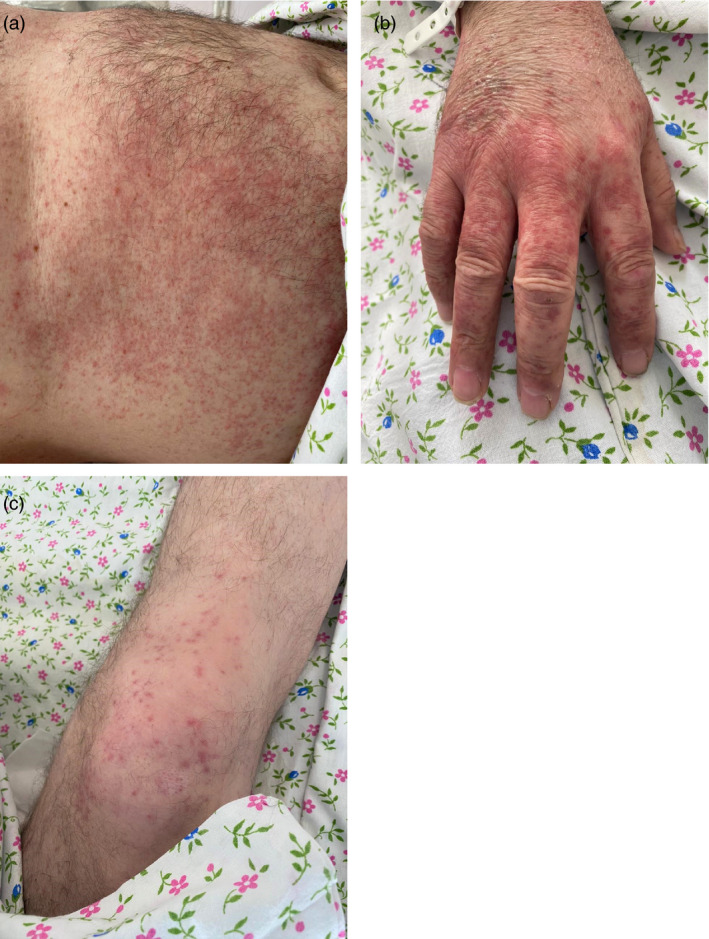
Case #7
A 47‐year‐old female patient with COVID‐19 pneumonia developed acral urticarial highly pruritic rash on upper and lower extremities 5 days after the laboratory diagnosis (Fig. 7a,b). The patient received oral glucocorticosteroids with quick resolution of the rash.
Figure 7.
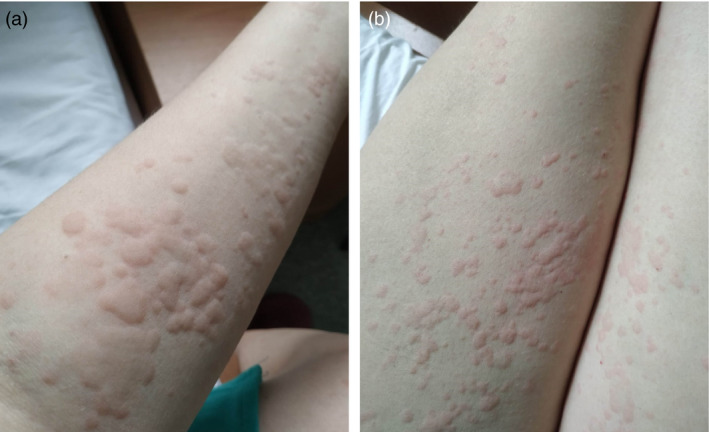
Urticarial rash of acral distribution in a 47‐year‐old female patient with involvement of upper (a) and lower (b) extremities
Case #8
A 71‐year‐old male patient, 2 weeks prior to our examination, developed papulovesicular eruptions while having escalating clinical symptoms of COVID‐19 infection (fever, weakness, cough, shortness of breath) (Fig. 8a,b). From the history, it is also known that papular and papulovesicular rashes occurred before hospitalization and treatment, which excludes CADR and indicates its correlation with the onset of COVID‐19 infection. The patient himself associated the skin eruption with profuse sweating. On exam abundant truncal rash was observed involving the chest, abdomen, back, and flanks, consisting of papules with surface erosions (as a result of destruction of the vesicular elements), as well as single remaining vesicles still present in these locations. During the patient's hospital stay, an improvement with cessation of the appearance of new lesions and a partial involution of the rash was observed.
