Abstract
The outcome of kidney transplant patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is still unclear. Here we describe the clinical characteristics, disease outcome, and risk factors for acute respiratory distress syndrome (ARDS) and death of a cohort of 53 kidney transplant patients with coronavirus disease 2019 (COVID-19). Eight of 53 have been handled as outpatients because of mild disease, on average with immunosuppression reduction and the addition of hydroxychloroquine and azithromycin; no patients required admission, developed ARDS, or died. Because of severe symptoms, 45/53 required admission: this cohort has been managed with immunosuppression withdrawal, methylprednisolone 16 mg/d, hydroxychloroquine, and antiviral drugs. Dexamethasone and tocilizumab were considered in case of ARDS. About 33% of the patients developed acute kidney injury, 60% ARDS, and 33% died. In this group, thrombocytopenia was associated to ARDS whereas lymphopenia at the baseline, higher D-dimer, and lack of C-reactive protein reduction were associated with risk of death. In the overall population, dyspnea was associated with the risk of ARDS and age older than 60 years and dyspnea were associated with the risk of death with only a trend toward an increased risk of death for patients on tacrolimus. In conclusion, SARS-CoV-2 infection may have a variable outcome in renal transplant patients, with higher risk of ARDS and death in the ones requiring admission.
KEYWORDS: clinical research/practice, infection and infectious agents – viral, infectious disease, kidney disease: infectious
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the general population may present a variable outcome ranging from mild to severe/life-threatening forms. We have recently described the first cohort of kidney transplant patients with coronavirus disease 2019 (COVID-19)1 treated with a therapeutic approach based on immunosuppression withdrawal, higher-dose steroids, antivirals, hydroxychloroquine, and dexamethasone with or without tocilizumab for the subgroup developing acute respiratory distress syndrome (ARDS) with progressive respiratory failure2; the mortality rate of this population was 25%. Since our preliminary report and at the time of the first version of this paper (end of April 2020), 2 further monocentric case series of kidney transplant patients had been published with variable outcomes: the Columbia and the Montefiore Medical Center cohorts, which included, respectively, 15 and 36 patients with mortality rates of 7% and 28%. In both of these cohorts, the antimetabolite was withdrawn, while the calcineurin inhibitors (CNIs) cessation was restricted to severe cases. The vast majority of the Columbia cohort patients received hydroxychloroquine and azithromycin, whereas these drugs were employed, respectively, in 86% and 46% of the Montefiore Medical Center cohort. Tocilizumab was administered only in a few cases.3 , 4
Here we describe the clinical characteristics and outcomes of a cohort of 53 kidney transplant patients (KTx) with COVID-19 followed within 3 centers of the Brescia Renal COVID task force and try to identify prognostic factors for poor outcome. The manuscript includes 20 patients already described in another report1 with extended follow-up and 33 patients who have not been, until now, the subject of publication.
2. METHODS
From March 1 to April 16, 2020, we enrolled all KTx with SARS-CoV-2 infection admitted within 3 centers of the Brescia Renal COVID task force (Spedali Civili of Brescia, Cremona Hospital, and Crema Hospital) and all the kidney transplant outpatients with proven SARS-CoV-2 infection followed at Spedali Civili of Brescia. To be eligible for this study, patients had to have symptoms compatible with COVID-19 and a positive reverse transcription polymerase chain reaction (RT-PCR) for SARS-CoV2; no asymptomatic patients identified during contact screening have been included in this survey.
The indication on patients’ management (inpatients vs outpatients) was based only on symptom severity as well as the presence of signs or symptoms of ongoing pneumonia.
The therapeutic strategy followed our protocol.2 Antiviral therapy with lopinavir/ritonavir associated with hydroxychloroquine (with dose adjusted according to kidney function) was considered for all patients requiring admission, if not contraindicated, for a treatment length of a minimum of 7 days up to a maximum of 15 according to clinical evolution or treatment with glucocorticoids and/or tocilizumab. In case of shortage of lopinavir/ritonavir, darunavir and ritonavir were employed.
Patients experiencing clinical deterioration after at least 7 days following symptom onset, or no fever for >72 hours, with escalating oxygen requirements, progression of the chest X-ray, and no signs of bacterial infection, were considered for dexamethasone (20 mg/daily for 5 days, then 10 mg/daily for 5 days) and up to 2 tocilizumab infusions at an interval of 12-24 hours (8 mg/kg of body weight, maximum dose per infusion 800 mg).
Considering the well-known potential of lopinavir/ritonavir and hydroxychloroquine for increasing QTc, a baseline electrocardiogram was performed before therapy commencement and, afterwards, every 2-3 days; in case of QTc prolongation a reduction or discontinuation of treatment was considered in a case-by-case manner.
The outpatients were monitored daily, using a telemedicine approach; in this subgroup baseline blood tests were not performed in order to promote social distancing.
Low dose of glucocorticoids was defined as methylprednisolone 4 mg or equivalent; medium dose of glucocorticoids was defined as 16 mg of methylprednisolone or equivalent. ARDS was defined as per previous publications.5
Ethical approval for this study was obtained according to Italian regulations.
2.1. Statistical analyses
Statistical analysis was performed using R software (https://www.r-project.org) and GraphPad Prism 7. Results are expressed as the number and percentage for categorical variables and the median (interquartile range [IQR]) for continuous variables.
Changes in variables were compared by a related sample Wilcoxon test, proportions of patients were compared using a chi-square or Fisher test, as appropriate.
