Abstract
Background
Post‐viral olfactory dysfunction (PVOD) is one of the most common causes of olfactory loss. Despite its prevalence, optimal treatment strategies remain unclear. This article provides a comprehensive review of PVOD treatment options and provides evidence‐based recommendations for their use.
Methods
A systematic review of the Medline, Embase, Cochrane, Web of Science, Scopus, and Google Scholar databases was completed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. Studies with defined olfactory outcomes of patients treated for PVOD following medical, surgical, acupuncture, or olfactory training interventions were included. The Clinical Practice Guideline Development Manual and Conference on Guideline Standardization (COGS) instrument recommendations were followed in accordance with a previously described, rigorous, iterative process to create an evidence‐based review with recommendations.
Results
From 552 initial candidate articles, 36 studies with data for 2183 patients with PVOD were ultimately included. The most common method to assess olfactory outcomes was Sniffin’ Sticks. Broad treatment categories included: olfactory training, systemic steroids, topical therapies, a variety of heterogeneous non‐steroidal oral medications, and acupuncture.
Conclusion
Based on the available evidence, olfactory training is a recommendation for the treatment of PVOD. The use of short‐term systemic and/or topical steroids is an option in select patients after careful consideration of potential risks of oral steroids. Though some pharmacological investigations offer promising preliminary results for systemic and topical medications alike, a paucity of high‐quality studies limits the ability to make meaningful evidence‐based recommendations for the use of these therapies for the treatment of PVOD.
Keywords: olfaction disorders, smell, evidence‐based medicine, olfactory training, budesonide, viral infection
Olfaction, 1 of the 5 principal human senses, serves a variety of critical health‐related roles ranging from the ability to detect health hazards such as fire or toxic fumes, to psychosocial implications such as the ability to enjoy food. Its importance is underscored by the well‐established association of olfactory dysfunction (OD) with multiple comorbidities, including depression, impaired cognition, and decreased nutrition. 1 , 2 Furthermore, OD is associated with a negative impact on quality of life, increased social isolation, and mortality in a “dose‐dependent” fashion. 1 , 3
Despite the importance and implications of OD, it is often overlooked by scientific and medical communities. Approximately 5% of the general population is believed to be affected by functional anosmia secondary to a variety of etiologies, including infectious, trauma, chronic rhinosinusitis (CRS), iatrogenic, and idiopathic causes. 4 Of these etiologies, post‐infectious OD is one of the most prevalent. 4 , 5 Though post‐infectious OD may be secondary to bacteria, fungi, or other rare organisms, viruses are the most common etiology. 4 , 6 Examples of causative viruses include human rhinovirus, coronavirus, parainfluenza, and Epstein‐Barr viruses. 7 Notably, the novel coronavirus severe acute respiratory syndrome (SARS)‐CoV‐2, responsible for the COVID‐19 pandemic, has been associated with at least temporary olfactory loss in a large proportion of affected patients from recently reported cohorts. 8 , 9 , 10 This pandemic has renewed interest in post‐viral olfactory dysfunction (PVOD) and evidence‐based treatments. At this time, the natural history of PVOD cannot be clearly anticipated. While some patients will experience transient dysosmia or parosmia, followed by return of olfactory function, many patients experience permanent OD. 4 , 11 The efficacy and evidence for treatment options are also lacking. Though corticosteroids are commonly used, there are a plethora of other potential therapies available, as well as evidence that suggests a role for olfactory training. 12 Study size, quality of evidence, and efficacy of these options are wide‐ranging.
This review sought to provide a comprehensive review of the supporting evidence for the treatment of PVOD, with accompanying, evidence‐based recommendations when possible. Though recommendations are provided, this review is not meant to replace clinical judgment, but rather arm clinicians with an improved understanding of the available treatment strategies for PVOD in an effort to optimize patient outcomes and identify areas of further inquiry for this common condition.
Materials and methods
Study design
Recommendations from The Clinical Practice Guideline Manual, 13 Conference on Guideline Standardization (COGS) instrument, 14 and the iterative process described by Rudmik and Smith 15 were used to create this evidence‐based review with recommendations. According to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines, 16 a systematic review of the literature was performed, guided by the PICOS (populations, interventions, comparisons, outcomes, and study design) described in Table 1.
TABLE 1.
Search strategy—PICOS (population, intervention, comparator, outcomes, study design) approach
| Population | Included | Patients with post‐viral olfactory dysfunction |
| Excluded | Alternate etiologies of olfactory dysfunction | |
| Intervention | Included |
Medical therapy Surgical intervention Olfactory training Acupuncture |
| Comparator | Included | Patients with post‐viral olfactory dysfunction who did not undergo treatment |
| Outcomes | Included |
Subjective olfactory measurements Objective olfactory scores |
| Studies | Included |
≥5 subjects Intervention for olfactory dysfunction |
| Excluded |
Non‐English Pre‐existing or alternate etiology of olfactory dysfunction Natural history cohorts |
Literature search strategy
A systematic search was conducted on March 26, 2020 using MEDLINE via PubMed, Embase, Cochrane Library, Web of Science, Scopus, and Google Scholar. The first 44 citations were extracted from Google Scholar. All other databases were searched from inception to search date. A focused literature search was performed using a combination of the following keywords: “post‐viral olfactory dysfunction,” “anosmia,” “dysosmia,” “parosmia,” “olfaction disorders,” “olfactory impairment,” “olfactory disturbance,” “olfactory loss,” “smell disorder,” “viral infection,” “virus,” “viral disease,” “common cold,” and “respiratory tract infection.” Additional records were identified by examining the references of articles obtained for review. Records were obtained by a qualified medical library informationist (S.M.S.).
Inclusion and exclusion criteria
Studies investigating the effects of medical, surgical, or olfactory training interventions on olfaction in patients with PVOD were included. Abstracts containing subjects with PVOD in addition to other etiologies of OD were included. Only studies with ≥5 subjects were included. Exclusion criteria included non‐English language and patient populations composed exclusively of those with OD secondary to etiologies other than PVOD (eg, idiopathic, trauma, and CRS). Studies without a defined intervention were excluded. Additionally, case reports, letters to the editor, abstracts, and book chapters were not included.
Data extraction, collection, and risk of bias assessment
Studies were managed in Covidence (Veritas Health Innovation Ltd, Melbourne, Australia) and duplicates were removed. Articles were independently reviewed by 3 authors (D.X.X., N.H., C.P.O.). Following abstract review, the remaining studies underwent a full text review. Outcome data were independently extracted from studies meeting inclusion criteria and disagreements were resolved by consensus. Studies were graded by quality in accordance with the 2011 Oxford Center for Evidence‐Based Medicine Criteria (Table 2). 17
TABLE 2.
Quality rating according to Oxford Center for Evidence‐Based Medicine 17
| 1 | Properly powered and conducted randomized clinical trial; systematic review with meta‐analysis |
| 2 | Well‐designed controlled trial without randomization; prospective comparative cohort trial |
| 3 | Case‐control studies; retrospective cohort study |
| 4 | Case series with or without intervention; cross‐sectional study |
| 5 | Opinion of respected authorities; case reports |
Risk of bias was assessed for each included study. Level 1 and 2 evidence studies were evaluated with the Modified Cochrane Collaboration Tool for Assess Risk of Bias (Table 3). 18 The Newcastle‐Ottawa Quality Assessment Scale was utilized for level 3 and 4 evidence studies (Table 4). 19
TABLE 3.
