To the Editor,
We would like to report our experience of two patients coinfected with Streptococcus pneumoniae (S. pneumoniae) and coronavirus disease 2019 (COVID‐19) in the United Kingdom. Our institution serves a population of 500 000 and at the time of writing, 20 June 2020, only two (0.4%) patients of a total of 450 who tested positive for COVID‐19 on nasopharyngeal swab specimen using real‐time reverse transcriptase‐polymerase chain reaction (PCR) have also been diagnosed with S. pneumoniae infection as determined by blood culture positivity upon hospitalization. Whether the surge in COVID‐19 cases has led to the overlooking of other respiratory illnesses or whether increased hygiene, social distancing, and face mask use has reduced the rate of transmission is open to debate.
We treated two older polymorbid men who presented with a short history of typical symptoms of COVID‐19 infection and tested positive for the virus on nasopharyngeal swab. In both cases, S. pneumoniae was subsequently isolated in blood culture. Urinary antigen tests were not performed. Both patients had been vaccinated against S. pneumoniae with a 23‐valent vaccine (PPSV23) (case 1 in 2005 and case 2 in 2003). Both were treated with antibiotics from admission. Case 1, a very frail, white British male aged 86 years, was admitted from a nursing home. He was treated with intravenous amoxicillin and clarithromycin and received a single dose of vancomycin on day 2, pending sensitivities. Blood culture grew Gram‐positive cocci in pairs and chains. Antibiotic treatment was subsequently rationalized to intravenous amoxicillin alone on day 3. Cited markers of cytokine release became prominent and the patient died in hospital after 15 days. Case 2 was a Pakistani male aged 82 years who had a better premorbid level of function and was living independently. He was treated with intravenous amoxicillin on admission which was switched to intravenous co‐amoxiclav on day 3 on clinical grounds. The patient deteriorated on day 8 and required continuous positive airway pressure support. He was discharged in a good condition after a protracted inpatient spell of 21 days. Patient demographics, clinical characteristics, and outcomes are demonstrated in Table 1.
Table 1.
Demographics, clinical characteristics, treatment, and outcomes
| Patient 1 | Patient 2 | ||
|---|---|---|---|
| Demographics | |||
| Age, y | 86 | 82 | |
| Sex | Male | Male | |
| Ethnicity | White British | Pakistani | |
| Comorbidities | Alzheimer's disease, hypertension (HTN), and disseminated malignancy of unknown origin | Type 2 diabetes mellitus, ischemic cardiomyopathy, HTN, and chronic kidney disease | |
| Pneumococcal vaccination (PPSV23) | 2005 | 2003 | |
| Clinical findings on admission | |||
| Duration of symptom, d | 2 | 2 | |
| Chest X‐ray |
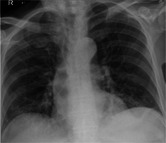
|
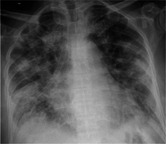
|
|
| Symptoms and initial observations | |||
| Symptoms | Dyspnea, fever, and fatigue | Chest pain, fever, and cough | |
| Respiratory rate, breaths/min | 24 | 22 | |
| O2 saturation in room air (%) | 92 | 95 | |
| Temperature, °C | 38 | 39 | |
| Supine blood pressure, mm Hg | 160/98 | 127/59 | |
| Heart rate, beats/min | 120 | 107 | |
| Initial laboratory results | |||
| WCC (cells per 106/L) | 2.01 | 14.5 | |
| Lymphocyte (cells per 106/L) | 0.19 | 0.8 | |
| Platelets (cells per 106/L) | 131 | 192 | |
| Lactate dehydrogenase, U/L | 396 | 149 | |
| CRP, mg/dL | 59 | 41 | |
| D dimer, ng/mL | 716 | ||
| Troponin, ng/mL | 114 | 62 | |
| Ferritin ng/mL | 1237 | 788 | |
| Blood culture organism | S. pneumoniae (serotype 38—not in any current pneumococcal vaccines) | S. pneumoniae (serotype 8—present in the 23‐valent plain polysaccharide vaccine but not in the 13‐valent conjugate vaccine) | |
| Sensitivities |
Erythromycin Penicillin |
Erythromycin Co‐trimoxazole Cefotaxime |
|
| Treatment and outcomes | |||
| Other antibiotics |
Amoxicillin Clarithromycin vancomycin |
Amoxicillin Co‐amoxiclav |
|
| Admitted to intensive care unit | No | No | |
| Invasive or noninvasive ventilation | No | CPAP | |
| Length of admission (nights) | 15 | 21 | |
| Outcome | Death | Discharged | |
Abbreviations: CPAP, continuous positive airway pressure; CRP, C‐reactive protein; WCC, white cell count.
