Editor
Skin manifestations have been increasingly reported in the setting of COVID‐19. However, their incidence and presentation are debated, and the role, direct or indirect, of SARS‐CoV‐2 in their pathogenesis has yet to be determined.
In this work, we aimed to analyse our experience in a French referral centre and to perform a systematic review of the literature to evaluate the incidence and prognosis of cutaneous lesions observed in COVID‐19 patients.
Cutaneous manifestations were assessed in COVID‐19 patients admitted to Cochin Hospital (Paris, France) between 16 March and 27 April 2020. Seven hundred and fifty‐nine confirmed moderate‐to‐severe COVID‐19 cases were diagnosed in our institution. Eight patients (1%, six males, two females, mean age 55.6) presented with skin lesions, mainly disseminated maculopapular exanthema, but also digitate papulosquamous rash (reported in Ref.1), herpes recurrence, papulovesicular rash and Grover's disease. The mean delay between respiratory/systemic and dermatological signs was 13 days.
Our systematic review of the literature identified 56 articles (including our series) evaluating 1020 patients (Table 1, WHOLE cohort) between 1 December 2019 and 9 of May 2020. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Diagnosis of COVID‐19 infection was confirmed in 47% of patients (Table 1, CONFIRMED cohort). The female‐to‐male ratio was 1.1 in both cohorts. Mean ages were 42 and 48 in the WHOLE and CONFIRMED cohorts, respectively. Rashes were the most frequent manifestations (54% and 70% in the WHOLE and CONFIRMED cohorts, respectively). These rashes were erythematous maculopapular/morbilliform, urticarial/annular, vesicular/varicelliform or petechial/purpuric by order of frequency. Trunk was the preferential localization of rashes. Other cutaneous manifestations included chilblains in 34% patients in the WHOLE cohort, but in only 11.5% cases in the CONFIRMED cohort. Digital necrosis was more frequently reported in the CONFIRMED cohort (11.5% vs. 5%). Transient livedo was uncommon (1%). About 70% of all patients experienced pruritus. Other symptoms were burning and pain.
Table 1.
Characteristics of COVID‐19 patients with cutaneous manifestations included in the WHOLE and CONFIRMED cohorts
| WHOLE cohort | CONFIRMED cohort | |
|---|---|---|
| Number of COVID‐19 patients, N | 1020 | 480 |
| Confirmed SARS‐CoV‐2 infection, N (%) | 480 (47%) | 480 (100%) |
| Suspected SARS‐CoV‐2 infection, N (%) | 540 (53%) | 0 (0%) |
| Age | ||
| Mean, years (range) | 42.2 (15 days to 98 years) | 48.0 (15 days to 98 years) |
| Not mentioned, N | 161/1020 | 45/480 |
| Sex | ||
| Male, N (%) | 407 (48%) | 174 (48%) |
| Female, N (%) | 438 (52%) | 189 (52%) |
| Not mentioned N | 175/1020 | 117/480 |
| Country | ||
| Belgium, N (%) | 3 (0.3%) | 3 (0.6%) |
| China, N (%) | 10 (1%) | 10 (2%) |
| France, N (%) | 308 (30%) | 57 (12%) |
| Iran, N (%) | 2 (0.2%) | 2 (0.4%) |
| Italy, N (%) | 164 (16%) | 85 (17.8%) |
| Kuwait, N (%) | 2 (0.2%) | 2 (0.4%) |
| Spain, N (%) | 518 (51%) | 308 (64%) |
| Thailand, N (%) | 1 (0.1%) | 1 (0.2%) |