Modified Cochrane Collaboration Tool for assessing risk of bias in level 1 and 2 evidence studies 18
| Study (year) | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessors | Incomplete outcome data | Selective outcome reporting | Other sources of bias |
|---|---|---|---|---|---|---|---|
| Nguyen and Patel 54 (2018) | Unclear | Unclear | High | High | Low | Low | Low |
| Philpott et al. 32 (2017) | Low | Low | Low | Low | Low | Low | Low |
| Konstantinidis et al. 52 (2016) | High | Unclear | High | Low | Low | Low | Low |
| Whitcroft et al. 33 (2016) | Unclear | Low | Low | High | Low | Low | Low |
| Altundag et al. 51 (2015) | Low | Unclear | High | High | Low | Low | Low |
| Damm et al. 49 (2014) | Low | Low | High | Low | Low | Low | Low |
| Reden et al. 44 (2012) | Unclear | Unclear | Low | Low | Low | Low | Low |
| Reden et al. 36 (2011) | Unclear | Unclear | Low | High | Low | Low | Low |
| Blomqvist et al. 29 (2003) | Low | Low | Low | Low | Low | Low | Low |
| Quint et al. 42 (2002) | Unclear | Unclear | High | Low | Low | Low | Low |
| Henkin et al. 40 (1976) | Low | Low | Low | Low | Low | Low | Low |
TABLE 4.
| Study (year) | Selection grade (maximum 4 asterisks) | Comparability grade (maximum 2 asterisks) | Exposure grade (maximum 3 asterisks) | Total |
|---|---|---|---|---|
| Wang et al. 37 (2018) | *** | 0 | ** | ***** |
| Kim et al. 22 (2017) | ** | N/A | *** | ***** |
| Poletti et al. 53 (2017) | **** | ** | *** | ********* |
| Whitcroft et al. 34 (2017) | **** | ** | ** | ******** |
| Dai et al. 56 (2016) | **** | ** | *** | ********* |
| Henkin et al. 39 (2017) | *** | 0 | *** | ****** |
| Schopf et al. 35 (2015) | **** | 0 | * | ***** |
| Geißler et al. 50 (2014) | ** | N/A | *** | ***** |
| Kollndorfer et al. 55 (2014) | ** | N/A | ** | **** |
| Konstantinidis et al. 48 (2013) | **** | * | *** | ******** |
| Fleiner et al. 47 (2012) | **** | ** | *** | ********* |
| Schriever et al. 23 (2012) | ** | N/A | ** | **** |
| Fleiner and Goktas 31 (2011) | *** | N/A | *** | ****** |
| Vent et al. 57 (2010) | *** | ** | ** | ******* |
| Henkin et al. 38 (2009) | *** | N/A | *** | ****** |
| Hummel et al. 46 (2009) | **** | ** | *** | ********* |
| Seo et al. 21 (2009) | ** | * | *** | ****** |
| Stenner et al. 25 (2008) | *** | 0 | ** | ***** |
| Fukazawa et al. 30 (2005) | * | N/A | * | ** |
| Heilmann et al. 24 (2004) | **** | 0 | ** | ****** |
| Hummel et al. 45 (2002) | ** | N/A | *** | ***** |
| Aiba et al. 41 (1998) | ** | * | *** | ****** |
| Mori et al. 28 (1998) | *** | ** | ** | ******* |
| Ikeda et al. 26 (1995) | * | N/A | *** | **** |
| Duncan et al. 43 (1962) | * | N/A | * | ** |
Higher number of asterisks indicate higher quality study. Maximum score for case control study is 9 asterisks, and maximum score for case series is 4 asterisks. For assessment of case series articles, questions regarding control group are not applicable.
Development of recommendations
Following the completion of the systematic review and evaluation of research quality, a summary was produced including the aggregate grade of evidence (A to D) and recommendations based on the American Academy of Pediatrics Steering Committee on Quality Improvement and Managements guidelines (Table 5). 20 An aggregate grade of evidence was not provided for any intervention investigated by only a single study.
TABLE 5.
Recommendations based on defined grades of evidence 20
| Grade | Research quality | Preponderance of benefit over harm | Balance of benefit and harm |
|---|---|---|---|
| A | Well‐designed RCTs | Strong recommendation | Option |
| B | Randomized controlled trials with minor limitations; overwhelming consistent evidence from observational studies | Strong recommendation/recommendation | Option |
| C | Observational studies (case control and cohort design) | Recommendation | Option |
| D | Expert opinion; case report; reasoning from first principles | Option | No recommendation |
The Clinical Practice Guideline Development Manual and COGS) instrument recommendations were followed, 13 and in accordance with a previously described iterative process, each subsequent author reviewed, critiqued, and refined the recommendations. 15 Any disagreements amongst the authors were debated electronically until a consensus was achieved. The goal of the recommendations aimed to incorporate the quality of the research in addition to the balance of benefit and harm. Recommendations were provided when sufficient evidence for an intervention was available.
Results
Search characteristics
Initial literature search yielded 524 manuscripts with an additional 28 manuscripts identified through other sources (Fig. 1). Following removal of duplicates and abstract screening, 99 studies underwent full text review and were assessed for eligibility. Thirty‐six studies were included with a total of 4640 patients with OD, of which 2183 patients (47.0%) had a post‐viral etiology.
FIGURE 1.
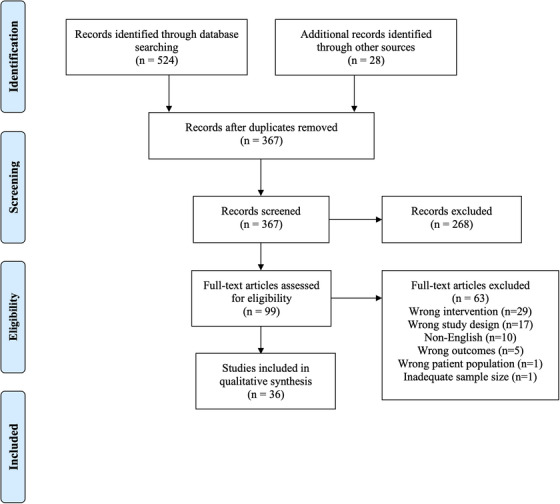
PRISMA flow diagram. PRISMA = Preferred Reporting Items for Systematic Reviews and Metaanalyses.
Of these 36 studies, 13 exclusively examined PVOD, whereas 23 studies considered OD of PVOD and other etiologies. In all studies, a patient was considered to have PVOD if they presented with olfactory loss following a viral infection. In many studies, further detail of how this diagnosis of PVOD was made was not offered; however, 15 studies specifically distinguished PVOD from “idiopathic” or “unknown” causes of OD and 2 studies excluded patients with diagnoses of “idiopathic” or “unknown” causes. Olfactory outcomes included 21 studies that utilized Sniffin’ Sticks, 3 with the University of Pennsylvania Smell Identification Test (UPSIT), 3 with the Toyota & Takagi olfactometer (T&T), 2 with the Cross‐Cultural Smell Identification Test (CCSIT), 2 with the Connecticut Chemosensory Clinical Research Center (CCCRC) test, 4 with olfactory thresholds from multiple odors, 1 with butanol threshold testing (BTT), and 8 with subjective symptoms using a Visual Analog Scale (VAS) and/or additional subjective scales. In studies that utilized Sniffin’ Sticks, a composite score, “TDI,” was calculated from the odor threshold (T), discrimination (D), and identification (I) subtest scores. Several studies utilized 2 or more olfactory outcome modalities. Regarding the quality of studies, 7 studies were level 1, 4 were level 2, 8 were level 3, and 17 were level 4. Mean follow‐up duration was 7.6 months (range 20 minutes‐72 months). One study did not specify a follow‐up duration. Summaries of included patients are included in Tables 6, 7, 8, 9, 10.
TABLE 6.
