At present, the medical approaches to cope with Covid‐19 infection caused by the respiratory syndrome coronavirus 2 (SARS‐CoV‐2) are mainly supportive. In the absence of specific anti‐viral therapies or vaccines, the medical care is complemented with different combinations of broad‐spectrum antiviral agents, antibiotics, hydroxychloroquine, and convalescent plasma transfusion (Jin et al., 2020).
Thymoquinone, the main constituent of Nigella sativa, has demonstrated anti‐inflammatory, anti‐oxidant, anti‐tumor, and antimicrobial activities (Banerjee et al., 2009; Chaieb, Kouidhi, Jrah, Mahdouani, & Bakhrouf, 2011). Thymoquinone was also effective and tolerable in children with intractable epilepsy in a randomized controlled clinical trial at a dose of 1 mg/kg/day orally (Akhondian et al., 2011). Interestingly, thymoquinone and Nigella sativa extract were found to be effective against avian influenza virus (H9N2 AIV) and a murine cytomegalovirus infection model (Salem & Hossain, 2000; Umar et al., 2016). Ulasli and co‐workers reported that the treatment of cells with Nigella sativa extract prior to infection with coronavirus decreases the replication of the virus (Ulasli et al., 2014). Moreover, gene expression analysis of the transient receptor potential proteins (TRPs) showed a reduction in virus loads upon extract treatments, which can decrease coronavirus survival inside cells. It should be noted, however, that these studies on the herbal extracts may not have been carried out according to the more recent scientific qualitative standards for plant‐derived products (Heinrich et al., 2020). Therefore, there is the possibility that high concentrations in vitro or doses in vivo, which are of no translational value have been used.
Thymoquinone as a compound (purity >99%) has unveiled a remarkable anti‐sepsis and immunomodulatory activities at specific doses (Alkharfy, Ahmad, Jan, & Raish, 2018; Alkharfy, Ahmad, Raish, & Vanhoutte, 2015; Alkharfy, Al‐Daghri, Al‐Attas, & Alokail, 2011). More specifically, thymoquinone modulates the production of nitric oxide (NO) and reactive oxygen species (ROS), and protects against multiple organ dysfunction syndrome (MODS). ROS including superoxide, hydrogen peroxide, and hydroxyl radicals are produced, among others, by xanthine oxidase and NADH/NADPH oxidases (Galley, 2011; Ichinose et al., 2007). The NADPH oxidases, uncoupled NO synthase (iNOS), and mitochondria are considered important mediators of ROS in sepsis and cardiovascular dysfunction (Kirkeboen & Strand, 1999; Munzel, Gori, Bruno, & Taddei, 2010; Tsolaki, Makris, Mantzarlis, & Zakynthinos, 2017). In fact, sepsis is characterized by the enhanced release of NO, which correlates with systemic dysfunction and tissue injury in humans and animal models (Rabuel et al., 2010; Tsolaki et al., 2017). NO can interact with the absorption of calcium in the myocytes and, therefore, can impede contractile activity (Forstermann & Sessa, 2012). In addition, NO plays a key role in the systemic inflammation of sepsis including vasodilatation, altered vascular permeability and extravasation, leukocyte migration, and activation (Ince et al., 2016). Notably, inflammatory cytokines such as TNF‐α, IL‐1α, IL‐2, IL‐6, and IL‐10 also enhance NO production via iNOS (Green et al., 1994). Thymoquinone has been shown to downregulate inflammatory cytokines, reduce NO levels, and improve organ functions and survival of sepsis in an animal model (Alkharfy et al., 2015). This perhaps through a redox mechanism, which decreases the systemic oxidative stress and inflammatory response. Consequently, thymoquinone decreases the levels of early‐stage sepsis biomarkers (e.g., ESM‐1, CRP, and VEGF) by ~30–50% (Alkharfy et al., 2018). Interestingly, thymoquinone has also been found to have a protective effect against lung fibrosis and collagen deposition by modulating the nuclear factor Kappa‐B (NF‐κB) and the antioxidant enzyme nuclear factor 2 heme oxygenase‐1 (Nrf2/HO‐1) signaling pathway (Ahmad et al., 2020).