Univariate and multiple logistic regression models were used to assess the ability of some predefined clinical characteristics to predict the risk of ARDS or death. All the statistically significant predictors at univariate analysis were entered in a multivariate model; age at disease diagnosis was added to the multivariate models as a factor likely to play an a priori role.6 Finally, the best multivariate model was identified by adopting a stepwise selection approach. Odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated from logistic regression analysis. P < .05 (2-tailed) were considered significant.
3. RESULTS
Fifty-three patients were included in this study; all the patients had symptoms suggestive for COVID-19 and had been assessed in the emergency room or in our clinics. In both settings, patients received the swab and were then admitted in case of severe symptoms and/or low blood oxygen levels or managed as outpatients in the case of mild symptoms and normal blood oxygen levels. For all the patients, diagnosis has been established on RT-PCR of nasopharyngeal swabs. Of the 53 patients, 42 have been managed as inpatients and 11 as outpatients; of the latter group, 3 were admitted within 3 days from the diagnosis because of aggravation of symptoms and were considered in the admitted cohort. Of the 45 patients admitted, 28 were followed at the Spedali Civili of Brescia, 13 at the Cremona hospital, and 4 at the Crema hospital. Patients’ characteristics are shown in Table 1.
TABLE 1.
Baseline clinical characteristics of 53 kidney transplant patients affected by SARS-CoV-2 infection and followed within 3 centers of the “Brescia Renal COVID task force”
| Characteristics | All patients (53) | Outpatients (8) | Admitted (45) |
|---|---|---|---|
| Male/female | 42 (79%) | 6 (75%) | 36 (80%) |
| Age (y) | 60 (IQR 50-67) | 62 (IQR 52-71) | 60 (IQR 50-67) |
| Cause of ESRD | |||
| ADPKD | 12 (23%) | 1 (13%) | 11 (24%) |
| Not determined | 17 (32%) | 2 (25%) | 15 (33%) |
| IgA | 8 (15%) | 3 (38%) | 5 (11%) |
| CAKUT | 5 (9%) | 0 | 5 (11%) |
| Other glomerulonephritis | 6 (11%) | 0 | 6 (13%) |
| Other | 5 (9%) | 2 (25%) | 3 (7%) |
| Comorbidities | |||
| Hypertension | 42/53 (79%) | 6/8 (75%) | 36/45 (80%) |
| Cardiac diseases | 10/53 (19%) | 1/8 (13%) | 9/45 (20%) |
| Previous DVT | 4/53 (8%) | 0/8 (0%) | 4/45 (9%) |
| Diabetes | 11/53 (21%) | 1/8 (13%) | 10/45 (22%) |
| Other | 4/53 (8%) | 0/8 (0%) | 4/45 (9%) |
| Type of transplant | |||
| Dead donor – single kidney | 45/53 (85%) | 8/8 (100%) | 37/45 (82%) |
| Live donor | 5/53 (9%) | 0 | 5/45 (11%) |
| Otherb | 3/53 (6%) | 0 | 3/45 (7%) |
| Induction | |||
| Thymoglobulin | 17/38 (45%) | 4/8 (50%) | 14/32 (44%) |
| Basiliximab | 14/38 (37%) | 3/8 (38%) | 12/32 (38%) |
| Alemtuzumab | 6/38 (16%) | 0 | 6/32 (19%) |
| Other | 1/38 (3%) | 1/8 (13%) | 0 |
| Baseline immunosuppression | |||
| Cyclosporine | 17/53 (32%) | 3/8 (38%) | 14/45 (31%) |
| Tacrolimus | 31/53 (58%) | 3/8 (38%) | 28/45 (62%) |
| mTORi | 6/53 (11%) | 1/8 (13%) | 5/45 (11%) |
| MMF | 32/53 (60%) | 6/8 (75%) | 26/45 (58%) |
| Low dose glucocorticoidsOthera | 30/53 (57%) | 6/8 (75%) | 24/45 (53%) |
| Baseline creatinine (mg/dL) | 1.83 (1.5-2.4) | 2.1 (1.8-2.5) | 1.8 (1.5-2.4) |
| Transplant age (y) | 9.2 (IQR 4-16) | 15 (IQR 4-17) | 9 (IQR 4-15) |
| SARS-CoV-2 infection symptoms at disease onset | |||
| Temperature (>37.5°C) | 51/53 (96%) | 7/8 (88%) | 44/45 (98%) |
| Cough | 26/53 (49%) | 3/8 (38%) | 23/45 (51%) |
| Gastrointestinal symptoms | 9/53 (17%) | 2/8 (25%) | 7/45 (16%) |
| Pharyngitis | 7/53 (13%) | 0/8 | 7/45 (16%) |
| Shortness of breath | 15/53 (28%) | 0/8 | 15/45 (33%) |
| Myalgia | 18/54 (33%) | 2/8 (25%) | 16/45 (36%) |
| Baseline chest X-ray | |||
| No infiltrates | — | — | 1/39 (3%) |
| Unilateral infiltrates | 12/40 (30%) | 1/1 (100%) | 11/39 (28%) |
| Bilateral infiltrates | — | — | 27/39 (69%) |
| Baseline chest CT | |||
| Infiltrates <50% | 4/8 (50%) | 4/8 (50%) | |
| Infiltrates >50% | 4/8 (50%) | 4/8 (50%) | |
| Baseline blood tests | |||
| WBC (NV 4.00-10.80 × 103/µL) | — | — | 5560 (4140-7400) |
| Neutrophils (NV 1.50-8.00 × 103/µL) | — | — | 4066 (IQR 2864-6790) |
| Lymphocytes (NV 0.90-4.00 × 103/µL) | — | — | 590 (IQR 430-1092) |
| Platelets (NV 130-400 × 103/µL) | — | — | 162 000 (IQR 129 000-219 000) |
| LDH (NV 135-225 U/L) | — | — | 263 (IQR 213-323) |
| CRP (NV < 5.0 mg/L) | — | — | 39 (IQR 16-103) |
| Creatinine | — | — | 2.4 (IQR 1.7-4) |
| Ferritin (µg/L) | — | — | 433 (IQR 284-872) |
| Fibrinogen (mg/dL) | — | — | 540 (IQR 380-625) |
| D-dimer (0-232 ng/mL) | — | — | 414 (IQR 101-677) |
| Antiviral therapy | |||
| Lopinavir/ritonavir | 18/53 (34%) | 0/8 (0%) | 18/45 (40%) |
| Darunavir + ritonavir | 14/53 (26%) | 0 (0%) | 14/45 (31%) |
| Hydroxychloroquine | 39/53 (79%) | 8/8 (100%) | 34/57 (60%) |
Note: Data are reported as n (%) for categorical variables and median (interquartile range) for continuous variables.
Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; CAKUT, congenital anomalies of the kidneys and of the urinary tract; CRP, C-reactive protein; CT, computed tomography; DVT, deep vein thrombosis; ESRD, end stage renal disease; IQR, interquartile range; LDH, lactate dehydrogenase; MMF, mofetil mycophenolate; mTORi, inhibitors of the mammalian target of rapamycin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WBC, white blood cells; NV, normal value.
4 mg of methylprednisolone or equivalent.
Includes 1 patient transplanted with a single kidney (dead donor) preventively, 1 patient transplanted with double kidneys (dead donor), and 1 with a combined kidney pancreas transplant.
3.1. Outpatients’ cohort
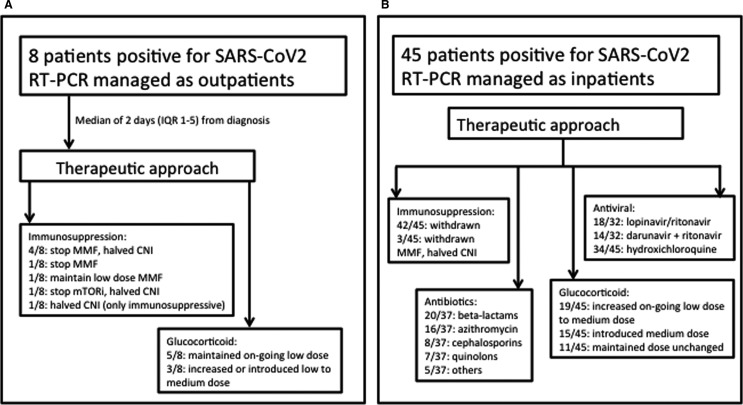
This group included 8 patients; the median time from symptoms onset to the diagnosis was 4.5 days (IQR 2.8-8). Only 1 patient of this group had a baseline chest X-ray available that showed unilateral infiltrates; baseline blood tests are not available for this group. Main clinical characteristics are shown in Table 1. Changes in patients immunosuppression and glucocorticoids dose are shown in Figure 1, panel A.
FIGURE 1.
Therapeutic approach in a cohort of 53 kidney transplant patients affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and followed within 3 centers of the “Brescia Renal COVID task force”
In this group, antivirals were not administered, whereas all the patients received hydroxychloroquine for 10 days after a median time of 3 days (IQR 2-5) from symptoms onset and azithromycin for a median length of 5 days (IQR 5-6) after a median of 5 days (IQR 4-10) from symptoms onset. No patients experienced adverse events because of the commencement of new drugs.
The median follow-up for this cohort has been 26 days (IQR 23-29) during which 6/8 patients experienced the onset of new symptoms compared to the baseline: cough in 4/6, myalgia or fatigue in 3/6, and gastrointestinal symptoms in 2/6. Five of 8 patients showed the first negative swab after a median of 21 days (IQR 19-27) from symptoms onset and 14 days (IQR 13-16) from the disease diagnosis with positive RT-PCR. No patients required admission or developed ARDS or died. At the last follow-up, 5/8 patients were still on the immunosuppressive scheme employed at the diagnosis of the SARS-CoV-2 infection, whereas in 3/8 the CNI was increased to the full dose.
3.2. Admitted cohort
Forty-five of 53 patients required admission; the median time from symptom onset to admission was 7 days (IQR 4-10), and the median time from symptom onset to the positive RT-PCR was 6 days (IQR 3-9). Main clinical characteristics are shown in Table 1. All the patients underwent a change of the immunosuppressive drugs at admission and after a median time of 7 days (IQR 3-9) from disease onset. The therapeutic approach is shown in Figure 1 panel B; immunosuppressive changes have been made at the moment of admission in 34/45 or within 24 hours from admission in 11/45. Antiviral therapy was administered in 32/45 (71%) after a median time of 6 days (IQR 3-9) from symptoms onset and of 1 day (IQR 0-2) from positive RT-PCR; 2/32 (6%) developed side effects requiring cessation of the therapy: antivirals and hydroxychloroquine were withdrawn because of vomiting in 1 case, hydroxychloroquine was suspended because of QTc prolongation in another case. A total of 23/45 (51%) received prophylactic heparin during the hospital stay, and 17/45 (38%) were on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers at admission.