Summary of systemic steroid studies
| Author (year) | Study design | LOE | PVOD subjects (n)/Total subjects (n) | Primary comparison | Treatment details | Follow‐up | Olfactory outcome | Results |
|---|---|---|---|---|---|---|---|---|
| Seo et al. 21 (2009) | Randomized, nonblinded, parallel group trial | 3 | 71/71 |
|
|
4 weeks | BTT, CCSIT |
|
| Kim et al. 22 (2017) | Retrospective case series | 4 | 178/491 |
|
|
1 month | CCCRC, CCSIT |
|
| Schriever et al. 23 (2012) | Retrospective case series | 4 | 27/425 | Oral methylprednisolone (all) | Methylprednisolone 40 mg daily with taper | 2 weeks | Sniffin’ Sticks | PVOD patients exhibited statistically significant increase in TDI after treatment with systemic steroids (mean increase 4.47 ± 7.09 points, p = 0.003) |
| Stenner et al. 25 (2008) | Retrospective case series | 4 | 31/89 |
|
|
12 weeks | Sniffin’ Sticks |
|
| Heilmann et al. 24 (2004) |
Retrospective, nonrandomized parallel group case series |
4 | 22/92 |
|
|
21‐330 days | Sniffin’ Sticks |
|
| Ikeda et al. 26 (1995) | Case series | 4 | 9/21 | Oral prednisolone (all) | Prednisolone 40‐60 mg daily with taper | Up to 1 year | T&T | No significant improvement in odor recognition and detection thresholds in PVOD patients |
Data presented as mean ± standard deviation.
BTT = butanol threshold test; CCCRC = Connecticut Chemosensory Clinical Research Center test; CCSIT = Cross‐cultural Smell Identification Test; LOE = level of evidence; PVOD = post‐viral olfactory dysfunction; TDI = threshold, discrimination, and identification score; T&T = Toyota & Takagi olfactometer; URI = upper respiratory infection.
TABLE 7.
Summary of topical or local therapy studies
| Author (year) | Study design | LOE | PVOD subjects (n) /Total subjects (n) | Primary comparison | Treatment details | Follow‐up | Olfactory outcome | Results |
|---|---|---|---|---|---|---|---|---|
| Corticosteroids | ||||||||
| Blomqvist et al. 29 (2003) | Randomized, double‐blinded, placebo‐controlled clinical trial | 1 | 23/40 |
All patients showed BTT improvement of at least 2 steps following a 10‐day course of oral prednisolone 40 mg daily with taper and fluticasone spray, prior to being randomized to:
|
Fluticasone proprionate 200 µg daily | 6 months | CCCRC, VAS | No difference in olfaction amongst the 3 groups |
| Fleiner and Goktas 31 (2011) | Prospective case series | 4 | 8/18 | Beclomethasone spray directed to the olfactory cleft (all) | Beclomethasone spray 250 µg twice daily | 4 weeks | Sniffin’ Sticks |
|
| Fukazawa et al. 30 (2005) | Prospective, noncontrolled case series | 4 | 133/133 | Dexamethasone or Betamethasone (all) | Dexamethasone 5 mg or Betamethasone 5 mg injections into olfactory cleft every 2 weeks for 16‐20 weeks | 16‐20 weeks | T&T, VAS | 49.6% of PVOD patients demonstrated improvement on T&T olfactometry after treatment |
| Mori et al. 28 (1998) | Retrospective case series | 4 | 244/889 | Topical corticosteroids (not otherwise specified, all) | Not otherwise specified | 2 weeks‐2 years | T&T, Alinamin test |
|
| Sodium citrate | ||||||||
| Philpott et al. 32 (2017) | Randomized, double‐blind, placebo‐ controlled trial | 1 | 26/55 |
|
|
120 minutes | Olfactory thresholds | Temporary improvement for all patients in detection threshold for 3 of 4 odorants after administration of intranasal sodium citrate |
| Whitcroft et al. 33 (2016) | Prospective, controlled trial | 2 | 7/57 |
|
|
20‐30 minutes | Sniffin’ Sticks | Significant improvement in identification scores for PVOD patients (p = 0.02) |
| Whitcroft et al. 34 (2017) | Prospective, single‐blind, internally‐controlled trial | 3 | 49/49 |
|
|
20‐30 minutes | Sniffin’ Sticks | Significant improvement in composite threshold and identification scores for patients receiving sodium citrate compared to placebo (p = 0.04) |
| Insulin | ||||||||
| Schopf et al. 35 (2015) | Prospective, controlled pilot study | 4 | 10/10 |
|
|
55 weeks | Sniffin’ Sticks |
|
BTT = butanol threshold test; CCCRC = Connecticut Chemosensory Clinical Research Center test; IU = international units; LOE = level of evidence; PVOD = post‐viral olfactory dysfunction; TDI = threshold, discrimination, and identification score; T&T = Toyota & Takagi olfactometer; VAS = visual analog scale.
TABLE 8.
Summary of nonsteroidal oral medication studies
| Author (year) | Study design | LOE | PVOD subjects (n)/Total subjects (n) | Primary comparison | Treatment details | Follow‐up | Olfactory outcome | Results |
|---|---|---|---|---|---|---|---|---|
| Antibiotics | ||||||||
| Reden et al. 36 (2011) | Double blinded, randomized, placebo‐controlled trial | 1 | 55/55 |
|
Minocycline 100 mg twice daily | 207 days | Sniffin’ Sticks | No difference in TDI between minocycline and placebo (p = 0.55) |
| Wang et al. 37 (2018) | Retrospective cohort study | 3 | 158/288 |
|
Antibiotic: either bacteriostatic or bactericidal |
Bactericidal antibiotics: 178 days Bacteriostatic antibiotic: 163 days |
UPSIT |
|
| Theophylline | ||||||||
| Henkin et al. 39 (2017) | Prospective case‐control trial | 3 | 10/44 | Theophylline (all) | Oral theophylline 200‐800 mg | 2‐10 months | Olfactometry, VAS | 61% of all patients reported improvement in subjective smell after treatment |
| Henkin et al. 38 (2009) | Prospective case series | 4 | 97/312 | Theophylline (all) | Oral extended release theophylline in divided doses 200‐800 mg daily | Up to 72 months | Olfactometry, VAS |
|
| Supplements: vitamins and antioxidants | ||||||||
| Reden et al. 44 (2012) | Double blinded, randomized, placebo‐controlled trial | 1 | 33/52 |
|
Vitamin A 10,000 IU daily | 5 months | Sniffin’ Sticks | No difference in TDI improvement between vitamin A and placebo for all etiologies (p = 0.47) |
| Henkin et al. 40 (1976) | Double blinded, randomized, placebo‐controlled crossover clinical trial | 1 | 45/106 |
|
|
3 and 6 months | Olfactometry, VAS | No statistically significant difference in mean changes in smell thresholds between baseline and 3 or 6 months for any group |
| Quint et al. 42 (2002) | Prospective, controlled trial | 2 | 38/77 |
|
|
4 weeks | Sniffin’ Sticks |
|
| Aiba et al. 41 (1998) | Retrospective, non‐blinded, non‐controlled parallel group, clinical trial | 3 | 184/426 |
|
|
>2 weeks | Subjective symptoms | No significant differences among the 3 treatment groups for PVOD patients |
| Hummel et al. 45 (2002) | Non‐blinded, non‐controlled prospective case series | 4 | 23/23 | Alpha‐lipoic acid (all) | Alpha‐lipoic acid 600 mg daily | 3‐11 months | Sniffin’ Sticks |
|
| Duncan et al. 43 (1962) | Non‐controlled case series | 4 | 21/56 | Vitamin A (all) | Vitamin A 100,000 IU/mL injectable preparation weekly followed by 50,000‐150,000 IU oral tablets/emulsions daily for 3‐12 weeks | Not listed | Subjective symptoms |
|
IU = international units; LOE = level of evidence; OD = olfactory dysfunction; PVOD = post‐viral olfactory dysfunction; TDI = threshold, discrimination, and identification score; UPSIT = University of Pennsylvania Smell Identification Test; VAS = visual analog scale.