Figure 8.
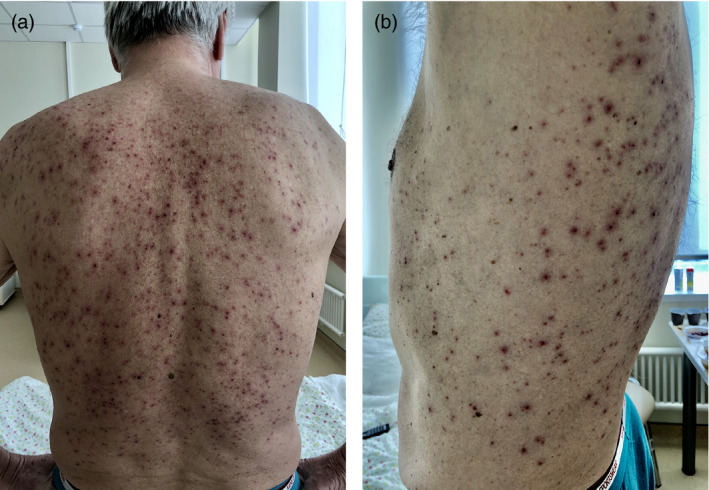
Papular and papulovesicular rash in a 71‐year‐old male patient on the back (a) and flanks (b)
Case #9
A 46‐year‐old female patient with confirmed COVID‐19 infection and bilateral pneumonia presented with papulovesicular elements in the chest area (Fig. 9). The appearance of a rash was noted after an increase in body temperature, accompanied by sweating.
Figure 9.
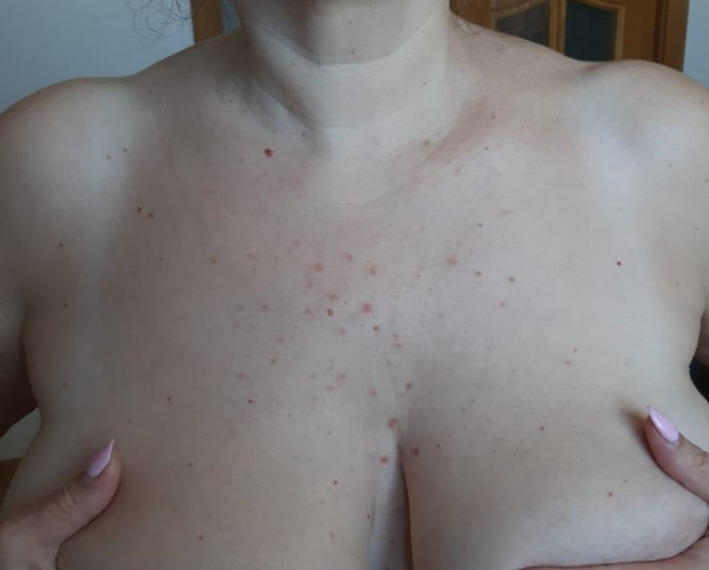
Papular and papulovesicular rash in a 46‐year‐old female patient
Case #10
A 49‐year‐old female patient with moderate COVID‐19 pneumonia developed papulosquamous rash 3 days prior to the development of other clinical symptoms of infection (Fig 10). Pityriasis rosea was suspected despite the absence of the herald patch. She received supportive treatment and showed significant improvement in both respiratory and dermatologic symptoms starting on day 5 of hospitalization.
Figure 10.