| USA, N (%) | 9 (0.9%) | 9 (2%) |
| Morocco, N (%) | 2 (0.2%) | 2 (0.4%) |
| Indonesia, N (%) | 1 (0.1%) | 1 (0.2%) |
| Cutaneous lesions morphology | ||
| Rash, N (%) | 555 (54%) | 337 (70%) |
| Vesicular/varicelliform, N (%) | 114 (11%) | 80 (17%) |
| Erythematous maculopapular/morbilliform, N (%) | 245 (24%) | 169 (36%) |
| Urticarial/annular/eczematiform, N (%) | 178 (17%) | 76 (14%) |
| Petechial/purpuric, N (%) | 18 (2%) | 12 (3%) |
| Vascular lesions, N (%) | 408 (40%) | 112 (24%) |
| Chilblains, N (%) | 342 (34%) | 53 (11.5%) |
| Transient/livedo reticularis, N (%) | 9 (1%) | 6 (1%) |
| Digital necrosis/ necrotic purpura, N (%) | 57 (5%) | 53 (11.5%) |
| Other findings, N (%) | 57 (6%) | 31 (6%) |
| Herpes recurrence, N (%) | 21 (2%) | 21 (4%) |
| Eruptive cherry haemangioma, N (%) | 8 (1%) | 1 (0.2%) |
| Acral dyshidrosis‐like lesions, N (%) | 20 (2%) | 1 (0.2%) |
| Ecthyma/impetigo, N (%) | 5 (0.5%) | 5 (1%) |
| Coma blisters, N (%) | 2 (0.2%) | 2 (0.4%) |
| Acute generalized exanthematous pustulosis (drug reaction), N (%) | 1 (0.1%) | 1 (0.2%) |
| Cutaneous involvement location | ||
| Trunk, N (%) | 186 (35%) | 125 (53%) |
| Lower limbs (excluding acral lesions), N (%) | 102 (19%) | 43 (18.4%) |
| Upper limbs, N (%) | 56 (10%) | 29 (12.4%) |
| Acral lesions (fingers, toes, hands, heels), N (%) | 322 (60%) | 44 (18.8%) |
| Buttocks, N (%) | 2 (0.2%) | 2 (0%) |
| Mucosa (oral, genitalia), N (%) | 6 (1.3%) | 6 (1.3%) |
| Face/head, N (%) | 26 (5%) | 16 (6.8%) |
| Widespread, N (%) | 26 (5%) | 23 (9.8%) |
| Flexural areas/folds, N (%) | 4 (0.75%) | 4 (1.7%) |
| Not mentioned, N | 485/1020 | 246/480 |
| Cutaneous symptoms | ||
| Itching, N (%) | 347 (73%) | 205 (68%) |
| Burning, N (%) | 66 (14%) | 23 (8%) |
| Pain, N (%) | 91 (19%) | 37 (12.2%) |
| Asymptomatic, N (%) | 62 (13%) | 38 (12.5%) |
| Not mentioned, N | 472/1020 | 177/480 |
| Mean delay of onset of cutaneous manifestations, days (range) | 6.8 (−15 to 25 days) | 6.7 (−15 to 25 days) |
| Mean duration of cutaneous manifestations, days (range) | 9.0 (20 min to 30 days) | 9.0 (20 min to 30 days) |
The mean delay between the onset of respiratory/systemic symptoms and cutaneous manifestations was around 6.8 days in both cohorts. In some cases, rashes preceded the occurrence of systemic symptoms. When mentioned, chilblains appeared most frequently as a late manifestation. The mean duration of skin lesions was 9 days in both cohorts.
We retrieved six series, including ours, in which the numbers of both infected patients and patients with skin signs were available. 4 , 6 , 7 , 9 , 10 Cutaneous lesions were observed in 38 patients over 2199 COVID‐19 cases. Therefore, the mean incidence of cutaneous manifestations in COVID‐19 patients was 1.7% (Fig. 1a).
Figure 1.
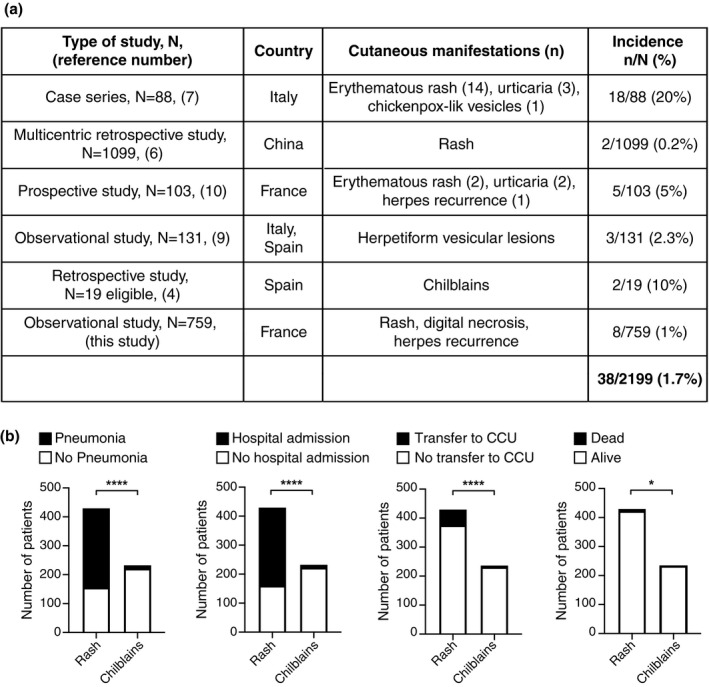
(a) Analysis of the incidence of cutaneous manifestations related to COVID‐19. (b) Pneumonia, hospital admission, transfer to CCU and death were analysed in patients of the WHOLE cohort presenting with rash or chilblains. Chi‐square test. *P‐value < 0.05; ****P‐value < 0.0001.