During a median in-hospital stay time of 11 days (IQR 7-16), 27/45 (60%) developed ARDS and 10/45 (22%) required intensive care unit (ICU) care, of whom 9/10 (90%) required mechanical ventilation and of these patients 8/9 died; of note, access to ICU and mechanical ventilation was associated with an increased risk of death (respectively, OR 21, P = .0007 and OR 33.1, P = .002). Regarding the ward-managed patients with ARDS, 7/17 (41%) subsequently died; of these 7 patients, 6 were not considered eligible for ICU because of comorbidities and 1 refused ICU care. Among the 45 patients, 15 (33%) developed AKI and 3/15 (20%) required hemodialysis.
Fifteen of 45 (33%) patients died; in 14/15 the cause of death appeared to be related to ARDS, whereas 1/15 died of likely bacterial sepsis (high C-reactive protein [CRP], high procalcitonin in the context of resolved ARDS).
For ARDS management and in the context of progressive respiratory failure, 18/45 (40%) received dexamethasone: 10/18 (56%) died, and 6/18 (33%) were subsequently discharged. Nine of 18 (50%) showed amelioration in terms of chest X-ray or respiratory failure improvement. Eight of 18 patients (44%) also received tocilizumab on top of dexamethasone: 3/8 (38%) died and 3/8 (38%) have been discharged. Five of 8 (63%) showed improvement in terms of chest X-ray or respiratory failure (reduction of infiltrates, improvement of the pO2/FIO2 ratio; data not shown). Patients who received dexamethasone and tocilizumab were more likely to experience an amelioration compared to the ones receiving only dexamethasone (5/8, 63% vs 4/10, 40%).
At the end of the follow-up, 27/45 patients (60%) were discharged. At discharge, all the patients continued the immunosuppressive scheme adopted during the hospitalization (Table 1). Among the discharged patients, 20/27 had a medium further follow-up of 19 days (IQR 15-22). During this further follow-up, the immunosuppression was modified in 17/20: 13/17 halved the methylprednisolone dose to 8 mg/d and introduced the CNI at half dose compared to the baseline, 2/17 halved the methylprednisolone dose to 8 mg/d and introduced the CNI and mycophenolate mofetil at half dose compared to the baseline, 1/17 maintained methylprednisolone at the dose of 16 mg/d and halved the CNI, and 1/17 maintained methylprednisolone at the dose of 16 mg/d and introduced the inhibitors of the mammalian target of rapamyci. Fifteen of 20 patients with extended follow-up experienced a negative RT-PCR after a median of 14 days (IQR 12-18).
3.3. Blood tests
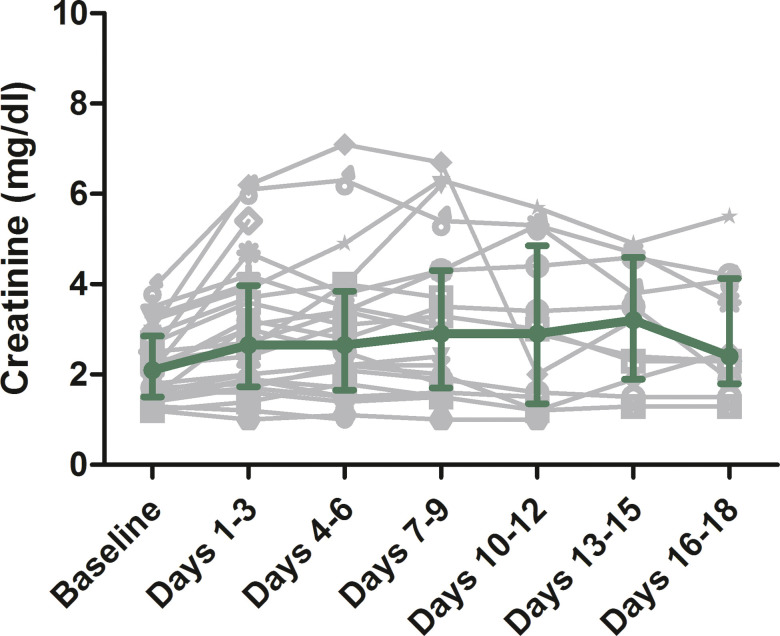
Baseline blood tests of the 45 admitted patients are shown in Table 1. Patients with platelet count lower than 160 × 103/µL were found at increased risk of ARDS (OR 6; 95% CI, 1.5-28.5; P = .02), whereas patients with lymphocyte count lower than 0.6 × 103/µL showed a trend toward a higher risk of death (OR 4.9; 95% CI, 0.95-37-8; P = .078) ( Table 2). From the renal function point of view, patients presented a medium creatinine level increase of +21% (IQR 7%-30%) compared to baseline, whereas the medium maximum creatinine during the stay in hospital compared to baseline was 0% (IQR 0%-13%); for the discharged patients, the serum creatinine compared to the baseline was −8% (IQR −20% to 7.1%); creatinine changes during admission and compared to baseline for the subgroup of 28 patiets admitted in the Spedali Civili of Brescia are shown in Figure 2.
TABLE 2.