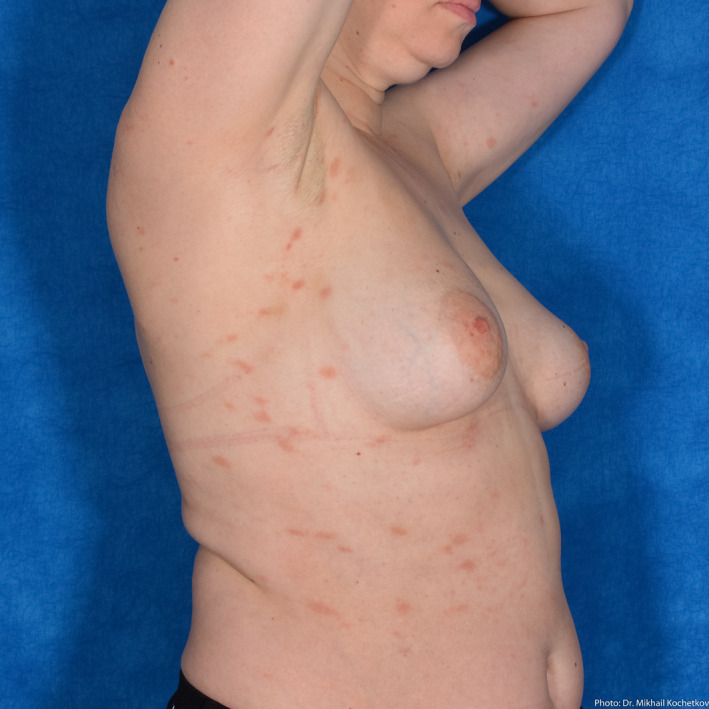
Pityriasis rosea‐like presentation in a 49‐year‐old female patient
Case #11
A 53‐year‐old woman presented with disseminated pink‐red maculopapular rash resembling that of measles that developed on day 7 of the disease, involving the trunk and upper and lower extremities (Fig. 11).
Figure 11.
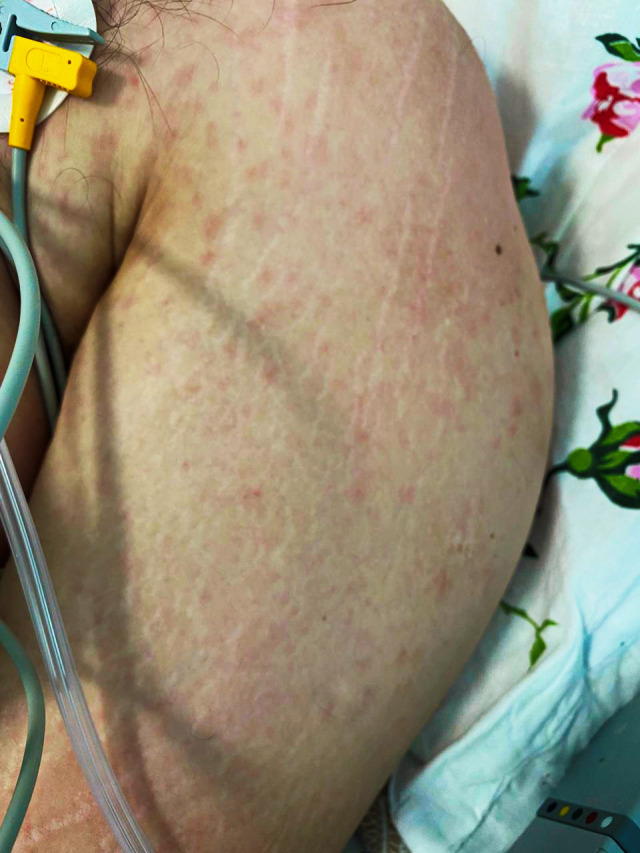
Measles‐like maculopapular rash in a 53‐year‐old patient
Case #12
An 83‐year‐old patient was admitted to the hospital in serious condition with 75% parenchymal pulmonary involvement. While receiving hydroxychloroquine and azithromycin, an extensive skin rash developed that involved the entire trunk with a transition to the shoulders and buttocks (Fig. 12a–c). The rash consisted of erythematous spots of bluish‐pink color, merging into extensive plaques. Some of the elements were ring shaped, which made the skin lesion resemble exudative erythema multiforme. The administration of parenteral glucocorticosteroids quickly led to regression of the rash.