Virus‐induced phagocyte activation is correlated with oxidative stress, not just because ROS is produced, but also because activated phagocytes also produce inflammatory cytokines by the activation of NF‐κB (S. F. Liu & Malik, 2006; Schwarz, 1996). Actually, many genes that are regulated by NF‐κB, including inflammatory cytokines, COX‐2, and iNOS, contribute to a rise in sepsis inflammatory responses (Ghosh, May, & Kopp, 1998; Schneider‐Stock, Fakhoury, Zaki, El‐Baba, & Gali‐Muhtasib, 2014). Thus, NF‐κB inhibition can suppress inflammatory genes, impede the cytokine storm, and reduce immune cells infiltration and activation, and, therefore, protecting against tissue and organ damage (T. Liu, Zhang, Joo, & Sun, 2017). While inhibition of NF‐κB activation has been suggested as a therapeutic strategy for sepsis, it should be noted that NF‐κB is an important component of normal immune defenses and that excessive blockade of NF‐κB regulatory activities can be strong immunosuppressive (Coldewey, Rogazzo, Collino, Patel, & Thiemermann, 2013). Therefore, a more selective modulation of NF‐κB activity is probably needed. Overall, existing evidence indicates that thymoquinone can favorably modulate NF‐κB expression during sepsis (Alkharfy et al., 2015). Consequently, thymoquinone can be a strong candidate to avert MODS and mortality of sepsis (Figure 1). Recently, molecular docking studies have also proposed that thymoquinone may inhibit SARS‐CoV‐2 and interfere with its binding to ACE2 receptors. This can prevent virus entry and replication inside the host cell (Bouchentouf & Missoum, 2020; Sekiou, Ismail, Zihad, & Abdelhak, 2020). Furthermore, SARS‐CoV‐2 spikes can bind to a cell surface heat shock protein (HSPA5), which is upregulated during viral infections. Molecular dynamics simulations showed that thymoquinone can interfere with the attachment of SARS‐CoV‐2 to the HSPA5 substrate‐binding domain b (SBDb) on the stressed cells, and thus may reduce the risk of infection (Elfiky, 2020). Therefore, the time is probably appropriate to move thymoquinone from experimentation on the bench to clinical testing for the Covid‐19 pandemic.
FIGURE 1.
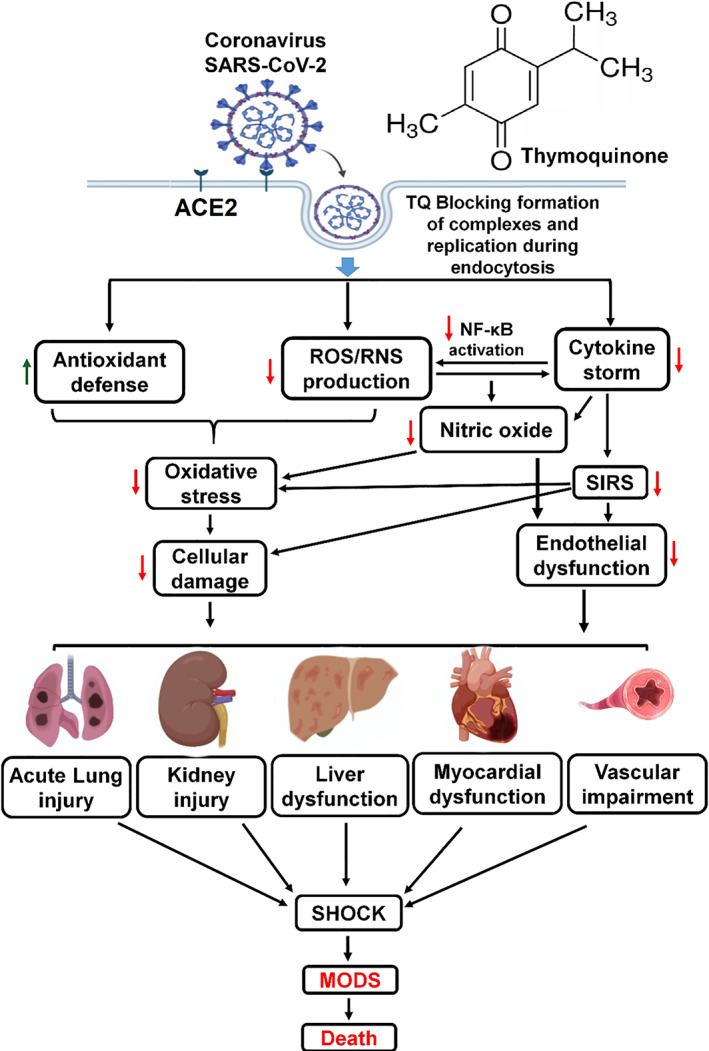
Thymoquinone potential protective mechanism against Covid‐19 infection. Progression from risk factors for severe SARS‐CoV‐2 infection mediated by oxidative stress and cytokine storm‐inducing multiple organ dysfunction syndrome (MODS). Thymoquinone inhibitory effects on viral infection and amelioration of MODS complications by restoration of the redox and immune balances. SARS‐CoV‐2 (Severe acute respiratory syndrome coronavirus 2); ACE (Angiotensin Converting Enzyme); ROS (reactive oxygen species); RNS (reactive nitrogen species); NF‐κB (nuclear factor kappa‐B); SIRS (systemic inflammatory response syndrome); green arrow indicates upregulation and red arrow indicates downregulation mediated by thymoquinone [Colour figure can be viewed at wileyonlinelibrary.com]