Besides, we investigated whether cutaneous manifestations could correlate with COVID‐19 severity. Therefore, we analysed severity criteria [hospital admission, pneumonia, transfer to Critical Care Unit (CCU), death] in patients of the WHOLE cohort with rashes or chilblains. In patients with rashes, severity was found in 64% of cases and death in 2%, while it was respectively found in 5% and 0% patients with chilblains. We found a statistically significant association between pneumonia, hospitalization, transfer to CCU or death and the occurrence of a rash as compared to chilblains (Fig. 1b).
The incidence of skin signs during COVID‐19 is variable in the literature, ranging from 0.2% in China to 20% in North Italy. Our literature analysis indicated that the worldwide incidence is low, around 1–2%, as we observed in our hospital. Skin lesions were dominated by rashes and chilblains, that seem to present opposite prognosis. However, rashes could not always be discriminated with drug‐induced exanthema. Likewise, chilblains pathogenesis in the setting of COVID‐19 remains poorly understood and its relationship with SARS‐CoV‐2 is still unclear. Importantly, in our systematic review of the literature, only 15% of chilblains cases had a proven SARS‐CoV‐2 infection.
In conclusion, this paper provides a comprehensive overview of cutaneous manifestations reported in COVID‐19 patients. However, their relationship with SARS‐CoV‐2 remains to be specified.
Conflicts of interest
Prof. Dupin, Dr. Matar and Dr. Sohier have nothing to disclose. Dr. Oulès reports personal fees from Novartis, outside the submitted work. Prof. Chosidow is a PI for PSOBIOTEQ. Prof. Beylot‐Barry reports personal fees from AbbVie, Celgene, Takeda, Janssen, Novartis, and Lilly, outside the submitted work. Prof. Aractingi reports grants from Novartis and Leo Pharma, and personal fees from Pierre Fabre, BMS and Lilly, outside the submitted work.
Funding source
None.
Authors' contributions
N.D. and S.A. designed the study with inputs from O.C. and M.B.B.. S.M. and B.O collected the data and performed the analysis. P.S. performed the pathological analysis. S.M., B.O., N.D. and S.A. wrote the manuscript with inputs from all the other authors.
Data availability statement
All data that support the conclusions, including the complete list of references, are available from the authors upon request.
References
- 1. Sanchez A, Sohier P, Benghanem S et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol 2020. 10.1001/jamadermatol.2020.1704 [DOI] [PubMed] [Google Scholar]
- 2. Recalcati S, Barbagallo T, Frasin LA et al. Acral cutaneous lesions in the time of COVID‐19. J Eur Acad Dermatology Venereol 2020. 10.1111/jdv.16533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piccolo V, Neri I, Filippeschi C et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatology Venereol 2020. 10.1111/jdv.16526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernandez‐Nieto D, Jimenez‐Cauhe J, Suarez‐Valle A et al. Characterization of acute acro‐ischemic lesions in non‐hospitalized patients: a case series of 132 patients during the COVID‐19 outbreak. J Am Acad Dermatol 2020. 10.1016/j.jaad.2020.04.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Galván Casas C, Català A, Carretero Hernández G et al. Classification of the cutaneous manifestations of COVID‐ 19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020. 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan W, Ni Z, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Recalcati S. Cutaneous manifestations in COVID‐ 19: a first perspective. J Eur Acad Dermatology Venereol 2020; 34. 10.1111/jdv.16387 [DOI] [PubMed] [Google Scholar]
- 8. de Masson A, Bouaziz J‐D, Sulimovic L et al. Chilblains are a common cutaneous finding during the COVID‐19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol 2020. 10.1016/j.jaad.2020.04.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tammaro A, Adebanjo GAR, Parisella FR, Pezzuto A, Rello J. Cutaneous manifestations in COVID‐ 19: the experiences of Barcelona and Rome. J Eur Acad Dermatology Venereol 2020. 10.1111/jdv.16530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hedou M, Carsuzaa F, Chary E, Hainaut E, Cazenave‐Roblot F, Masson Regnault M. Comment on “Cutaneous manifestations in COVID‐ 19: a first perspective” by Recalcati S. J Eur Acad Dermatology Venereol 2020. 10.1111/jdv.16519 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the conclusions, including the complete list of references, are available from the authors upon request.