Figure 12.
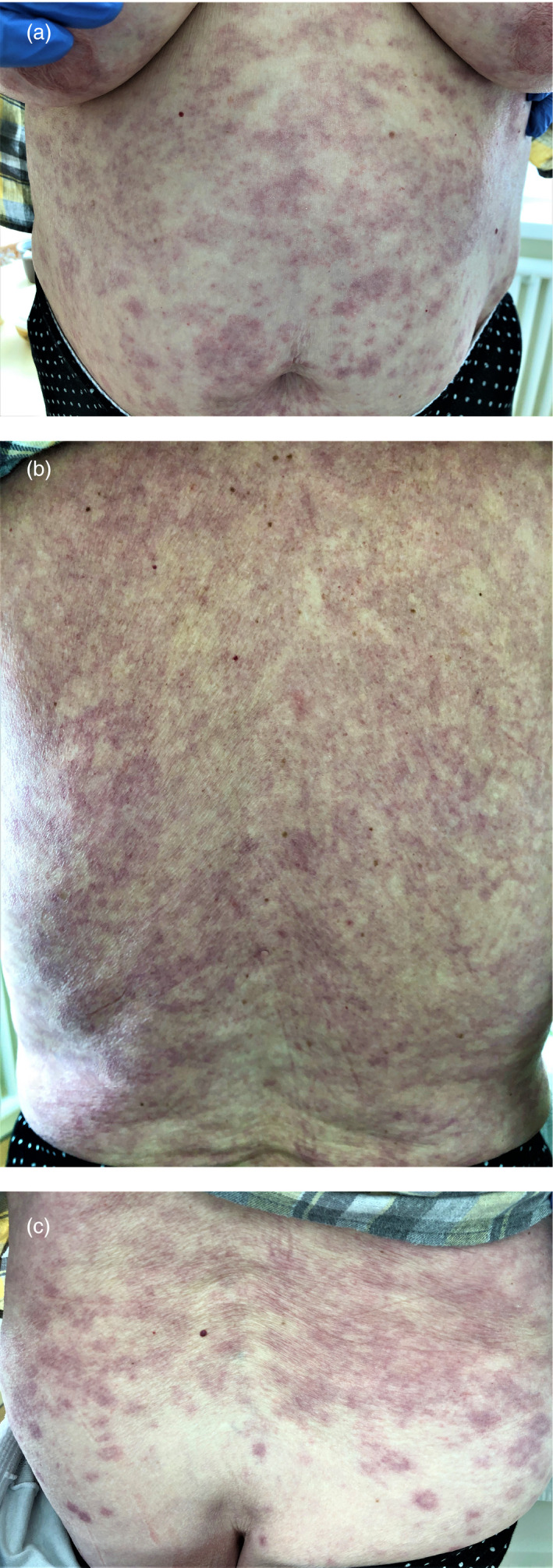
Probable CADR in an 83‐year‐old patient with elements resembling exudative erythema multiforme
Case #13
A 56‐year‐old male patient with COVID‐19 infection presented with skin lesions, also called “bilateral inguinal purple rash.” Large pink–red plaques were observed in both inguinal folds with a transition to the inner thighs, with a brownish tint and abundance of follicular papules along the periphery (Fig. 13). The patient was receiving hydroxychloroquine, ceftriaxone, and azithromycin. Rashes in the inguinal areas appeared during treatment, gradually spreading via formation of follicular papules along the periphery of the foci, and underwent regression when systemic corticosteroids were administered.
Figure 13.