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Ajaz Ahmad and Khalid M. Alkharfy: Conceptualization. Muneeb U. Rehman and Parvaiz Ahmad: Resources. Ajaz Ahmad and Muneeb U. Rehman: Writing. Parvaiz Ahmad and Khalid M. Alkharfy: Review and Editing. Ajaz Ahmad and Khalid M. Alkharfy: Supervision.
REFERENCES
- Ahmad, A. , Alkharfy, K. M. , Jan, B. L. , Ahad, A. , Ansari, M. A. , Al‐Jenoobi, F. I. , & Raish, M. (2020). Thymoquinone treatment modulates the Nrf2/HO‐1 signaling pathway and abrogates the inflammatory response in an animal model of lung fibrosis. Experimental Lung Research, 46(3–4), 53–63. 10.1080/01902148.2020.1726529 [DOI] [PubMed] [Google Scholar]
- Akhondian, J. , Kianifar, H. , Raoofziaee, M. , Moayedpour, A. , Toosi, M. B. , & Khajedaluee, M. (2011). The effect of thymoquinone on intractable pediatric seizures (pilot study). Epilepsy Research, 93(1), 39–43. 10.1016/j.eplepsyres.2010.10.010 [DOI] [PubMed] [Google Scholar]
- Alkharfy, K. M. , Ahmad, A. , Jan, B. L. , & Raish, M. (2018). Thymoquinone reduces mortality and suppresses early acute inflammatory markers of sepsis in a mouse model. Biomedicine & Pharmacotherapy, 98, 801–805. 10.1016/j.biopha.2018.01.028 [DOI] [PubMed] [Google Scholar]
- Alkharfy, K. M. , Ahmad, A. , Raish, M. , & Vanhoutte, P. M. (2015). Thymoquinone modulates nitric oxide production and improves organ dysfunction of sepsis. Life Sciences, 143, 131–138. 10.1016/j.lfs.2015.08.007 [DOI] [PubMed] [Google Scholar]
- Alkharfy, K. M. , Al‐Daghri, N. M. , Al‐Attas, O. S. , & Alokail, M. S. (2011). The protective effect of thymoquinone against sepsis syndrome morbidity and mortality in mice. International Immunopharmacology, 11(2), 250–254. 10.1016/j.intimp.2010.11.032 [DOI] [PubMed] [Google Scholar]
- Banerjee, S. , Kaseb, A. O. , Wang, Z. , Kong, D. , Mohammad, M. , Padhye, S. , … Mohammad, R. M. (2009). Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Research, 69(13), 5575–5583. 10.1158/0008-5472.CAN-08-4235 [DOI] [PubMed] [Google Scholar]
- Bouchentouf, S. , & Missoum, N. (2020). Identification of compounds from Nigella sativa as new potential inhibitors of 2019 novel corona virus (Covid‐19): Molecular docking study. ChemRxiv Preprint. Retrieved from https://doi.org/10.26434/chemrxiv.12055716.v1
- Chaieb, K. , Kouidhi, B. , Jrah, H. , Mahdouani, K. , & Bakhrouf, A. (2011). Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complementary and Alternative Medicine, 11, 29. 10.1186/1472-6882-11-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldewey, S. M. , Rogazzo, M. , Collino, M. , Patel, N. S. , & Thiemermann, C. (2013). Inhibition of IκB kinase reduces the multiple organ dysfunction caused by sepsis in the mouse. Disease Models & Mechanisms, 6(4), 1031–1042. 