Abstract
Objective
Concerns have been raised by healthcare organisations in New Zealand that routine mask use by healthcare workers (HCW) may increase the risk of transmission of SARS‐CoV‐2 through increased face touching. Routine mask use by frontline HCW was not recommended when seeing ‘low risk’ patients. The aim of this review was to determine the carriage of respiratory viruses on facemasks used by HCW.
Methods
A systematic review was conducted with structured searches of medical and allied health databases. Two authors independently screened articles for inclusion, with substantial agreement (k = 0.66, 95% CI 0.54–0.79). Studies that at least one author recommended for full text review were reviewed in full for inclusion. Two authors independently extracted data from included studies including the setting, method of analysis and results. There was exact agreement on the proportion of virus detected on masks.
Results
We retrieved 1233 titles, 47 underwent full text review and five studies reported in four articles were included. The studies were limited by small numbers and failure to test all eligible masks in some studies. The proportion in each study ranged from 0 (95% CI 0–10) to 25% (95% CI 8–54). No study reported clinical respiratory illness as a result of virus on the masks.
Conclusions
Although limited, current evidence suggests that viral carriage on the outer surface of surgical masks worn by HCW treating patients with clinical respiratory illness is low and there was not strong evidence to support the assumption that mask use may increase the risk of viral transmission.
Keywords: contamination; healthcare workers; mask; review, systematic; virus
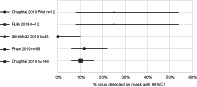
A systematic review found the rate of virus detection on surgical masks of healthcare workers averaged 10.2% (95%CI 7 to 14%), five studies, n = 283.
Key findings.
There is a low chance of viral carriage on masks worn by healthcare workers.
There were no studies relating viral carriage on masks to increased risk of clinical respiratory illness.
Introduction
During the current novel coronavirus disease 2019 (SARS‐CoV‐2, COVID‐19) pandemic, the Ministry of Health (MOH) and District Health Boards have not recommended routine use of surgical masks for healthcare workers (HCW) in the ED in New Zealand (NZ). Such advice was contrary to the experience of countries that had faced similar pandemics previously who recommended use of masks for ED staff within days of the first cases presenting. 1 , 2
The initial drivers for this were a belief that the risk to HCW from patients without epidemiological (travel/known contact) risk factors and clinical respiratory illness (CRI) and fever was very low and that overuse of masks could jeopardise the available supply later in the pandemic when the prevalence of CRI in the population presenting to ED would be higher. This advice was consistent with the contemporaneous World Health Organization (WHO) guidelines on rational use of personal protective equipment (PPE) for coronavirus disease 2019, based on droplets being the most likely mode of virus transmission. 3 However, emerging evidence from the current SARS‐CoV‐2 pandemic suggests that aerosol and asymptomatic spread are both possible. 4 , 5 , 6
The case definition in NZ subsequently changed to include any respiratory illness regardless of fever or epidemiological risk. Evidence has also emerged that as many of 50% of infected people are asymptomatic. 6 , 7 This prompted a change in advice such that currently mask use is permitted, with warnings that incorrect use of masks may be harmful, including concerns that mask use may ‘actually increase your risk of COVID‐19’. 8 This is consistent with the advice given by the MOH that:
‘Incorrect use of PPE may cause more harm than good and may contribute to an increased spread of disease. If PPE is not used properly health care workers can put themselves, clients/patients, their colleagues, family/whānau and community at greater risk. For example, wearing the same pair of gloves for long periods of time may lead to transfer of infectious material from one surface to another. Similarly, wearing a mask for long periods may lead to contamination of the wearer's face if they rub their nose or eyes after their hand has touched the mask’. 9
The aim of this review was to determine the carriage of respiratory viruses on facemasks used by HCW in acute care settings, to inform a recommendation on mask usage in the ED in the setting of an emerging viral pandemic. The primary outcome is the proportion of masks positive for any respiratory viruses. The secondary aim was to determine whether viral carriage on masks used by HCW increased or decreased the risk of CRI for staff.
Method
Literature search and data extraction
Structured searches were conducted in Medline, Embase and CInAHL using free text and MeSH terms for ‘mask’; ‘touch’; ‘nosocomial infection’; ‘contamination’ and ‘virus’ (Appendix S1). The final search was run on 23 April 20. These were supplemented by a citation search of included articles. There was no restriction on year or language.