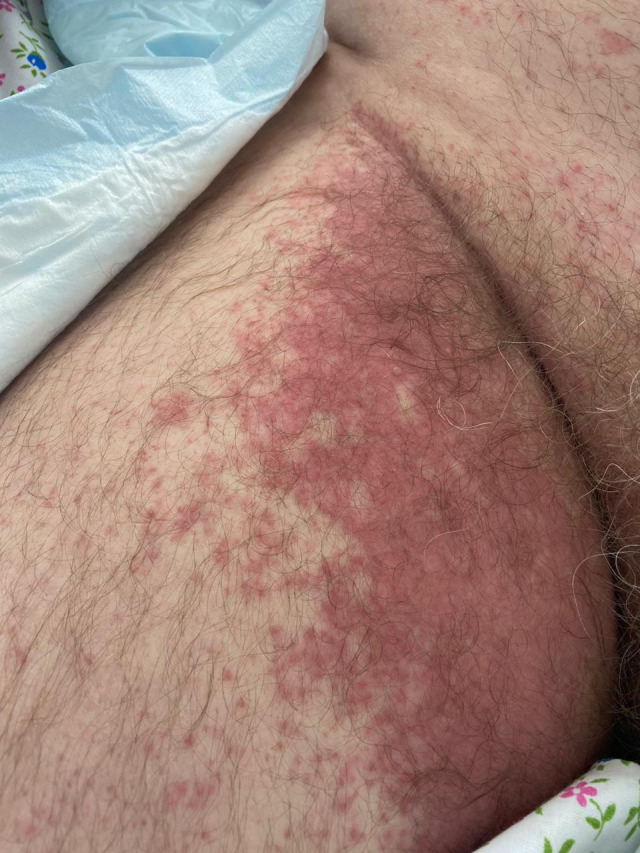
Bilateral inguinal purple rash in a 56‐year‐old male patient
Case #14
A 60‐year‐old female patient with COVID‐19 infection presented with large purple plaques on the abdomen with umbilical sparing with numerous thick papules along the periphery. Skin lesions appeared several days after the start of combined antiviral and antibacterial therapy for pneumonia, and in the second week of treatment, disseminated erythematic papules appeared on the breasts, chest, abdomen, and hips (Fig. 14).
Figure 14.
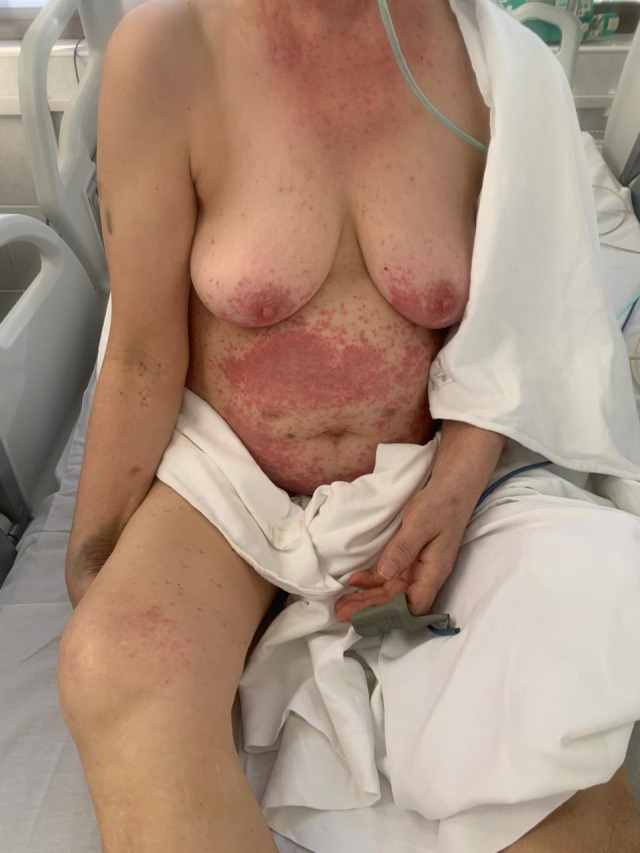
Disseminated purple rash in a 60‐year‐old female patient
Case #15
A 55‐year‐old male patient with COVID‐19 pneumonia developed large bright red foci on the extensor surfaces of both upper extremities, which subsequently became pale after the addition of systemic glucocorticosteroids to the treatment regimen (Fig. 15). At the time of the onset of skin rash, the patient was not receiving any concomitant therapy.