TABLE 9.
Summary of olfactory training studies
| Author (year) | Study design | LOE | PVOD subjects (n)/Total subjects (n) | Primary comparison | Treatment details | Follow‐up | Olfactory outcome | Results |
|---|---|---|---|---|---|---|---|---|
| Nguyen and Patel. 54 (2018) | Randomized control trial | 1 | 62/133 |
|
|
6 months | UPSIT |
|
| Damm et al. 49 (2014) | Randomized, single‐blind, controlled crossover clinical trial | 1 | 144/144 |
|
|
18 and 36 weeks | Sniffin’ Sticks, subjective symptoms |
|
| Konstantinidis et al. 52 (2016) | Prospective, randomized controlled trial | 2 | 111/111 |
|
Exposure to 4 odors twice daily
|
56 weeks | Sniffin’ Sticks |
|
| Altundag et al. 51 (2015) | Prospective, randomized, controlled clinical trial | 2 | 85/85 |
|
|
12, 24, and 36 weeks | Sniffin’ Sticks |
|
| Poletti et al. 53 (2017) | Prospective, pseudo‐randomized trial | 3 | 70/96 |
|
Exposure to 3 odors twice in the morning and twice in the evening
|
5 months | Sniffin’ Sticks | For PVOD patients, training with high molecular weight molecules produced significantly improved PEA threshold compared to low molecular weight molecules (p = 0.004) |
| Konstantinidis et al. 48 (2013) | Prospective controlled trial | 3 | 81/119 |
|
|
8 and 16 weeks | Sniffin’ Sticks |
|
| Hummel et al. 46 (2009) | Prospective controlled, nonblinded trial | 3 | 35/56 |
|
|
12 weeks | Sniffin’ Sticks |
|
| Geißler et al. 50 (2014) | Prospective, nonrandomized case series | 4 | 39/39 | Olfactory training (all) | Exposure to 4 odors twice daily | 16 and 32 weeks | Sniffin’ Sticks |
|
| Kollndorfer et al. 55 (2014) | Prospective case series | 4 | 7/7 | Olfactory training (all) | Exposure to 4 odors twice daily | 13 weeks | Sniffin’ Sticks |
|
| Fleiner et al. 47 (2012) | Retrospective case series | 4 | 16/16 |
|
|
4 and 8 months | Sniffin’ Sticks |
|
Data presented as mean ± standard deviation unless otherwise specified.
mean ± standard error of the mean.
COT = classical olfactory training; LOE = level of evidence; MOT = modified olfactory training; PEA = phenyl ethyl alcohol; PVOD = post‐viral olfactory dysfunction; TDI = threshold, discrimination, and identification score; UPSIT = University of Pennsylvania Smell Identification Test.
TABLE 10.
Summary of acupuncture studies
| Author (year) | Study design | LOE | PVOD subjects (n)/Total subjects (n) | Primary comparison | Treatment details | Follow‐up | Olfactory outcome | Results |
|---|---|---|---|---|---|---|---|---|
| Dai et al. 56 (2016) | Randomized, nonblinded controlled trial | 3 | 50/50 |
|
20 minutes of acupuncture 3 times per week for a course of 10 times, with 3‐5 days of rest between courses, continued for 3 months | 3 months | UPSIT |
|
| Vent et al. 57 (2010) | Retrospective nonblinded, non‐controlled, parallel group trial | 4 | 15/15 |
|
|
12 weeks | Sniffin’ Sticks | TDI score increase significantly better with TCA treatment (17.9 ± 6.5 from 13.5 ± 5.4) compared to vitamin B complex (15.8 ± 4.8 from 13.0 ± 3.5, p = 0.02) |
Data presented as mean ± standard deviation.
LOE = level of evidence; PVOD = post‐viral olfactory dysfunction; TCA = traditional Chinese acupuncture; UPSIT = University of Pennsylvania Smell Identification Test.
Systemic steroids
Six studies were performed using systemic steroids. Duration of follow‐up, olfactory testing, and dosing of systemic steroids varied widely (Table 6). Overall, 4 studies showed mild benefit with systemic steroids, 21 , 22 , 23 , 24 while study design in others prevented definitive conclusions. 25 , 26 Notably, Schriever et al. showed a statistically significant increase in TDI score (from 14.39 to 18.86; p = 0.003) after 2 weeks of treatment with oral methylprednisolone 40 mg daily and taper, though there was no comparison group. Furthermore, the mean improvement in TDI scores failed to reach the minimal clinically important difference (MCID) of 5.5. 27 Olfactory improvement secondary to combination therapy of oral prednisolone and mometasone spray with Gingko biloba was found to be similar to that after oral prednisolone and mometasone spray alone. 21
Summary:
Aggregate evidence: C (Level 3: 1 study, Level 4: 5 studies)
Benefit: Improved objective olfaction across multiple psychophysical tests
Harm: Potential side effects relating to systemic corticosteroids
Cost: Minimal medication cost
Benefit‐Harm assessment: Balance of benefit and harm
Value judgments: Provider must consider the risk of systemic corticosteroids in setting of patient medical comorbidities, notably in setting of heterogeneous studies with limited use of controls and unknown clinical significance.
Recommendation level: Option
Intervention: In absence of additional risk factors, can offer short course of systemic therapy after thoroughly considering potential outcomes and treatment‐associated risks.
Topical or local therapies
Topical or local steroids
Four studies assessed the effect of topical or local corticosteroids, including fluticasone proprionate, beclomethasone spray, and dexamethasone or betamethasone injections (Table 7). 28 , 29 , 30 , 31 One study did not specify the steroids used for treatment. 28 Blomqvist et al. conducted a randomized control trial (RCT) of 40 patients that included 23 patients (57.5%) with PVOD and 7 patients (17.5%) with idiopathic loss. All patients had experienced a 2‐step improvement in BTT scores following a 10‐day taper of oral prednisolone from 40 mg/day and 200 µg/day of fluticasone proprionate and were subsequently randomized into continued nasal steroids, placebo, or no further treatment for an additional 2 months. There was no difference in olfactory outcomes amongst the 3 treatment groups. 29
The remaining 3 studies were all level 4 evidence with no control groups, with the largest cohort of PVOD patients (n = 244) studied by Mori et al. 28 This study was limited by its retrospective nature and did not detail the specific topical corticosteroids or average time for follow‐up; in this setting, it is difficult to interpret the “slight improvement,” “improvement,” or “cured” nature of OD found in 57.8% of their PVOD patients. All 133 patients in the case series by Fukazawa et al. had a PVOD etiology of OD, with improvement seen in 49.6% of patients using T&T olfactometry, and an average improvement of 10.2 to 39.5 points on VAS, after injection of dexamethasone or betamethasone into the olfactory cleft. 30 Fleiner and Goktas utilized a directed beclomethasone spray therapy and demonstrated that 2/8 PVOD patients had TDI score improvement of greater than 6 points. 31
Summary: topical or local steroid therapy
Aggregate evidence: C (Level 1: 1 study, Level 4: 3 studies)
Benefit: Improved TDI and T&T scores
Harm: Minimal treatment‐related side effects (eg, local irritation, possible epistaxis), minor inconvenience
Cost: Minimal
Benefit‐Harm assessment: Balance of benefit and harm
Value judgments: Heterogeneous studies and difficult to interpret Level 1 study secondary to prior usage of systemic steroids make providing a recommendation challenging given minimal likely benefit in conjunction with the minimal harm.
Recommendation level: Option
Intervention: Low risk intervention, with potential improved olfaction, but potential benefit is also limited. Can be offered to patients with PVOD, but if no initial improvement, limited evidence suggesting benefit with chronic use.