Univariate analyses of the association between baseline blood tests and the risk of ARDS or death in 45 kidney transplant patients admitted for SARS-CoV-2 infection in 3 centers of the “Brescia Renal COVID task force”
| Variable | Outcome ARDS | Outcome death | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| WBC (≤6 vs >6 × 103/µL) | 0.5 (0.13-1.89) | .31 | 0.78 (0.16-3.3) | .74 |
| Lymphocytes (≤0.6 vs >0.6 × 103/µL) | 3.1 (0.76-13.9) | .124 | 4.9 (0.95-37.8) | .078 |
| Platelets (≤160 vs >160 × 103/µL) | 6 (1.5-28.5) | .016 | 1.4 (0.34-6) | .64 |
| LDH (>260 vs ≤260 U/L) | 0.8 (0.15-4.16) | .79 | 1.25 (0.24-6.79) | .79 |
| Ferritin (>450 vs ≤450 µg/L) | 0.8 (0.06-9.3) | .85 | 1.25 (0.11-14.8) | .85 |
| D-dimer (>400 vs ≤400 ng/mL) | 1.7 (0.19-15.7) | .64 | 1.2 (0.13-12.8) | .87 |
| CRP (>40 vs ≤40 mg/L) | 1.41 (0.38-5.34) | .603 | 1.4 (0.34-6) | .64 |
| Creatinine (>2.5 vs ≤2.5 mg/L) | 1.25 (0.33-4.9) | .74 | 0.78 (0.17-3.27) | .74 |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; CRP, C-reactive protein; LDH, lactate dehydrogenase; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WBC, white blood cells.
Bold values: statistically significant.
FIGURE 2.
Creatinine changes during admission and compared to baseline in 28 kidney transplant patients admitted for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the Spedali Civili of Brescia. Each patient is represented by a grey symbol. The green dots represent the overall median value, whiskers the interquartile range [Color figure can be viewed at wileyonlinelibrary.com]
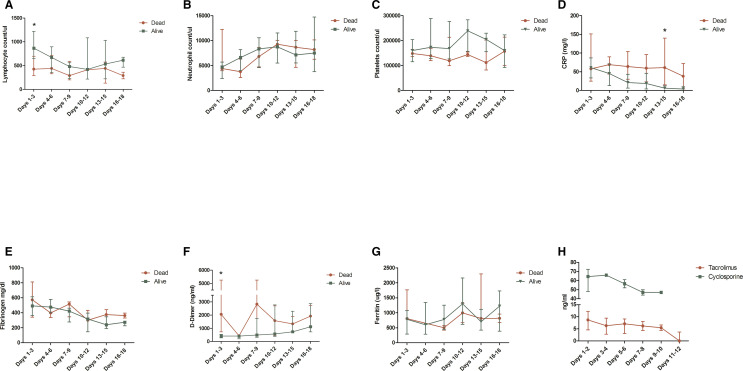
For the subgroup of 28 patients admitted in the Spedali Civili of Brescia, longitudinal blood tests have been available ( Figure 3). In the subgroup of patients who eventually died, baseline lymphocyte count was significantly lower (P = .008, Figure 3, panel A), baseline D-dimer was significantly higher (P = .02, Figure 3, panel F) and the CRP tended to remain higher during the follow-up, although the difference was statistically significant only at the time point “Days 13-15” (P = .018, Figure 3, panel D). The through levels of tacrolimus and cyclosporine tended to slowly decrease during the follow-up, despite CNIs were withdrawn at admission (Figure 3, panel H).
FIGURE 3.
Longitudinal blood tests value in a cohort of 28 kidney transplant patients admitted for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the Spedali Civili of Brescia stratified according to the outcome. Data are presented as median and interquartile range (whiskers). *Difference between groups statistically significant. CRP, C-reactive protein [Color figure can be viewed at wileyonlinelibrary.com]
3.4. Clinical characteristics associated to the risk of ARDS and death
In the overall population of 53 patients, at univariate analysis, the only clinical characteristic associated to the risk of developing ARDS was shortness of breath at disease onset (OR 5.9; 95% CI, 1.6-29.1; P = .015). A trend for increased risk of ARDS was recorded in patients on tacrolimus (OR 2.77; 95% CI 0.91-8.9, P = .077) and in patients with pharyngitis at disease onset (OR 6.9; 95% CI, 0.76-61.7; P = .09), whereas a protective trend toward the risk of ARDS was a transplant vintage >10 years (OR 0.37; 95% CI, 0.12-1.1; P = .078) and the presence of gastrointestinal symptoms at disease onset (OR 0.21; 95% CI, 0.04-1.1; P = .07) ( Table 3). At the multivariate analyses including age as risk factor identified a priori, dyspnea at disease onset was associated with the risk of development of ARDS (OR 6.61; 95% CI, 1.7-35; P = .013) ( Table 4).
TABLE 3.