Figure 15.
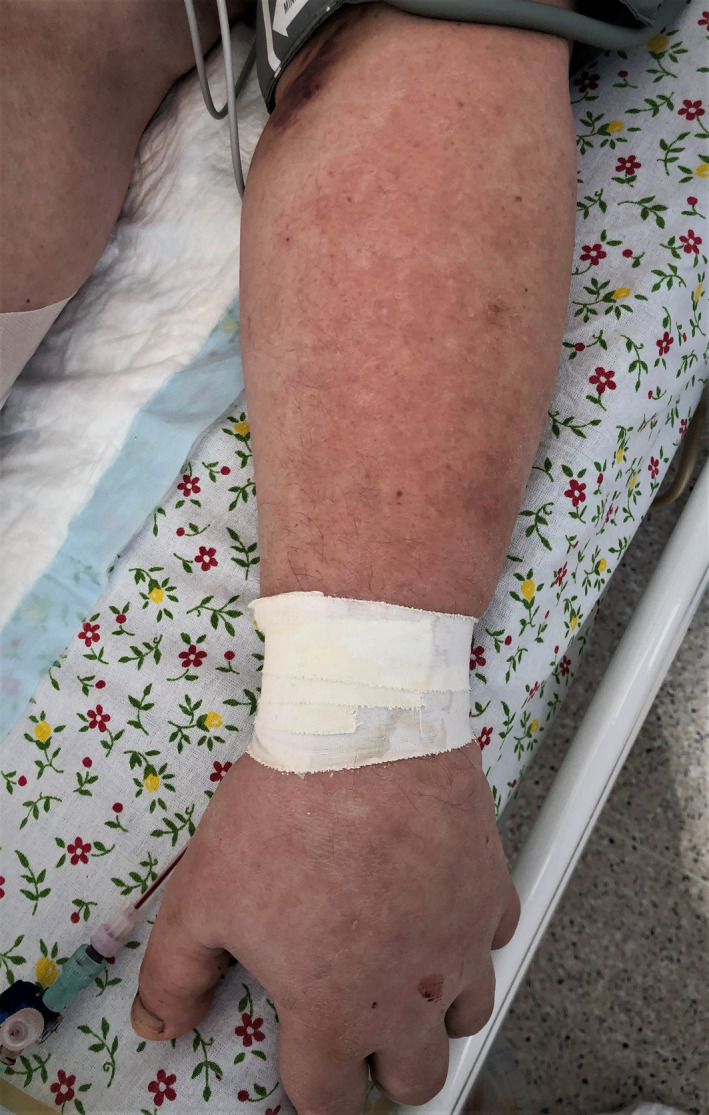
Posteruptive erythematous rash of the upper limbs in a 55‐year‐old male patient
Discussion
Given that millions of patients have been infected with COVID‐19 and no associated pathognomonic dermatologic phenomenon has been identified, it is unlikely that one exists. Nevertheless, literature review of documented dermatologic manifestations patients with COVID‐19 infection, as well as our own experience of the ongoing dynamic observations of the patients suffering from this devastating viral illness, allows us to conclude that skin manifestations are sometimes the first manifestations of COVID‐19 infection. Thus, while the data on dermatologic involvement in COVID‐19 infection continue to accumulate, it is important to try and categorize the already documented findings.
We propose that the described dermatoses and skin rashes may be divided into six groups depending on their etiology and pathogenesis:
Group 1 – Cutaneous vasculitides
Margo et al. 7 examined skin from three patients with severe COVID‐19 characterized by purpuric skin rash. The purpuric skin lesions showed a pauci‐inflammatory thrombogenic vasculopathy, with deposition of C5b‐9 and C4d in both grossly involved, and normal‐appearing skin. In addition, there was colocalization of COVID‐19 spike glycoproteins with C4d and C5b‐9 in the interalveolar septa and the cutaneous microvasculature of two cases examined. They concluded that at least a subset of patients with sustained, severe COVID‐19 may define a type of catastrophic microvascular injury syndrome mediated by activation of complement pathways and an associated procoagulant state.