Two authors independently screened titles and abstracts for relevance and selected articles for full text review. Articles were included if they were clinical studies that reported virus detection on masks worn by HCW. Experimental studies and computer simulation studies were excluded, as were letters to the editor or opinion pieces. Agreement between authors on study selection was substantial, 10 with 97.6% exact agreement (k = 0.66, 95% CI 0.54–0.79). All studies that at least one author recommended for full text review were reviewed in full for inclusion. Two authors independently extracted data from included studies into a table including the setting, type of study, method of analysis and results. There was exact agreement on the proportion of virus detected on masks from the included studies.
Data analysis
Data from included studies were shown using descriptive statistics: n, proportion, 95% confidence interval (calculated using Graphpad https://www.graphpad.com/quickcalcs/confInterval1/, San Diego, CA, USA).
Unit of analysis issues
When it was unclear whether studies reported virus detection on multiple sites on the same mask, we reported the highest and lowest possible proportions.
Risk of bias
Risk of bias was assessed in the studies based on mask selection, method of sampling and detection, and reporting and rated as high, low or unclear.
Ethics and study registration
As a secondary analysis of published aggregate data, ethical approval was not required. This review was not registered in a review registry.
Patient and public involvement
Patients and the public were not involved in the present study.
Results
The searches retrieved 1233 titles and abstracts, 1186 were either irrelevant or duplicates and 47 underwent full text review (Fig. 1). Forty studies did not report viral presence on masks and three were simulation or theoretical modelling studies 11 , 12 , 13 so were excluded. Five studies reported in four articles met the inclusion criteria and were included. 14 , 15 , 16 , 17
Figure 1.
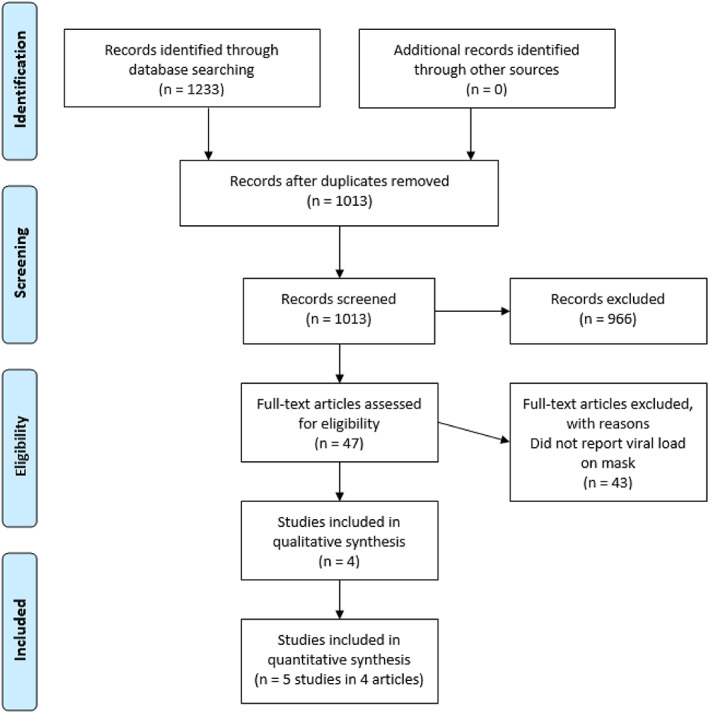
Study selection.
The settings, methods, proportion positive and types of viruses are presented in Table 1. The risk of bias for each study is also shown in this table.
TABLE 1.