Univariate analyses of the association between clinical characteristics and the risk of ARDS or death in 53 kidney transplant patients with SARS-CoV-2 infection
| Variable | Outcome ARDS | Outcome death | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Sex | 0.52 (0.12-1.99) | .35 | 0.62 (0.15-2.74) | .51 |
| Age (>60 vs ≤60) | 1.47 (0.5-4.41) | .49 | 2.06 (0.62-7.29) | .24 |
| History of hypertension | 0.83 (0.21-3.18) | .79 | 0.38 (0.08-1.3) | .93 |
| History of cardiac disease | 1.57 (0.39-6.89) | .53 | 1.94 (0.43-8.15) | .37 |
| History of DVT | 3.13 (0.37-65.6) | .34 | 2.77 (0.31-25.1) | .33 |
| History of diabetes | 3.23 (0.81-16.34) | .12 | 1.61 (0.36-6.48) | .51 |
| Number of comorbidities (0-1 vs >1) | 0.94 (0.3-3) | .92 | 0.96 (0.25-3.34) | .95 |
| CNIs last follow-up | 1.63 (0.25-13.2) | .61 | 5.1 (0.26-98) | .99 |
| Cyclosporin last follow-up | 0.39 (0.11-1.26) | .122 | 0.43 (0.09-1.64) | .24 |
| Tacrolimus last follow-up | 2.77 (0.91-8.9) | .077 | 4 (1.1-19.7) | .05 |
| mTORi last follow-up | 0.96 (0.16-5.64) | .96 | 0.47 (0.02-3.3) | .51 |
| MMF last follow-up | 1.25 (0.41-3.81) | .7 | 2.23 (0.63-9.2) | .23 |
| Low dose steroids last follow-up | 1.7 (0.57-5.19) | .343 | 1.8 (0.53-6.71) | .36 |
| Baseline creatinine (≤2 vs >2 mg/dL) | 1.37 (0.46-4.2) | .58 | 1.03 (0.29-3.56) | .96 |
| Age transplant (>10 vs ≤10 y) | 0.37 (0.12-1.1) | .078 | 0.5 (0.13-1.69) | .28 |
| Cough at disease onset | 1.17 (0.39-3.5) | .78 | 1.76 (0.53-6.24) | .36 |
| Pharyngitis at disease onset | 6.9 (0.76-61.7) | .09 | 2.06 (0.36-10.7) | .39 |
| Shortness of breath at disease onset | 5.9 (1.6-29.1) | .015 | 7.75 (2.1-32.2) | .003 |
| Myalgia or fatigue at disease onset | 1.25 (0.4-4) | .703 | 0.6 (0.14-2.14) | .45 |
| Gastrointestinal symptoms at disease onset | 0.21 (0.04-1.1) | .07 | 0.26 (0.01-1.62) | .22 |
| Chest X-ray (bilateral infiltrates vs unilateral or no infiltrates) | 1.7 (0.46-6.7) | .44 | 3.24 (0.68-23.7) | .18 |
| Time from symptoms to antiviral (>5 vs ≤5 d) | 0.82 (0.22-2.95) | .76 | 0.8 (0.20-3) | .74 |
| Antiviral therapy | 2.38 (0.78-7.6) | .133 | 1.45 (0.43-5.43) | .56 |
| Hydroxychloroquine | 1.56 (0.46-5.54) | .48 | 0.98 (0.26-4.2) | .98 |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; CNI, calcineurin inhibitor; DVT, deep vein thrombosis; MMF, mofetil mycophenolate; mTORi, inhibitors of the mammalian target of rapamycin; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Bold values: statistically significant.
TABLE 4.
Two models of multivariate analyses of the association between clinical characteristics and the risk of ARDS or death in 53 kidney transplant patients with SARS-CoV-2 infection
| Variable | Outcome ARDS | |
|---|---|---|
| OR (95% CI) | P value | |
| Model 1 | ||
| Age (>60 vs ≤60) | 1.05 (0.99-1.12) | .10 |
| Shortness of breath | 6.61 (1.7-35) | .013 |
| Variable | Outcome death | |
|---|---|---|
| OR (95% CI) | P value | |
| Model 2 | ||
| Age (>60 vs ≤60) | 1.12 (1.03-1.24) | .01 |
| Tacrolimus last follow-up | 4.8 (0.97-32) | .07 |
| Shortness of breath | 13.7 (2.7-68.9) | .004 |
Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2;
Bold values: statistically significant
Clinical characteristics associated to the risk of death at univariate analysis were immunosuppression with tacrolimus (OR 4; 95% CI, 1.1-19.7; P = .05) and shortness of breath at disease onset (OR 7.75; 95% CI, 2.1-32.2; P = .003) (Table 3). At multivariate analysis including age as risk factor identified a priori, age >60 and shortness of breath have been identified as risk factors for death (OR 1.12; 95% CI, 1.03-1.24); P = .01 and OR 13.7; 95% CI, 2.7-68.9); P = .004, respectively); only a trend as risk factor for treatment with tacrolimus has been identified (OR 4.8; 95% CI, 0.97-32; P = .07) (Table 4).
4. DISCUSSION
The first version of this paper was drafted at the end of April 2020 when, apart from our preliminary cohort of 20 patients,1 reports of only 2 further cohorts of kidney transplant patients had been published;3 , 4 at that stage the outcome of this population was still relatively unclear with mortality ranging from 7% to 28%. Since then, further data became available and the mortality of kidney transplant patients with SARS-CoV-2 infection appears now to have been consistently described as around 25%-28% across different cohorts with a sufficient sample size and follow-up.1 , 3 , 4 , 7, 8, 9 Our findings suggest a clear difference in terms of outcome according to disease severity: patients with mild symptoms and manageable at home experienced a benign disease course, whereas patients requiring hospitalization experienced a mortality rate of 33%; the overall fatality rate of our population was 28%. Of interest, this wide spectrum of disease severity is in keeping with what is described in the general population as well as in other KTx transplant cohorts10 although the mortality rate of the latter group, when presenting with severe disease, appears to be higher;11 of note, this variability has been observed also in the hemodialysis population.12
In our admitted kidney transplant population, we adopted from day one a policy based on immunosuppression cessation, introduction/increase of the glucocorticoid dose to methylprednisolone 16 mg or equivalent dose of prednisone, antivirals, and hydroxychloroquine.2 In our outpatient population, we opted, on average, to withdraw the antiproliferative, reduce the CNI dose, and start hydroxychloroquine and azithromycin. Of note, in both our subgroups adverse events due to the therapeutic approach were rare and no cardiac toxicity has been documented. The interaction between antivirals and CNIs metabolism has been well documented and this may explain the slow reduction rates of cyclosporine and tacrolimus in our population; the impact of this on kidney function evolution as well as the overall outcome is unclear. The combined approach of protease inhibitors start and CNIs withdrawn seems reasonable because of the interactions in the metabolism of the 2 drugs; however, caution in terms of CNIs withdrawn in different clinical contexts where protease inhibitors are not employed should be advised. Of note, heterogeneous approaches in terms of CNIs management for renal transplant patients during COVID-19 outbreaks have been described ranging from our approach to their maintenance.3
In the general population, the role of antivirals is debated. Remdesivir seems a promising option13 while the benefits of lopinavir/ritonavir are contradictory;14 , 15 of note, for the latter a trend toward a reduced risk of death was observed in a randomized controlled trial in the general population although the difference was not statistically significant compared to placebo. It should be stressed that the viral load may represent a risk factor for severe disease16 and impacting on this might reduce the risk of ARDS.15 The mortality rate of our cohort was similar to that of one of the other kidney transplant case series9 and of interest was higher compared to carriers of liver transplant.17 Of interest, case fatality rate was similar to that of the Montefiore Medical Center, where no antivirals were employed:4 this might be interpreted as in support for a lack of role for the antivirals commercially available at the moment. However, in our opinion, data are still scarce and more information is needed including long-term outcomes in terms of patients’ survival, renal outcome, and residual lung damages as a consequence of the infection.