It is well established that seasonal viral respiratory tract infections have been linked to increased risk of bacterial coinfection 1 and current evidence suggests that the innate immune response against severe acute respiratory syndrome coronavirus 2 can similarly compromise host defense of the respiratory tract against bacteria. Variable incidence of concurrent bacterial infection in COVID‐19 has been reported. 2 , 3 , 4 , 5 , 6
The fear of misdiagnosis or missed diagnoses of coinfections, when faced with COVID‐19 infection, remains high; however, the detrimental effects of widespread empirical antibiotic therapy and propagation of antimicrobial resistance should be considered. The traditional markers used to guide antibiotic treatment such as white cell count, C‐reactive protein, and chest radiograph abnormalities may be unhelpful in COVID‐19 infection and decision‐making surrounding antimicrobial therapy can be challenging. The role of procalcitonin is still unclear.
The National Institute for Healthcare and Excellence (UK) acknowledges that at first presentation, it may be difficult to differentiate between COVID‐19 pneumonitis and bacterial pneumonia and recommend that empirical antibiotics should be administered if there is clinical suspicion of added bacterial infection. 7
We present two (0.4%) COVID‐19 patients with confirmed S. pneumoniae coinfection, one of whom died. Older adults can respond and present differently (often subclinically) compared to young adults. These are due to differences in physiological and immune responses, which are beyond the scope of our article. Notably, however, it has been argued, although not validated in large sample sizes, that older adults are more prone to bacterial superinfection and that empirical treatment with antibiotics may be justified. 8 In keeping with good medical practice, these should only be continued when there is strong clinical or microbiological evidence of bacterial infection, regardless of COVID‐19 test results, and should be de‐escalated once the specific organisms are identified.
Although these cases might suggest bacterial coinfection is rare and difficult to clinically distinguish from COVID‐19, larger studies are required to clarify this. The necessity of microbiological diagnostic testing, perhaps with a panel consisting of multiplex PCR for seasonal respiratory viruses on swab and urinary antigens for S. pneumoniae and Legionella pneumophila alongside blood cultures for characterization of bacterial coinfections, is highlighted. Emphasis is also laid on the importance of influenza and pneumococcal vaccination.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1. Beadling C, Slifka MK. How do viral infections predispose patients to bacterial infections? Curr Opin Infect Dis. 2004;17(3):185‐191. 10.1097/00001432-200406000-00003 [DOI] [PubMed] [Google Scholar]
- 2. Cucchiari D, Pericàs JM, Riera J, et al. Pneumococcal superinfection in COVID‐19 patients: A series of 5 cases [published online ahead of print June 5, 2020]. Med Clin (Barc). 2020;S0025‐7753(20):30349‐3. 10.1016/j.medcli.2020.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adler H, Ball R, Fisher M, Mortimer K, Vardhan MS. Low rate of bacterial co‐infection in patients with COVID‐19. Lancet Microbe. 2020;1:e62. 10.1016/S2666-5247(20)30036-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu X, Ge Y, Wu T, et al. Co‐infection with respiratory pathogens among COVID‐2019 cases. Virus Res. 2020;285:198005. 10.1016/j.virusres.2020.198005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xing Q, Li G, Xing Y, et al. Precautions are needed for COVID‐19 patients with coinfection of common respiratory pathogens [published online ahead of print February 29, 2020]. Medrxiv. 2020. 10.1101/2020.02.29.20027698 [DOI]
- 7. NICE . COVID‐19 rapid guideline: antibiotics for pneumonia in adults in hospital. NICE guideline [NG173]. 2020. https://www.nice.org.uk/guidance/ng173/evidence. Accessed June 16, 2020. [PubMed]
- 8. Liu K, Chen Y, Lin R, Han K. Clinical features of COVID‐19 in elderly patients: A comparison with young and middle‐aged patients. J Infect. 2020;80(6):e14‐e18. [DOI] [PMC free article] [PubMed] [Google Scholar]