Studies reporting virus detection on the facemasks of HCW
| Study, n, site | Sampling and detection method | Virus detected | Proportion of masks with detectable virus | CRI | Risk of bias |
|---|---|---|---|---|---|
|
Chughtai 2019 15 Main study China, n = 158 staff Respiratory, internal medicine and paediatric wards |
Prospective observational study Standard medical mask used continuously 99/148 used same mask >6 h 49/148 used mask ≤6 h Laboratory experiments to identify position on mask more likely to have virus Masks collected in zip lock bags and frozen until tested. Outer layer of mask removed and placed in phosphate buffered saline to elute viral particle. Centrifuged and filtrate used for PCR. Viral DNA/RNA extracted using Kingfisher Flex 96 viral purification kit. rtPCR to amplify 15 target genes including Influenza A/B; Rhinovirus, RSV, Corona virus, Metapneumovirus |
Adenovirus 7 Bocavirus 2 Metapneumovirus 1 Influenza B and Parainfluenza 1 Influenza B and H1N1 1 RSV 2 Parainfluenza 1 |
10 excluded for multiple samples, 148 analysed 15/148 (10.1%, 95% CI 6–16%) positive >6 h 14/99 (14%, 95% CI 8–22%) ≤6 h 1/49 (2%, 95% CI 0–15%) Adjusted analysis found independent associations with Continuous mask use: more than 6 h OR 7.9 (95% CI 1.01–62) and Seeing more than 25 patients a shift OR 5 (95% CI 1.35–18.6) |
Not reported |
Low for sampling Unclear for detection (no mention of limit of detection) Low for reporting |
|
Chughtai 2019 15 Pilot study n = 12 staff in Sydney, Australia (ID, ICU and respiratory wards) |
Prospective observational study Standard medical mask for at least 30 min Laboratory experiments to identify mask sites likely to have virus. Masks divided into six sections, samples from the upper 3 sections x 12 masks (n = 36) Viral extraction as above Total nucleic acid extracted on Kingfisher Flex using MagNA Pure total Nucleic Acid Isolation Kit. Respiratory virus detected using the Seegene Allplex Respiratory Panel Assay |
Enterovirus |
3/36 samples positive (two from outer sections, one from middle section). Unclear if these were from the same or different masks, so virus detection per mask ranges from 1 to 3 of 12 1/12 (8.3%, 95% CI 0–38%) to 3/12 (25%, 95% CI 8–54%) |
Not reported |
Unclear for sampling and detection (no mention of limit of detection) Low for reporting |
|
Phan 2019 16 USA, n = 59 staff Inpatient wards caring for 52 CRI patients with Influenza A (n = 23) Influenza B (n = 8) Rhinovirus (n = 15) Parainfluenza (n = 1) Coronavirus (n = 1) RSV (n = 1) Adenovirus (n = 1) |
Prospective observational study Type of mask not stated Staff were swabbed with Copan swabs before and after doffing masks and other PPE, including a 2 cm area of the face where the mask edge had been Samples stored on ice. RNA extracted from swab. Preamplification and qPCR analysis, Ct = 40 was limit of detection |
Influenza 6/42 masks 2/21 faces Rhinovirus 1/19 masks 0/8 faces ‘Other’ 1/7 masks 0/1 faces |
59 staff with 70 care episodes 8/68 (12%, 95% CI 6–22%) positive, mean 25 viral copies/cm3 Post doffing: 2/30 6.6% (95% CI 1–22%) faces positive |
Not reported |
Low for sampling and detection Low for reporting |
|
Rule 2018 17 USA, n = 30 clean‐shaven ED staff who saw ILI patients in ED during a shift in the influenza season |
Prospective observational study Staff wore bioaerosol samplers Surgical Masks and FFR (N95), worn for 6 h Masked in zip‐lock bags stored frozen until analysed 25 mm coupon punched from mask, four coupons placed in 8 mL Hanks solution. Viral particles eluted overnight at 4°C MagMax‐96 viral isolation kit Final viral volume 32 μL, transcribed to cDNA for qPCR analysis, Limit of detection 10 viral copies per sample |
Influenza A |
30 staff 12/128 FFR analysed (9.4%) 0/205 Surgical masks analysed (0%) 3/12 FFR 25% (95% CI 8–54%), mean 20 viral copies/cm2 ‘low’ |
Not reported |
High for sampling and detection (only used masks with high risk of contamination) Low for reporting |
|
Ahrenholz 2018 14 USA, n = 12 staff in student health centre, 2 weeks in the influenza season |
Prospective observational study Surgical masks Surgical mask worn at start of shift for 10 min as control Surgical mask worn after ILI patient stored frozen for analysis Surgical masks worn for <30 to >60 min on any day (much variation) 25 mm coupon punched from mask, four coupons placed in 8 mL Hanks solution. Viral particles eluted overnight at 4°C MagMax‐96 viral isolation kit Final viral volume 40 μL, transcribed to cDNA for qPCR analysis. Limit of detection 10 viral copies per sample |
Influenza |
12 staff 295/381 surgical masks submitted for analysis 43/295 study surgical masks analysed (15%), including all 4 directly exposed to cough/sneeze 11/11 controls analysed (100%) 0/43 0% (95% CI 0–10%) study surgical masks positive 0/11 0% (95% CI 0–30%) control surgical masks positive |
Not reported |
High for sampling and detection (only sampled 15% of available masks) Low for reporting |
CRI, clinical respiratory illness; FFR, filtering facepiece respirator; HCW, healthcare workers; OR, odds ratio; qPCR, quantitative polymerase chain reaction.