Nonsteroidal topical therapies
In regard to nonsteroidal topical therapies (Table 7), 3 studies investigated intranasal sodium citrate. 32 , 33 , 34 Philpott et al. conducted a double‐blinded, randomized, placebo‐controlled trial of 55 patients with nonconductive OD, the majority of which had PVOD (42%) or idiopathic loss (26%). 32 Though subgroup analyses for patients with PVOD was not completed, there was a significant improvement in 3 of the 4 odor thresholds in the intervention arm compared to the control arm (p < 0.05). Two additional studies utilizing intranasal sodium citrate performed by Whitcroft et al. used Sniffin’ Sticks to evaluate objective olfactory outcomes. The first prospective, controlled trial demonstrated significant improvement in 7 patient's odor identification scores (p = 0.02), but no change in odor threshold scores (p = 0.08) in the treatment group compared to placebo. 33 A follow‐up, prospective, single‐blind, internally‐controlled study comprised exclusively of patients with PVOD identified significant improvement in composite threshold and identification scores compared to placebo (p = 0.04), but no change in odor identification or threshold compared to placebo (p = 0.11 and p = 0.23, respectively). 34 Composite TDI scores were not calculated.
Despite commonalities in treatment modality and dosages across these 3 placebo‐controlled studies, key differences must be acknowledged. While the RCT utilized bilateral sodium citrate spray versus sterile water placebo, the 2 studies by Whitcroft et al. had each patient serve as their own control‐sodium citrate spray applied to 1 nasal cavity and saline solution to the other. 33 , 34 The choice to use sterile water as the control agent instead of saline was acknowledged by Philpott et al., describing that the ionic composition of saline could have a local influence on the sodium ion concentrations involved with olfaction. 32 Additionally, timing of olfactory testing differed in these studies. Philpott et al. demonstrated peak effect of sodium citrate at 30 to 60 minutes after application; 32 however, both Whitcroft studies evaluated olfaction only 20 to 30 minutes after treatment. 33 , 34 How these findings translate to longer, clinically relevant outcomes, is unclear. Across all studies, sodium citrate spray was well tolerated, with common side effects including transient rhinorrhea, sore throat, and nasal obstruction.
Furthermore, 1 identified study investigated intranasal insulin compared to saline placebo. 35 Despite a small sample size of 10 PVOD patients, their findings supported increased odor intensity perception (p = 0.043) after intranasal insulin compared to placebo. Interestingly, there was a significant correlation between BMI and identification scores following administration of insulin (ρ = 0.909, p = 0.005). As this is a single pilot study, there is insufficient evidence for it to be included in the evidence‐based summary.
Summary: intranasal sodium citrate
Aggregate evidence: B (Level 1: 1 study, Level 2: 1 study, Level 3: 1 study)
Benefit: Short‐term and temporary improvement of post‐treatment objective olfactory measures
Harm: Minimal, including transient rhinorrhea, sore throat, and nasal congestion
Cost: Minimal
Benefit‐Harm assessment: Balance of benefit and harm
Value judgments: Though the level of evidence and rigor of these studies demonstrate promise for intranasal sodium citrate, the transient nature and short‐term follow‐up of these studies makes the prolonged clinical utility of these medications difficult to determine, but certainly further study is warranted.
Recommendation level: Option
Intervention: Likely a low risk intervention with demonstrated temporary improved olfaction, but long‐term benefit is unclear. Assessment of long‐term benefit is necessary before more definitive clinical recommendations can be made.
Nonsteroidal oral medications
Numerous nonsteroidal oral medications have been evaluated for treatment of OD, primarily composed of vitamins and antioxidants (Table 8). Antibiotics, phosphodiesterase inhibitors, and muscle relaxants have also been investigated. Though these therapies do not belong to the same treatment class, these medications benefit from wide accessibility and are generally well‐tolerated.
In regard to antibiotic treatment, 1 RCT of 55 patients with PVOD studying minocycline demonstrated that the medication was well tolerated, but there was no difference in overall TDI scores between the group receiving minocycline and the group receiving the placebo (p = 0.55). 36 Another study retrospectively assessed various antibiotics and similarly found no overall improvement in UPSIT scores after treatment; however, patients with PVOD had significantly improved odor detection thresholds after treatment with bactericidal antibiotics relative to patients who received bacteriostatic antibiotics or no treatment (p = 0.023). 37 The study did not mention which antibiotics were evaluated or the duration.
Additionally, theophylline, a bronchodilator typically reserved for chronic respiratory disease, was investigated in 2 studies. 38 , 39 Both were prospective evaluations by Henkin et al. using oral theophylline doses between 200‐800 mg: the first a case series and the second a case‐control trial. Both reports demonstrated an overall improved subjective sense of smell, as well as detection and recognition thresholds following treatment. 38 , 39 There was no comparison group in either study.
Six total studies investigated oral supplements, with 3 on zinc sulfate, 40 , 41 , 42 2 on Vitamin A, 43 , 44 and 1 on alpha‐lipoic acid. 45 One RCT from 1976 found no significant difference in olfactory thresholds between patients who received 100 mg zinc sulfate daily compared to placebo, at 3 or 6‐month follow‐up. 40 Similar results were demonstrated by Aiba et al. when comparing 300 mg zinc sulfate to zinc sulfate in combination with topical corticosteroids and vitamin B. 41 A third study by Quint et al. intended to investigate the efficacy of caroverine, a quinoxaline derivate and NMDA antagonist, used a group of patients receiving zinc sulfate as the control group in a cohort of patients with a mixed etiology of OD. 42 While caroverine was associated with improved odor thresholds (p = 0.005) and identification (p = 0.042) in anosmic patients and improved identification in hyposmic patients (p = 0.041), zinc sulfate did not have a significant effect on thresholds or identification in either anosmic or hyposmic patients (p = 0.10, p = 0.428 respectively). Specific comparative analyses for PVOD patients in each treatment group could not be fully captured, though thresholds became measurable in 13 anosmic patients after caroverine treatment, of which 6 (46%) had PVOD.
In regard to vitamin A, a case series conducted by Duncan et al. in 1962 reported improvement in subjective olfactory function. 43 Though “marked improvement” was described in 35 of 52 patients with OD of various etiologies, there was no standardized treatment protocol or dosage described. Decades later, a double‐blinded, randomized, placebo‐controlled trial by Reden et al. examined the utility of 10,000 IU of Vitamin A daily for 3 months compared to placebo for the treatment of PVOD. 44 While TDI scores increased significantly in all patients (p < 0.001), there was no significant difference between the placebo and treatment groups (p = 0.47).
Furthermore, alpha‐lipoic acid, typically used to treat diabetic neuropathy, was investigated by Hummel et al. in 23 non‐blinded patients with PVOD. 45 TDI scores significantly improved after an average of 4.5 months on 600 mg/day alpha‐lipoic acid (pre‐treatment mean: 21.05, post‐treatment mean: 24.58; p = 0.002), though they did not reach an MCID for the Sniffin’ Sticks instrument. While the duration of PVOD did not appear to influence outcomes, patients under 60 years of age had improved recovery as compared to those older than 60 years old (p = 0.018). All patients received the intervention; there was no comparison group. This is in contrast to several other studies, including those by Heilmann et al., Fleiner and Goktas, and Mori et al., who reported no correlation between olfactory outcome and patient age in their patient cohorts. 24 , 28 , 31
Because of the heterogeneity in this treatment type, we elected to summarize oral zinc sulfate independently from the other treatments that each totaled no >2 investigations.