Thus, there is a likely causal relationship with COVID‐19 infection, as a result of the damage of the small vessels of the dermis by circulating immune complexes, activation of complement pathways, and procoagulant milieu. Tissue hypoxemia may also be contributing. Acrovasculitis and acronecrosis are some of the forms with COVID‐19 induced vasculitis.
Group 2 – Papulosquamous rashes and pityriasis rosea
These are infectious and/or allergic skin lesions associated with COVID‐19 infection. A clinical feature of pityriasis rosea presentation in COVID‐19 infection is the possible absence of a “herald patch.” Sanchez et al. 8 suggested that most patients with severe cases of COVID‐19 show a high level of proinflammatory cytokines and infection‐related biomarkers, and thus skin manifestations may represent body's immune response to the virus. It is noteworthy that the rashes observed by the authors differed from classical pityriasis rosea with the absence of the initial herald patch. We have presented a similar case where no herald patch could be identified. Thus, this peculiar form can be classified as paraviral dermatosis, and practitioners should be aware of this new clinical association. At the same time, the Spanish atlas, which includes 375 observations, presents a significant number of observations of typical pityriasis rosea cases in COVID‐19‐infected patients. 9
Group 3 – Measles‐like rashes
In the case of COVID‐19 infection, the clinical characteristics of the rashes resemble measles rash and, thus, indicate a pathogenetic proximity to other viral exanthems. Hunt and Koziatek reported a case of COVID‐19 infection in a 20‐year‐old patient that manifested with fever and a diffuse measles‐like rash of the trunk and extremities, excluding the face. 10 The rashes were patchy‐papular in nature, accompanied by pain, but without pruritis, which, according to the authors, is consistent with the characteristics of a viral exanthem. Avellana et al. 11 described the occurrence of a measles‐like rash in a 32‐year‐old patient with COVID‐19 on the sixth day from the onset of the disease with typical clinical symptoms of fever, dry cough, myalgia, and fatigue. Common petechial and maculopapular eruptions with an erythematous base were accompanied by pruritis and were localized to the scalp, face, neck, chest, abdomen, buttocks, and limbs, including the palms and soles. The clinical course was characterized by worsening pruritus and regression of erythema intensity. Peeling was noted on day 4, and rashes subsequently dissipated. Amatore et al. 12 described the manifestation of COVID‐19 infection in a 39‐year‐old patient as a single clinical symptom of “febrile” rash. Rashes were clinically characterized by erythematous edematous papules and ring‐shaped plaques, which were localized on the skin of the upper extremities, chest, neck, abdomen, and palms, without involvement of the face or of mucous membranes. Histologically, the changes were nonspecific and similar to those of viral exanthems: superficial perivascular lymphocytic infiltrates without eosinophils, edema of the papillary dermis, spongiosis, lymphocyte exocytosis, and single sites of dyskeratosis in the basal layer were detected. The patient that we presented has very similar dermatologic findings.
Group 4 – Papulovesicular rashes
These rashes occur in the presence of elevated body temperatures and consequential excessive sweating. Unlike the classic presentation of miliaria rubra (heat rash), which are usually local, these rashes may have wider anatomical distribution. The widely cited data by Casas et al. 9 indicate that vesicular eruptions with COVID‐19 infection occur in 9% of examined patients with skin manifestations. Moreover, according to the authors, the predominant localization is truncal. Marzano A.V. et al. 13 presented data on 22 patients with COVID‐19 infection who had papulovesicular eruptions similar to those of chickenpox. Our observations confirm the above clinical features of skin eruption with COVID‐19 infection.
Group 5 – Urticarial rash
Depending on its origin, the etiology can be twofold. On the one hand, urticarial eruptions may be a precursor of the onset of COVID‐19 infection or occur along with its first symptoms. On the other hand, urticaria often develops because of drug intolerance and, in this case, refers to one of the clinical manifestations of CADR. In our case, the acral arrangement of the blisters suggests viral etiology.