The proportion in each study ranged from 0 (95% CI 0–10) to 25% (95% CI 8–54). For the largest study with 148 participants, the proportion was 10.1% (95% CI 6–16), shown in Figure 2. None of the included studies reported whether any staff subsequently developed CRI related to detectable virus on their masks.
Figure 2.
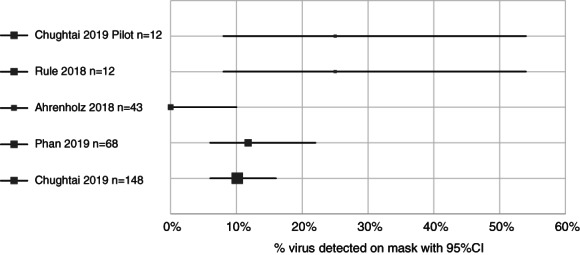
Proportion of virus detection on facemasks worn by healthcare workers.
Discussion
This is the first systematic review of viral detection on masks worn by HCW to the best of our knowledge.
No studies were conducted in the ED setting in the context of an emerging viral pandemic, which means the evidence relating to ED is indirect.
The available evidence suggests that between 0 and 25% of masks worn by staff seeing patients with symptomatic viral illness had a detectable virus and few had virus detected on their faces after doffing masks. Where reported, the viral loads on masks were small, and infectivity was not reported. Without a control group not wearing masks (which may be considered unethical) it is not possible to say whether this was better or worse than not wearing a mask.
The studies ranged in quality, with the main methodological concern being lack of testing of all eligible masks in several studies. There was a tendency for more testing in higher risk settings and masks that were more likely to be contaminated, which would bias towards finding a higher proportion of viral carriage on the tested masks. While all five studies used molecular methods to detect viral particles, the method of sampling differed with two studies reported in one article removing the outer layer of the mask, 15 two punching full‐thickness 25 mm coupons from the mask 14 , 17 and one swabbing the surface of the outer layer. 16 Three studies reported the level of detection for the polymerase chain reaction assay. 14 , 16 , 17 This limits the comparison between studies.
With respect to whether wearing masks increases facial touching by HCW, one study found that HCW wearing masks touched their faces during 29% and heads in 8% of care episodes for patients with CRI. The median number of mask contacts ranged from one per hour in the near patient zone and five per hour in the far patient zone. 18 In the present study, there was no control group to see how often staff touched their faces or heads when not wearing masks. In comparison, a study of medical students in a lecture found the rate of face touching to be 23 times per hour per student (without masks). 19 Another study found that gloves (31%) and gowns (21%) of HCW had more detectable virus than masks after single use caring for a patient with CRI (12%). 16
Wearing masks for more than 6 h continuously and seeing more than 25 patients per shift were associated with a higher chance of mask contamination in one study. 15 None of the included studies reported CRI in the staff studied, so it is not possible to say whether detecting virus on the mask leads to a higher risk of contracting CRI.
Systematic review evidence from a previous coronavirus pandemic suggests that general use of masks may be protective for HCW in this setting, 20 , 21 with a Number Needed to Treat of six to prevent one HCW infection (meta‐analysis of case control studies). 22 In contrast, there is one case report of a HCW who contracted Middle Eastern Respiratory Syndrome‐related coronavirus (MERS‐CoV) after performing resuscitation for 1 h in full PPE on a patient with cardiac arrest because of MERS‐CoV pneumonia with gross haemoptysis. 23 During the resuscitation the staff member was seen to adjust their mask and goggles with a heavily soiled glove. General use of masks by staff early in the course of the current 1 and previous 2 , 24 coronavirus pandemics has been associated with prevention of nosocomial infection, when used as part of a bundle of infection control measures, including: pre‐triage assessments to facilitate streaming of patients according to risk and prevent febrile patients entering general areas of the ED, placement of patients in single rooms, rigorous attention to hand hygiene and staff education around correct use of all PPE.