Summary: oral zinc sulfate
Aggregate evidence: B (Level 1: 1 study, Level 2: 1 study, Level 3: 1 study)
Benefit: no studies demonstrating improved olfactory outcomes
Harm: no reported therapy‐related risks, though zinc toxicity is plausible
Cost: medication cost
Benefit‐Harm assessment: Preponderance of harm over benefit
Value judgments: None
Recommendation level: Recommendation against
Summary: oral antibiotics, theophylline, vitamin A, caroverine, alpha‐lipoic acid
Aggregate evidence: D Level 1: 2 studies, Level 2: 1 study, Level 3: 2 studies, Level 4: 3 studies
Benefit: improvement TDI scores, olfactometry, and subjective scores
Harm: minimal side effects of medications reported, but not rigorously assessed in all studies
Cost: minimal to moderate depending on cost of medication, many available without prescription
Benefit‐Harm assessment: balance of benefit and harm
Value judgments: An assortment of studies examining different medications, completed with varying degrees of rigor and quality. Despite several studies with encouraging results, interpretation of this collection of studies is challenging, though trials with promising outcomes likely warrant further study (eg, alpha‐lipoic acid).
Recommendation level: No recommendation
Olfactory training
Ten total studies assessed olfactory training (OT), with 2 level 1 studies, 2 level 2 studies, 3 level 3 studies, and 3 level 4 studies (Table 9). 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 All but 1 study used Sniffin’ Sticks to test olfactory outcomes, with the other using UPSIT. Most studies employed an OT protocol involving exposure to 4 odors twice daily for at least 12 weeks. 46
In 4 studies comparing OT to no treatment, OT was found to have statistically superior olfactory outcomes. 46 , 48 , 51 , 52 Two of these studies in particular had multiple treatment groups of solely PVOD patients: Altundag et al. compared a classical olfactory training (COT) group (36 weeks of OT) to a modified olfactory training (MOT) group (3 sets of 12 weeks of OT with different odors), 51 and Konstantinidis et al. compared a long‐term training group (56 weeks) to a short‐term training group (16 weeks). 52 Though the former study did not find significant differences in composite TDI score between the MOT and COT groups at 24 or 36 weeks, the MOT group had significantly better odor discrimination and odor identification scores at these time points. 51 The latter study concluded that long‐term training was superior to short‐term training with a significantly higher average TDI score at 56 weeks (short term: 24.1 ± 1.5 from 15 ± 2.2 baseline, long term: 27.3 ± 1.5 from 15.9 ± 2.2 baseline; p = 0.038), though both training groups showed the most olfactory improvement within the first 16 weeks. 52 Interestingly, both studies commented that a shorter duration of olfactory loss prior to treatment initiation was associated with greater improvement in olfactory function after OT treatment. In a crossover RCT, Damm et al. demonstrated greater improvement in OT with high concentration odors compared to low‐concentration odors in patients with a duration of PVOD less than 12 months (p = 0.03). 49
Just as Damm et al. studied different concentrations of the odors used in OT, 49 Poletti et al. conducted a prospective, pseudo‐randomized trial in which patients underwent OT with either low molecular weight (<150 g/mol) or high molecular weight (>150 g/mol) odorants. In this study, they concluded that high molecule weight odorants (eg, ethyl vanilline) were superior in improving the phenyl ethyl alcohol (PEA) threshold relative to low molecular weight odorants (eg, ethyl maltol) in PVOD patients (p = 0.004). 53 Nguyen and Patel also attempted to optimize the OT protocol with a RCT comparing OT with budesonide irrigation compared to OT with saline irrigation. 54 Though this study did not perform subgroup analysis for PVOD patients, they found that 43.9% of patients had olfactory improvement with budesonide irrigation and OT compared to 26.9% of controls (p = 0.039); additionally, a shorter duration of olfactory loss was significantly associated with olfactory improvement (p < 0.0001). 54 Interestingly, even in absence of robust TDI score improvement, Kollndorfer et al. demonstrated enhanced organization of neural connectivity to the piriform cortices on functional magnetic resonance imaging following traditional OT. 55
Overall, OT was found to improve olfactory functioning in all 10 studies. Higher concentrations and molecular weights of the odors, longer duration of OT, and a variety of odors used for OT found to be most helpful in improving olfactory function. A shorter duration of OD prior to initiation of OT was also repeatedly associated with better olfactory function outcomes.
Summary:
Aggregate evidence: B (Level 1: 2 studies, Level 2: 2 studies, Level 3: 3 studies, Level 4: 3 studies)
Benefit: Improved Sniffin’ Sticks and UPSIT scores
Harm: minimal, inconvenience of daily training
Cost: Minimal with good access to training kits, though in countries with limited proprietary kits available, costs may be increased.
Benefit‐Harm assessment: Preponderance of benefit over harm
Value judgments: Given that this is an inexpensive option with minimal/no harm and likely benefit, the value of this option is high.
Recommendation level: Recommendation
Intervention: Begin OT following identification of patient with lasting PVOD. Consider augmenting OT with topical budesonide therapy, however further investigation into optimal OT treatment protocol is warranted.
Acupuncture
Traditional Chinese acupuncture (TCA) was evaluated as a treatment for PVOD in 2 level 4 studies after failure to respond to 1 to 6 months of oral steroids, vitamin B, olfactory training, or topical steroids (Table 10). 56 , 57 Vent et al. demonstrated an increase in mean TDI score from 13.5 to 17.9 after TCA. 57 Additionally, 8/15 patients attained an increase in TDI score of ≥6 points (MCID), significantly improved compared to controls receiving vitamin B complex (p = 0.02). 57 Dai et al. produced similar results, with 11/25 patients in the TCA group having improved olfactory function compared to 4/25 in the no treatment group (p = 0.031). 56 Frequency of TCA treatment differed between these 2 studies, and neither paper reported any adverse events related to TCA.
Summary:
Aggregate evidence: D (Level 3: 1 study, Level 4: 1 study)
Benefit: Improved TDI and UPSIT scores
Harm: Minimal harm or treatment‐related risk
Cost: Minimal to moderate, depending on cost of therapy.
Benefit‐Harm assessment: Balance of benefit and harm
Value judgments: Limited low‐level evidence is beneficial, but challenging to make a firm recommendation given few studies and low level of evidence. Much like surgical interventions, blinding proves challenging in treatment with TCA.
Recommendation level: No recommendation
Discussion
This evidence‐based review with recommendations spans 7 decades of research on PVOD and includes 36 investigations on diverse medical and non‐traditional therapies. In this review, olfactory training has emerged as the most efficacious treatment option for PVOD, supported by the highest level of evidence, a low risk profile, and is a recommendation for the treatment of PVOD. Our review revealed a common theme that a shorter duration of OD prior to OT was found to be associated with improved olfactory outcomes, such that earlier intervention with OT yields better outcomes. 48 , 49 , 50 , 51 , 52 , 54 Though not identified in the included studies, treatment compliance with OT is challenging in some reports. 58
Moreover, systemic or topical steroids are among the most widely acknowledged treatment options for OD, thought to be effective in PVOD by reducing subclinical inflammation. 4 , 59 , 60 However, given the weak evidence available, the potential for olfactory improvement after systemic steroid therapy must be considered against the tangible risks and side effects related to these medications. Despite an encouraging safety profile of topical steroid application, the heterogeneous data presented here makes conclusions regarding their use challenging. One exception may be the addition of topical budesonide therapy to OT, which showed good efficacy in the RCT by Nguyen and Patel. 54 Overall, this suggests that use of short‐term systemic and/or topical steroids is an appropriate option in a select subset of patients without underlying risk factors, after a thorough discussion on the potential risks of steroids has taken place with the provider.