10.1242/dmm.012435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfiky, A. A. (2020). Natural products may interfere with SARS‐CoV‐2 attachment to the host cell. Journal of Biomolecular Structure & Dynamics. 10.1080/07391102.2020.1761881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstermann, U. , & Sessa, W. C. (2012). Nitric oxide synthases: Regulation and function. Eur Heart J, 33(7), 829–837, 837a‐837d. 10.1093/eurheartj/ehr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galley, H. F. (2011). Oxidative stress and mitochondrial dysfunction in sepsis. British Journal of Anaesthesia, 107(1), 57–64. 10.1093/bja/aer093 [DOI] [PubMed] [Google Scholar]
- Ghosh, S. , May, M. J. , & Kopp, E. B. (1998). NF‐κB and REL proteins: Evolutionarily conserved mediators of immune responses. Annual Review of Immunology, 16, 225–260. 10.1146/annurev.immunol.16.1.225 [DOI] [PubMed] [Google Scholar]
- Green, S. J. , Scheller, L. F. , Marletta, M. A. , Seguin, M. C. , Klotz, F. W. , Slayter, M. , … Nacy, C. A. (1994). Nitric oxide: Cytokine‐regulation of nitric oxide in host resistance to intracellular pathogens. Immunology Letters, 43(1‐2), 87–94. 10.1016/0165-2478(94)00158-8 [DOI] [PubMed] [Google Scholar]
- Heinrich, M. , Appendino, G. , Efferth, T. , Furst, R. , Izzo, A. A. , Kayser, O. , … Viljoen, A. (2020). Best practice in research ‐ Overcoming common challenges in phytopharmacological research. Journal of Ethnopharmacology, 246, 112230. 10.1016/j.jep.2019.112230 [DOI] [PubMed] [Google Scholar]
- Ichinose, F. , Buys, E. S. , Neilan, T. G. , Furutani, E. M. , Morgan, J. G. , Jassal, D. S. , … Bloch, K. D. (2007). Cardiomyocyte‐specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circulation Research, 100(1), 130–139. 10.1161/01.RES.0000253888.09574.7a [DOI] [PubMed] [Google Scholar]
- Ince, C. , Mayeux, P. R. , Nguyen, T. , Gomez, H. , Kellum, J. A. , Ospina‐Tascon, G. A. , … Workgroup, A. X. (2016). The endothelium in sepsis. Shock, 45(3), 259–270. 10.1097/SHK.0000000000000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, Y. H. , Cai, L. , Cheng, Z. S. , Cheng, H. , Deng, T. , Fan, Y. P. , … Health, C. (2020). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019‐nCoV) infected pneumonia (standard version). Military Medical Research, 7(1), 4. 10.1186/s40779-020-0233-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkeboen, K. A. , & Strand, O. A. (1999). The role of nitric oxide in sepsis ‐An overview. Acta Anaesthesiologica Scandinavica, 43(3), 275–288. 10.1034/j.1399-6576.1999.430307.x [DOI] [PubMed] [Google Scholar]
- Liu, S. F. , & Malik, A. B. (2006). NF‐kappa B activation as a pathological mechanism of septic shock and inflammation. American Journal of Physiology. Lung Cellular and Molecular Physiology, 290(4), L622–L645. 10.1152/ajplung.00477.2005 [DOI] [PubMed] [Google Scholar]