The current WHO advice on use of surgical masks emphasises that these should be prioritised for HCW rather than for general public use in the community. The advice for HCW is to wear a surgical mask when entering rooms ‘where patients with suspected or confirmed COVID‐19 are admitted’ but does not address the use of surgical masks by HCW in ED who are seeing other patients. 25 , 26
Given the low proportion of virus detection on masks and lack of evidence that this is linked to CRI, it may be prudent for HCW in the ED to wear masks routinely in clinical areas as part of a comprehensive bundle of measures to prevent nosocomial infection. This is especially so when appropriate physical distancing is not possible. For example in settings such as the ED, when the prevalence of a pandemic virus in the general population is high or unknown, especially in the early phase of a pandemic when the exact routes of transmission are unclear.
To reduce the chance of self‐inoculation viral carriage, staff should be trained in the correct use of mask donning and doffing (removing mask from behind). Masks should be changed at regular intervals while reviewing patients in the ED setting; expert opinion is after a session of care 27 (or a maximum of 4 h). Staff should remove the mask if visibly soiled, it becomes damp or when taking breaks. Hand hygiene before donning and after doffing the mask is most important to help reduce the risk of hand‐face‐hand contamination.
Limitations
There were only five relevant studies with a small number of masks tested and not all studies reported the level of detection used. The current evidence of viral carriage on masks is indirect with respect to SARS‐CoV‐2 as only one patient with proven coronavirus infection was included in any of the studies, none of the detected viruses were coronaviruses and the settings were not ED in the time of a coronavirus pandemic. All of the studies were done in settings where staff were treating patients with CRI, so the proportion of viral detection reported is likely to be higher than in settings where staff are treating patients with no CRI symptoms.
Conclusion
Although limited, current evidence suggests that viral carriage on the outer surface of surgical masks worn by HCW treating patients with CRI is between 0 and 25%.
Competing interests
PJ is a section editor for Emergency Medicine Australasia.
Supporting information
Appendix S1. Search strategy in Ovid Medline 23/4/2020.
Peter Jones, MBChB, PhD, FACEM, Emergency Physician; Sally Roberts, MBChB, FRACP, FRCPA, Clinical Microbiologist; Cheri Hotu, MBChB, FRACP, Physician; Sinan Kamona, MBChB, FACEM, Emergency Physician.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
- 1. Cheng VCC, Wong SC, Chen JHK et al. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID‐19) due to SARS‐CoV‐2 in Hong Kong. Infect. Control Hosp. Epidemiol. 2020; 41: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ki HK, Han SK, Son JS, Park SO. Risk of transmission via medical employees and importance of routine infection‐prevention policy in a nosocomial outbreak of Middle East respiratory syndrome (MERS): a descriptive analysis from a tertiary care hospital in South Korea. BMC Pulm. Med. 2019; 19: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Rational use of personal protective equipment for coronavirus disease 2019 (COVID‐19). Interim guidance 27 February 2020, 2020. [Cited 14 Apr 2020.] Available from URL: https://apps.who.int/iris/handle/10665/331215
- 4. Zhen‐Dong G, Zhong‐Yi W, Shou‐Feng Z et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. J. 2020; 26: 1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoot N, Aronsky D. An early warning system for overcrowding in the emergency department. AMIA Annu. Symp. Proc. 2006; 2006: 339–43. [PMC free article] [PubMed] [Google Scholar]
- 6. Lavezzo E, Franchin E, Ciavarella C et al. Suppression of COVID‐19 outbreak in the municipality of Vo, Italy. medRxiv 2020. 10.1101/2020.04.17.20053157. [DOI] [Google Scholar]
- 7. Heneghan C, Brassey J, Jefferson T. COVID‐19: What proportion are asymptomatic? 2020. [Cited 12 Apr 2020.] Available from URL: https://www.cebm.net/covid-19/covid-19-what-proportion-are-asymptomatic/