Studies of nonsteroidal oral and topical medications are heterogeneous in nature. Though there is reassuring pilot data for oral medications like alpha‐lipoic acid, 45 phosphodiesterase inhibitors, 38 , 39 and caroverine, 42 these studies are limited in both size and study design. The data for intranasal sodium citrate spray shows great promise from initial studies, but more definitive data is needed with clinically relevant long‐term outcomes. There is not enough evidence at this time to warrant a recommendation of these treatments for clinical use. Based on current evidence, antibiotic treatment, zinc sulfate, vitamin A, and Gingko biloba failed to demonstrate clinical efficacy in controlled studies and do not appear to play a role in the management of PVOD.
At the time of this writing, in the face of the COVID‐19 pandemic, we now know that a significant proportion of patients infected with SARS‐CoV‐2 have at least a temporary olfactory loss. 8 , 9 , 10 It is plausible that if even a small fraction of patients experience lasting OD, this could represent an enormous total number. 53 Given the relatively recent appreciation of this form of viral‐associated OD, definitive outcomes of COVID‐associated PVOD are not yet fully understood. It is nonetheless notable though, that evidence from investigations of prior coronavirus outbreaks (SARS‐CoV‐1 and Middle East respiratory syndrome (MERS)), suggests that systemic corticosteroid treatment may impair viral clearance from the body. 61 As such, based on our current understanding of the available evidence, there may be additional risk associated with systemic steroid therapy for the treatment of COVID‐19‐associated PVOD in the acute setting, and it should likely be avoided. We believe in light of the efficacy of OT and relative paucity of other effective pharmacotherapies for non‐COVID PVOD, this knowledge should serve as an impetus to increase the prompt implementation of OT in patients experiencing PVOD following infection with SARS‐CoV‐2.
Conclusion
This review evaluated all reported treatment options for the management of PVOD and their associated outcomes, based on a specific protocol for evidence‐based review and recommendations. An evidence‐based treatment algorithm of patients with PVOD includes a recommendation of the use of OT, ideally with early utilization following the onset of the PVOD. Furthermore, in the appropriate setting, healthcare providers may offer a course of systemic or topical steroids, after acknowledging the risks associated with systemic steroids and the potential lack of added benefit.
Potential future research options should directly investigate patients with POVD, distinct from other etiologies, and include:
Evaluation of optimal timing of initiation of olfactory training.
Evaluation of strategies to improve OT compliance and accessibility in regions where OT is less commonly utilized (eg, United States)
Further evaluation of adjunctive therapies (eg, oral or topical steroids) to olfactory training that may augment treatment outcomes.
More rigorous evaluation and longer‐term outcomes of promising therapeutic strategies such as alpha‐lipoic acid and topical therapies (eg, sodium citrate).
Evaluation of impact of timing of initial therapies on treatment outcomes.
How to Cite this Article:Hura N, Xie DX, Choby GW, et al. Treatment of post‐viral olfactory dysfunction: an evidence‐based review with recommendations. Int Forum Allergy Rhinol. 2020;10:1065–1086.
Funding sources for the study: RJS is a consultant for Stryker, Optinose, GSK, a medical director for Healthy Humming and has received grant support from Stryker, Optinose and Healthy Humming. GWC is a consultant for Tissium, LLC, and Intersect ENT, Inc.
Potential conflict of interest: None provided.
To be submitted for a podium presentation at the 2020 annual American Rhinologic Society Meeting in Boston, MA.
References
- 1. Van Regemorter V, Hummel T, Rosenzweig F, et al. Mechanisms linking olfactory impairment and risk of mortality. Front Neurosci. 2020;14:140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kohli P, Soler ZM, Nguyen SA, et al. The association between olfaction and depression: a systematic review. Chem Senses. 2016;41(6):479‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pinto JM, Wroblewski KE, Kern DW, et al. Olfactory dysfunction predicts 5‐year mortality in older adults. PLoS One. 2014;9(10):e107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinology. 2017;54:1‐30. [DOI] [PubMed] [Google Scholar]
- 5. Deems DA, Doty RL, Settle G, et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Neck Surg. 1991;117(5):519‐528. [DOI] [PubMed] [Google Scholar]
- 6. Doty R. Handbook of Olfaction and Gustation. 3rd ed. Wiley‐Blackwell, Malden, MA, 2015. [Google Scholar]
- 7. Suzuki M, Saito K, Min W, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klopfenstein T, Kadiane‐Oussou NJ, Toko L, et al. Features of anosmia in COVID‐19. Médecine Mal Infect. 2020. 10.1016/j.medmal.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moein ST, Hashemian SMR, Mansourafshar B, et al. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. 2020:alr.22587. 10.1002/alr.22587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Oto‐Rhino‐Laryngology. 2020. 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reden J, Mueller A, Mueller C, et al. Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch Otolaryngol Neck Surg. 2006;132(3):265‐269. [DOI] [PubMed] [Google Scholar]
- 12. Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta‐analysis. Int Forum Allergy Rhinol. 2016;6(3):299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosenfeld RM, Shiffman RN, Robertson P. Clinical practice guideline development manual, third edition: a quality‐driven approach for translating evidence into action. Otolaryngol ‐ Head Neck Surg. 2013;148(Suppl 1):S1‐55. [DOI] [PubMed] [Google Scholar]
- 14. Shiffman RN, Shekelle P, Overhage JM, et al. Standardized reporting of clinical practice guidelines: a proposal from the conference on guideline standardization. Ann Intern Med. 2003;139(6):493‐498. [DOI] [PubMed] [Google Scholar]
- 15. Rudmik L, Smith TL. Development of an evidence‐based review with recommendations using an online iterative process. Int Forum Allergy Rhinol. 2011;1(6):431‐437. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2.
- 18. Savović J, Weeks L, Sterne JAC, et al. Evaluation of the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials: focus groups, online survey, proposed recommendations and their implementation. Syst Rev. 2014;3(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2019.