An inflammatory syndrome is likely to be central in the development of ARDS and progressive respiratory failure in a subgroup of patients. Longitudinal blood tests showed persistently high CRP levels in the subgroup that would die compared to the one with a more favorable outcome. In this context, a role for anti-inflammatory approaches have been proposed:2 in our cohort patients with ARDS were treated with high-dose steroids, with or without tocilizumab. Although this subgroup experienced, on average, an unfavorable outcome, the combination of these 2 drugs was associated with higher rates of chest X-ray and respiratory failure improvements compared to the use of dexamethasone alone (63% vs 40%); these results are too preliminary to draw conclusions on the potential role of combining tocilizumab with glucocorticoids in this clinical setting and further studies will be needed to clarify this aspect.
Moreover, in the light of recent reports, the role of glucocorticoids in COVID-19 patients is debated and on average discouraged during the viremic phase;18 however, our approach has been to employ moderate dose of glucocorticoids only in the patients with ARDS and progressive respiratory failure at distance from the likely viremic phase. Of note, the benefits of such approach has been confirmed in the preliminary results of the RECOVERY trial that showed a role for dexamethasone in reducing mortality in mechanically ventilated patients and in the ones receiving oxygen.19 It should, however, be stressed that, although in our therapeutic protocol moderate doses were reserved only to the subgroup with ARDS, lower doses have been employed from the day of admission because of the concerns of increased risk of rejection secondary to the reduction of the immunosuppression; the impact of this change at early stages of hospitalization will need to be clarified.
Our study provides some information on the role of baseline and longitudinal blood tests on the outcome. Low platelets and lymphocytes were found, respectively, associated with an increased risk of ARDS and to a trend toward an increased risk of death. In a subgroup of 28 patients, the ones who eventually died had lower baseline lymphocyte counts compared to the rest of the population; of interest the trend toward a further reduction of the lymphocyte count during follow-up was similar in the two groups. In the same population, D-dimer at admission was higher. These findings are in keeping with what was already described in the general population.20 The mechanisms causing lymphopenia are still to be clarified and range from potential direct viral infection of the lymphocytes to a possible role for the inflammatory milieu associated with the disease.21
With the first wave of SARS-CoV-2 infections diminishing in Italy, the focus is now moving to how to routinely manage renal transplant patients in the COVID-19 era with a special focus on how and if modifications of the baseline immunosuppression would be required in the entire transplant population. In our cohort, the use of tacrolimus as baseline CNI showed a trend as a risk factor for ARDS and a significant association as a risk factor for death at univariate analysis; the latter maintained a trend as a risk factor also after multivariate analysis. In a model of multivariate analyses not shown in the results and including baseline therapy with tacrolimus, age, transplant vintage, and dyspnea at disease onset, the trend of treatment with tacrolimus as a risk factor for death has been maintained (OR 6.9; 95% CI, 0.99-67; P = .07). The reasons for this association are unclear. For both cyclosporine and tacrolimus, an antiviral effect in general, and toward the coronaviruses more specifically, was described in vitro.22 , 23 However, data in vivo or regarding their effects on SARS-CoV-2 are not available and importantly this should be balanced against the higher potency of tacrolimus compared to cyclosporine.24 Of note, this finding may be a consequence of our population characteristics; however, of interest, a benign outcome of COVID-19 during a cyclosporine-based therapy in kidney transplant patients has been proposed also in other cohorts.25 Despite that, this result will need to be interpreted with caution and further investigations will be required in order to provide confirmation.
Our study has some strengths: it includes a big cohort of kidney transplant patients, the therapeutic approach has been homogenous allowing robust data interpretation and comparisons with other published cohort with similar consistent approaches.
Limitations need to be acknowledged: despite the cohort size, this is still a relatively small study; moreover, our population has on average a severe disease profile and therefore our findings may not necessarily be extended to subgroups with mild symptoms or even who are asymptomatic at the moment of the diagnosis. Importantly, our findings are restricted to a short follow-up time and long-term observations will need to be performed in order to better clarify the outcome of kidney transplant patients with COVID-19. Of note, the mortality rates of the patients with ARDS managed in ICU and in the ward (respectively, 80% and 41%) may be a consequence of the different disease severity in the 2 subgroups; however, an impact for delayed ICU referral as well as reduced ICU availability at some stages of the emergency may have played a role in the overall fatality rates of our population with ARDS. It is necessary to underline, however, that among the 7 patients with ARDS and managed in the ward who would later die, 6 had not been considered eligible for ICU because comorbidities and therefore an unfavorable risk benefit balance; however, it should be acknowledged that this happened in the context, at least at some point of the emergency, of reduced resources. One of these 7 patients refused ICU care.