- 8. Chen PS, Lin MH. Development of simulation optimization methods for solving patient referral problems in the hospital‐collaboration environment. J. Biomed. Inform. 2017; 73: 148–58. [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health, New Zealand. Guidelines for the use of personal protective equipment for frontline health care workers 7th April 2020. 2020. [Cited 11 Apr 2020.] Available from URL: https://www.health.govt.nz/our‐work/diseases‐and‐conditions/covid‐19‐novel‐coronavirus/covid‐19‐novel‐coronavirus‐information‐specific‐audiences/covid‐19‐advice‐essential‐workers‐including‐personal‐protective‐equipment/personal‐protective‐equipment‐use‐health‐care
- 10. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159–74. [PubMed] [Google Scholar]
- 11. Blachere FM, Lindsley WG, McMillen CM et al. Assessment of influenza virus exposure and recovery from contaminated surgical masks and N95 respirators. J. Virol. Methods 2018; 260: 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blanco N, Eisenberg MC, Stillwell T, Foxman B. What transmission precautions best control influenza spread in a hospital? Am. J. Epidemiol. 2016; 183: 1045–54. [DOI] [PubMed] [Google Scholar]
- 13. Zhang N, Li Y. Transmission of influenza A in a student office based on realistic person‐to‐person contact and surface touch behaviour. Int. J. Environ. Res. Public Health 2018; 15: 1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahrenholz SH, Brueck SE, Rule AM et al. Assessment of environmental and surgical mask contamination at a student health center – 2012–2013 influenza season. J. Assoc. Occup. Health Prof. Healthc. 2018; 38: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chughtai AA, Stelzer‐Braid S, Rawlinson W et al. Contamination by respiratory viruses on outer surface of medical masks used by hospital healthcare workers. BMC Infect. Dis. 2019; 19: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Phan LT, Sweeney D, Maita D et al. Respiratory viruses on personal protective equipment and bodies of healthcare workers. Infect. Control Hosp. Epidemiol. 2019; 40: 1356–60. [DOI] [PubMed] [Google Scholar]
- 17. Rule AM, Apau O, Ahrenholz SH et al. Healthcare personnel exposure in an emergency department during influenza season. PLoS One 2018; 13: e0203223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phan LT, Maita D, Mortiz DC, Bleasdale SC, Jones RM. Environmental contact and self‐contact patterns of healthcare workers: implications for infection prevention and control. Clin. Infect. Dis. 2019; 69: S178–S84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwok YL, Gralton J, McLaws ML. Face touching: a frequent habit that has implications for hand hygiene. Am. J. Infect. Control 2015; 43: 112–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Offeddu V, Yung CF, Low MSF, Tam CC. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta‐analysis. Clin. Infect. Dis. 2017; 65: 1934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bin‐Reza F, Lopez Chavarrias V, Nicoll A et al. The use of masks and respirators to prevent transmission of influenza: a systematic review of the scientific evidence. Influenza Other Respir. 2012; 6: 257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jefferson T, Foxlee R, Del Mar C et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nam H‐S, Yeon M‐Y, Park JW, Hong JY, Son JW. Healthcare worker infected with Middle East respiratory syndrome during cardiopulmonary resuscitation in Korea, 2015. Epidemiol. Health 2017; 39: e2017052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yen MY, Lin YE, Lee CH et al. Taiwan's traffic control bundle and the elimination of nosocomial severe acute respiratory syndrome among healthcare workers. J. Hosp. Infect. 2011; 77: 332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization . Infection prevention and control during health care when COVID‐19 is suspected. Interim guidance 19 March 2020, 2020. [Cited 14 Apr 2020.] Available from URL: https://www.who.int/publications‐detail/infection‐prevention‐and‐control‐during‐health‐care‐when‐novel‐coronavirus‐(ncov)‐infection‐is‐suspected‐20200125
- 26. World Health Organization . Advice on the use of masks in the context of COVID‐19. Interim guidance 6 April 2020, 2020. [Cited 14 Apr 2020.] Available from URL: https://apps.who.int/iris/handle/10665/331693
- 27. National Health Service . COVID‐19: infection prevention and control guidance. Version 2 (27/4/2020), 2020. [Cited 28 Apr 2020.] Available from URL: https://www.gov.uk/government/publications/wuhan‐novel‐coronavirus‐infection‐prevention‐and‐control
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search strategy in Ovid Medline 23/4/2020.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