- Liu, T. , Zhang, L. , Joo, D. , & Sun, S. C. (2017). NF‐κB signaling in inflammation. Signal Transduction and Targeted Therapy, 2, 12073. 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel, T. , Gori, T. , Bruno, R. M. , & Taddei, S. (2010). Is oxidative stress a therapeutic target in cardiovascular disease? European Heart Journal, 31(22), 2741–2748. 10.1093/eurheartj/ehq396 [DOI] [PubMed] [Google Scholar]
- Rabuel, C. , Samuel, J. L. , Lortat‐Jacob, B. , Marotte, F. , Lanone, S. , Keyser, C. , … Mebazaa, A. (2010). Activation of the ubiquitin proteolytic pathway in human septic heart and diaphragm. Cardiovascular Pathology, 19(3), 158–164. 10.1016/j.carpath.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Salem, M. L. , & Hossain, M. S. (2000). Protective effect of black seed oil from Nigella sativa against murine cytomegalovirus infection. International Journal of Immunopharmacology, 22(9), 729–740. 10.1016/s0192-0561(00)00036-9 [DOI] [PubMed] [Google Scholar]
- Schneider‐Stock, R. , Fakhoury, I. H. , Zaki, A. M. , El‐Baba, C. O. , & Gali‐Muhtasib, H. U. (2014). Thymoquinone: Fifty years of success in the battle against cancer models. Drug Discovery Today, 19(1), 18–30. 10.1016/j.drudis.2013.08.021 [DOI] [PubMed] [Google Scholar]
- Schwarz, K. B. (1996). Oxidative stress during viral infection: A review. Free Radical Biology & Medicine, 21(5), 641–649. 10.1016/0891-5849(96)00131-1 [DOI] [PubMed] [Google Scholar]
- Sekiou, O. , Ismail, B. , Zihad, B. , & Abdelhak, D. (2020). In‐silico identification of potent inhibitors of COVID‐19 main protease (M pro ) and Angiotensin converting enzyme 2 (ACE2) from natural products: Quercetin, Hispidulin, and Cirsimaritin exhibited better potential inhibition than Hydroxy‐Chloroquine against COVID‐19 main protease active site and ACE2. ChemRxiv. Preprint. Retrieved from https://doi.org/10.26434/chemrxiv.12181404.v1
- Tsolaki, V. , Makris, D. , Mantzarlis, K. , & Zakynthinos, E. (2017). Sepsis‐induced cardiomyopathy: Oxidative implications in the initiation and resolution of the damage. Oxidative Medicine and Cellular Longevity, 2017, 7393525. 10.1155/2017/7393525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulasli, M. , Gurses, S. A. , Bayraktar, R. , Yumrutas, O. , Oztuzcu, S. , Igci, M. , … Arslan, A. (2014). The effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) extracts on the replication of coronavirus and the expression of TRP genes family. Molecular Biology Reports, 41(3), 1703–1711. 10.1007/s11033-014-3019-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar, S. , Munir, M. T. , Subhan, S. , Azam, T. , Nisa, Q. , Umar, W. , … Shah, Y. (2016). Protective and antiviral activities of Nigella sativa against avian influenza (H9N2) in turkeys. Journal of the Saudi Society of Agricultural Sciences. 10.1016/j.jssas.2016.09.004 [DOI] [Google Scholar]