Group 6 – CADR
Drug rashes are not directly associated with COVID‐19 infection and are the result of individual patients' intolerance to medications. Customarily, antibacterial agents are the most common offenders. The above clinical examples of skin rashes that developed on the background of COVID‐19 infection do not exclude the possibility of CADR to antiviral, antibacterial, and other drugs.
Spanish researchers highlighted the so‐called “bilateral axillary purple rash” as a separate group of skin manifestations of COVID‐19 infection. 9 However, in our opinion, its characteristic appearance and localization is most likely a manifestation of CADR. In our patient, the course of the infection and while receiving concomitant therapy, further spread of the rash took place, going beyond the inguinal lines.
The weaknesses of this case series stem from the lack of histological analysis of observed cutaneous lesions and, in some cases, from inability to evaluate the patients at the time of symptom onset. In three cases, despite our suspicion of viral etiology, CADR could not be excluded. As medical facilities become more comfortable with handling patients with COVID‐19 infection, the aforementioned limitations will likely become less significant.
In conclusion it is important to reiterate that the significance of described dermatologic manifestations is still being deliberated. It is unclear if their presence can aid in the diagnosis, management, or prognosis for the patients with COVID‐19 infection. Still, we should remain vigilant and share our observations and new findings as we continue to take care of our patients.
Conflict of interest: None.
Funding source: None.
References
- 1. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34 5:e212–e213. 10.1111/jdv.16387 [DOI] [PubMed] [Google Scholar]
- 2. Su C‐J, Lee C‐H. Viral exanthem in COVID‐19, a clinical enigma with biological significance. J Eur Acad Dermatol Venereol 2020. doi: 10.1111/jdv.16469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Estébanez A, Pérez‐Santiago L, Silva E, et al Cutaneous manifestations in COVID‐19: a new contribution. J Eur Acad Dermatol Venereol 2020, Apr 15. doi: 10.1111/jdv.16474 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manalo I, Smith M, Cheeley J, et al A dermatologic manifestation of COVID‐19: Transient livedo reticularis. J Am Acad Dermatol 2020. Apr 10; S0190‐9622 (20) 30558‐2. doi: 10.1016/j.jaad.2020.04.018 Online ahead of print. Affiliations expand PMID: 32283229 PMCID: PMC7146700. doi: 10.1016/j.jaad.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for Dengue. J Am Acad Dermatol 2020. Mar 22. [Epub ahead of print] pii: S0190–9622 (20) 30454–0. doi: 10.1016/j.jaad.2020.03.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magro C, Mulvey JJ, Berlin D, et al Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases [published online ahead of print, 2020 Apr 15]. Transl Res 2020; 5244: 1–13. doi: 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanchez A, Sohier P, Benghane S, et al Digitate papulosquamous eruption associated with severe acute respiratory syndrome Coronavirus 2 infection. JAMA Dermatol. Published online April 30, 2020. doi: 10.1001/jamadermatol.2020.1704 [DOI] [PubMed] [Google Scholar]
- 9. Galván Casas C, Català A, Carretero Hernández G, et al Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases [published online ahead of print, 2020 Apr 29]. Br J Dermatol 2020. doi: 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. And HM, Koziatek C. A case of COVID‐19 pneumonia in a young male with full body rash as a presenting symptom. Clin Pract Cases Emerg Med. doi: 10.5811/cocemos.2020.3.47349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Avellana Moreno R, Villa E, Avellana Moreno V, et al Cutaneous manifestation of COVID‐19 in images: a case report. J Eur Acad Dermatol Venereol 2020. Apr 24. doi: 10.1111/jdv.16531 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amatore F, Macagno N, Mailhe M, et al SARS‐CoV‐2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol 2020. Apr 24. doi: 10.1111/jdv.16528 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marzano AV, Genovese G, Fabbrocini G, et al Varicellalike exanthem as a specific COVID‐ 19‐associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol 2020. doi: 10.1016/j.jaad.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]