Of note, Italy in general and the province of Brescia in particular has been among the first western areas hit by this epidemic, urging physicians in employing treatment protocols based on partial evidence. At the moment of the writing, data do not further support a role for hydroxychloroquine in preventing the development of severe disease or in preventing the development of symptoms as postexposure prophylaxis;26 , 27 however, protocols designed before this publication have extensively employed this approach in kidney transplant population.3 , 4 It should also be underlined that data on the general population may not necessarily be applicable to the kidney transplant population.
In conclusion, renal transplant patients may experience a heterogeneous range of disease severity; the ones requiring admission may experience a poor outcome with high ARDS and mortality rates. The ideal therapeutic approach other than supportive care is still unclear and the impact of the single interventions to be determined.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflict of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Nicola Bossini and Federico Alberici contributed equally to this work.
APPENDIX 1. The Brescia Renal COVID task force
Federico Alberici1,2, Elisa Delbarba2, Chiara Manenti2, Laura Econimo2, Francesca Valerio2, Alessandra Pola2, Camilla Maffei2, Stefano Possenti2, Vincenzo Terlizzi2, Mattia Zappa2, Chiara Saccà2, Elena Pezzini2, Eleonora Calcaterra2, Paola Piarulli2, Marianna Moscato2, Stefano Pasquali2, Margherita Venturini2, Nicole Zambetti2, Alice Guerini2, Francesca Boni2, Agnese Gallico2, Michela Tonoli2, Alberto Mucchetti2, Stefania Affatato2, Sergio Bove3, Martina Bracchi4, Ester M. Costantino5, Fabio Malberti6, Giorgio Depetri7, Roberto Zubani1,2, Corrado Camerini2, Paola Gaggia2, Ezio Movilli2, Nicola Bossini2, Mario Gaggiotti2, Francesco Scolari1, 2
1Department of Medical and Surgical Specialties, Radiological Sciences and Public Health, University of Brescia, Brescia Italy
2Nephrology Unit, Spedali Civili Hospital, ASST Spedali Civili di Brescia, Brescia, Italy
3Nephrology Unit, Montichiari Hospital, ASST Spedali Civili di Brescia, Montichiari, Italy
4Nephrology Unit, ASST Franciacorta, Chiari, Italy
5Nephrology Unit, ASST del Garda, Manerbio, Italy
6Nephrology Unit, ASST di Cremona, Cremona, Italy
7Nephrology Unit, ASST di Crema, Crema, Italy
REFERENCES
- 1.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97(6):1083–1088. doi: 10.1016/j.kint.2020.04.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberici F, Delbarba E, Manenti C, et al. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep. 2020;5(5):580–585. doi: 10.1016/j.ekir.2020.04.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Columbia University Kidney Transplant Program Early description of coronavirus 2019 disease in kidney transplant recipients in New York. JASN. 2020;31(6):1150–1156. doi: 10.1681/ASN.2020030375. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 6.McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020;382(21):2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tschopp J, L’Huillier AG, Mombelli M, et al. First experience of SARS-CoV-2 infections in solid organ transplant recipients in the Swiss Transplant Cohort Study. Am J Transplant. 2020. 10.1111/ajt.16062 [DOI] [PMC free article] [PubMed]
- 8.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maggiore U, Abramowicz D, Crespo M, et al. How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion. Nephrol Dial Transplant. 2020;35(6):899–904. doi: 10.1093/ndt/gfaa130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Husain SA, Dube G, Morris H, et al. Early outcomes of outpatient management of kidney transplant recipients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020. 10.2215/CJN.05170420 [DOI] [PMC free article] [PubMed]
- 11.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020. 10.1001/jama.2020.4683 [DOI] [PubMed]
- 12.Alberici F, Delbarba E, Manenti C, et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98(1):20–26. doi: 10.1016/j.kint.2020.04.030. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020. 10.1056/NEJMoa2007764 [DOI] [PubMed]
- 14.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020:e200994. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed]
- 16.Liu Y, Yan L-M, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20(6):656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becchetti C, Zambelli MF, Pasulo L, et al. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020. 10.1136/gutjnl-2020-321923 [DOI] [PMC free article] [PubMed]
- 18.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statement from the Chief Investigators: low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. https://www.recoverytrial.net/files/recovery_dexamethasone_statement_160620_v2final.pdf. Accessed 25 June, 2020 [press release].
- 20.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan LI, Wang QI, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Wilde AH, Zevenhoven-Dobbe JC, van der Meer Y, et al. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol. 2011;92(Pt 11):2542–2548. doi: 10.1099/vir.0.034983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbajo-Lozoya J, Muller MA, Kallies S, Thiel V, Drosten C, von Brunn A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012;165(1):112–117. doi: 10.1016/j.virusres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winkler M, Christians U. A risk-benefit assessment of tacrolimus in transplantation. Drug Saf. 1995;12(5):348–357. doi: 10.2165/00002018-199512050-00006. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Cubillo B, Moreno de la Higuera MA, Lucena R, et al. Should cyclosporine be useful in renal transplant recipients affected by SARS-CoV-2? Am J Transplant. 2020. 10.1111/ajt.16141 [DOI] [PMC free article] [PubMed]
- 26.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020. 10.1056/NEJMoa2016638 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.