- 20. Classifying recommendations for clinical practice guidelines. Pediatrics. 2004;114(3):874‐877. [DOI] [PubMed] [Google Scholar]
- 21. Seo BS, Lee HJ, Mo JH, et al. Treatment of postviral olfactory loss with glucocorticoids, Ginkgo biloba, and mometasone nasal spray. Arch Otolaryngol ‐ Head Neck Surg. 2009;135(10):1000‐1004. [DOI] [PubMed] [Google Scholar]
- 22. Kim DH, Kim SW, Hwang SH, et al. Prognosis of olfactory dysfunction according to etiology and timing of treatment. Otolaryngol ‐ Head Neck Surg (United States). 2017;156(2):371‐377. [DOI] [PubMed] [Google Scholar]
- 23. Schriever VA, Merkonidis C, Gupta N, et al. Treatment of smell loss with systemic methylprednisolone. Rhinology. 2012;50(3):284‐289. [DOI] [PubMed] [Google Scholar]
- 24. Heilmann S, Huettenbrink KB, Hummel T. Local and systemic administration of corticosteroids in the treatment of olfactory loss. Am J Rhinol. 2004;18(1):29‐33. [PubMed] [Google Scholar]
- 25. Stenner M, Vent J, Hüttenbrink KB, et al. Topical therapy in anosmia: relevance of steroid‐responsiveness. Laryngoscope. 2008;118(9):1681‐1686. [DOI] [PubMed] [Google Scholar]
- 26. Ikeda K, Sakurada T, Suzaki Y, Takasaka T. Efficacy of systemic corticosteroid treatment for anosmia with nasal and paranasal sinus disease. Rhinology. 1995;33(3):162‐165. [PubMed] [Google Scholar]
- 27. Gudziol V, Lötsch J, Hähner A, et al. Clinical significance of results from olfactory testing. Laryngoscope. 2006;116(10):1858‐1863. [DOI] [PubMed] [Google Scholar]
- 28. Mori J, Aiba T, Sugiura M, et al. Clinical study of olfactory disturbance. Acta Otolaryngol Suppl. 1998;538:197‐201. [PubMed] [Google Scholar]
- 29. Blomqvist EH, Lundblad L, Bergstedt H, Stj̈arne P. Placebo‐controlled, randomized, double‐blind study evaluating the efficacy of fluticasone propionate nasal spray for the treatment of patients with hyposmia/anosmia. Acta Otolaryngol. 2003;123(7):862‐868. [DOI] [PubMed] [Google Scholar]
- 30. Fukazawa K. A local steroid injection method for olfactory loss due to upper respiratory infection. Chem Senses. 2005;30(suppl 1):212‐213. [DOI] [PubMed] [Google Scholar]
- 31. Fleiner F, Goktas O. Topical beclomethasone in the therapy of smelling disorders—a new application technique. Indian J Otolaryngol Head Neck Surg. 2011;63(1):5‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Philpott CM, Erskine SE, Clark A, et al. A randomised controlled trial of sodium citrate spray for non‐conductive olfactory disorders. Clin Otolaryngol. 2017;42(6):1295‐1302. [DOI] [PubMed] [Google Scholar]
- 33. Whitcroft KL, Merkonidis C, Cuevas M, et al. Intranasal sodium citrate solution improves olfaction in post‐viral hyposmia. Rhinology. 2016;54(4):368‐374. [DOI] [PubMed] [Google Scholar]
- 34. Whitcroft KL, Ezzat M, Cuevas M, et al. The effect of intranasal sodium citrate on olfaction in post‐infectious loss: results from a prospective, placebo‐controlled trial in 49 patients. Clin Otolaryngol. 2017;42(3):557‐563. [DOI] [PubMed] [Google Scholar]
- 35. Schöpf V, Kollndorfer K, Pollak M, et al. Intranasal insulin influences the olfactory performance of patients with smell loss, dependent on the body mass index: a pilot study. Rhinology. 2015;53(4):371‐378. [DOI] [PubMed] [Google Scholar]
- 36. Reden J, Herting B, Lill K, et al. Treatment of postinfectious olfactory disorders with minocycline: a double‐blind, placebo‐controlled study. Laryngoscope. 2011;121(3):679‐682. [DOI] [PubMed] [Google Scholar]
- 37. Wang J‐J, Chen J, Doty RL. Impact of antibiotics on smell dysfunction. World J Otorhinolaryngol ‐ Head Neck Surg. 2018;4(1):33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Henkin RI, Velicu I, Schmidt L. An open‐label controlled trial of theophylline for treatment of patients with hyposmia. Am J Med Sci. 2009;337(6):396‐406. [DOI] [PubMed] [Google Scholar]
- 39. Henkin RI, Hosein S, Stateman WA, et al. Improved smell function with increased nasal mucus sonic hedgehog in hyposmic patients after treatment with oral theophylline. Am J Otolaryngol. 2017;38(2):143‐147. [DOI] [PubMed] [Google Scholar]
- 40. Henkin RI, Schecter PJ, Friedewald WT, et al. A double blind study of the effects of zinc sulfate on taste and smell dysfunction. Am J Med Sci. 1976;272(3):285‐299. [DOI] [PubMed] [Google Scholar]
- 41. Aiba T, Sugiura M, Mori J, et al. Effect of zinc sulfate on sensorineural olfactory disorder. Acta Otolaryngol. 1998;118(538):202‐204. [DOI] [PubMed] [Google Scholar]
- 42. Quint C, Temmel AFP, Hummel T, Ehrenberger K. The quinoxaline derivative caroverine in the treatment of sensorineural smell disorders: a proof‐of‐concept study. Acta Otolaryngol. 2002;122(8):877‐881. [PubMed] [Google Scholar]
- 43. Duncan RB, Briggs M. Treatment of uncomplicated anosmia by vitamin A. Arch Otolaryngol. 1962;75(2):116‐124. [DOI] [PubMed] [Google Scholar]
- 44. Reden J, Lill K, Zahnert T, et al. Olfactory function in patients with postinfectious and posttraumatic smell disorders before and after treatment with vitamin A: a double‐blind, placebo‐controlled, randomized clinical trial. Laryngoscope. 2012;122(9):1906‐1909. [DOI] [PubMed] [Google Scholar]
- 45. Hummel T, Heilmann S, Hüttenbriuk KB. Lipoic acid in the treatment of smell dysfunction following viral infection of the upper respiratory tract. Laryngoscope. 2002;112(11):2076‐2080. [DOI] [PubMed] [Google Scholar]
- 46. Hummel T, Rissom K, Reden J, et al. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009;119(3):496‐499. [DOI] [PubMed] [Google Scholar]
- 47. Fleiner F, Lau L, Ö Göktas. Active olfactory training for the treatment of smelling disorders. Ear Nose Throat J. 2012;91(5):198‐215. [DOI] [PubMed] [Google Scholar]
- 48. Konstantinidis I, Tsakiropoulou E, Bekiaridou P, et al. Use of olfactory training in post‐traumatic and postinfectious olfactory dysfunction. Laryngoscope. 2013;123(12):85‐90. [DOI] [PubMed] [Google Scholar]
- 49. Damm M, Pikart LK, Reimann H, et al. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 2014;124(4):826‐831. [DOI] [PubMed] [Google Scholar]
- 50. Geißler K, Reimann H, Gudziol H, et al. Olfactory training for patients with olfactory loss after upper respiratory tract infections. Eur Arch Oto‐Rhino‐Laryngology. 2014;271(6):1557‐1562. [DOI] [PubMed] [Google Scholar]
- 51. Altundag A, Cayonu M, Kayabasoglu G, et al. Modified olfactory training in patients with postinfectious olfactory loss. Laryngoscope. 2015;125(8):1763‐1766. [DOI] [PubMed] [Google Scholar]
- 52. Konstantinidis I, Tsakiropoulou E, Constantinidis J. Long term effects of olfactory training in patients with post‐infectious olfactory loss. Rhinology. 2016;54(2):170‐175. [DOI] [PubMed] [Google Scholar]
- 53. Poletti SC, Michel E, Hummel T. Olfactory training using heavy and light weight molecule odors. Perception. 2017;46(3‐4):343‐351. [DOI] [PubMed] [Google Scholar]
- 54. Nguyen TP, Patel ZM. Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. Int Forum Allergy Rhinol. 2018;8(9):977‐981. [DOI] [PubMed] [Google Scholar]
- 55. Kollndorfer K, Kowalczyk K, Hoche E, et al. Recovery of olfactory function induces neuroplasticity effects in patients with smell loss. Neural Plast. 2014;2014:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dai Q, Pang Z, Yu H. Recovery of olfactory function in postviral olfactory dysfunction patients after acupuncture treatment. Evidence‐Based Complement Altern Med. 2016;2016:4986034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vent J, Wang D‐W, Damm M. Effects of traditional Chinese acupuncture in post‐viral olfactory dysfunction. Otolaryngol Neck Surg. 2010;142(4):505‐509. [DOI] [PubMed] [Google Scholar]
- 58. Schriever VA, Lehmann S, Prange J, Hummel T. Preventing olfactory deterioration: olfactory training may be of help in older people. J Am Geriatr Soc. 2014;62(2):384‐386. [DOI] [PubMed] [Google Scholar]
- 59. Harless L, Liang J. Pharmacologic treatment for postviral olfactory dysfunction: a systematic review. Int Forum Allergy Rhinol. 2016;6(7):760‐767. [DOI] [PubMed] [Google Scholar]
- 60. Yan CH, Overdevest JB, Patel ZM. Therapeutic use of steroids in non‐chronic rhinosinusitis olfactory dysfunction: a systematic evidence‐based review with recommendations. Int Forum Allergy Rhinol. 2019;9(2):165‐176. [DOI] [PubMed] [Google Scholar]
- 61. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet. 2020;395(10223):473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]


