Abstract
Objectives
Coronavirus disease 2019 (COVID‐19) has spread globally and become a pandemic. The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) not only infects the gastrointestinal (GI) tract and causes GI symptoms, but also increases nosocomial transmission risk during endoscopic procedures for aerosol generation. We hereby share our infection control strategies aiming to minimize COVID‐19 transmission in the endoscopy center.
Methods
We established our infection control strategies based on the guidance of Chinese Society of Digestive Endoscopy and inputs from hospital infection control experts: admission control through the procedure and patient triage, environmental control to reduce possible virus exposure, proper usage of personal protective equipment (PPE), and scope disinfection and room decontamination. All endoscopic procedures accomplished during COVID‐19 outbreak and progress of stepwise resumption of elective endoscopy procedures were retrospectively reviewed.
Results
Only urgent or semi‐urgent procedures were performed during COVID‐19 outbreak. After no local new‐onset COVID‐19 case in Beijing for four weeks, we reopened the endoscopy center for elective procedures and monitored the outbreak continuously while maintaining a sustainable endoscopy service.
Conclusions
It is imperative that all endoscopy centers should establish standard infection control strategies in order to fight COVID‐19 pandemic based on national guidance and academic society guidelines and tailor them to individual resources. These measures and setup can also be reserved for future pandemics.
Keywords: COVID‐19, endoscopy, infection control strategy, personal protective equipment, triage
Introduction
Since December 2019, a novel coronavirus designated as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spread rapidly throughout China and beyond. 1 , 2 The outbreak of coronavirus disease 2019 (COVID‐19) was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020. 3 , 4
The most common symptoms for COVID‐19 include fever, dry cough, and shortness of breath. The incidence of gastrointestinal (GI) symptoms of COVID‐19 ranged from 3% (1/41) to 79% (159/201), including anorexia, diarrhea, nausea, vomiting, abdominal pain and GI bleeding, 5 which may precede the onset of fever and dyspnea by 1–2 days. 6
It is widely accepted that COVID‐19 is mainly spread via droplets and direct contact, 7 but there is evidence that airborne spread is possible during aerosol generating procedures (AGPs). 8 Emerging evidence suggests that SARS‐CoV‐2 can be detected in the feces, which leads to the possibility of transmission via the fecal–oral route. 9 , 10 , 11 , 12 This may be due to the high levels of expression of angiotensin converting enzyme 2 (ACE2) protein in the epithelium throughout the GI tract, 13 which is a receptor SARS‐CoV‐2 requires for cell entry. 14 , 15
Nosocomial transmissions were common in pandemic regions of COVID‐19: 3.8% of confirmed cases in China were health care personnel (HCP), 16 whose infection rate was approximately three times of that of the general population 17 ; similar trends have been observed in Italy, with an estimated 20% of COVID‐19 infections occurring in HCP. 18
Although endoscopy staff are not directly involved during a pandemic, they face great challenge and risk: large viral loads in pulmonary aerosols and even fecal materials, with endoscopy procedure believed to be an AGP. 19 , 20 Infection control measures must be implemented to protect both the patient and HCP.
We therefore created our infection control strategies (ICSs) to minimize nosocomial transmission risk since January 24, 2020, when Beijing Municipal Health Commission activated the first‐level response mechanism for public health emergencies. Although multiple endoscopy societies and expert groups have offered recommendations and position statements for GI endoscopy during the pandemic, 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 we aim to share local experience on ICS in the endoscopy center.
Methods
We established our ICSs based on the guidance of Chinese Society of Digestive Endoscopy (CSDE) and inputs from hospital infection control experts in the Peking Union Medical College Hospital (PUMCH). 26 , 28
Strategies to prevent transmission in the endoscopy center
Admission control
This is the basic yet most important measure to avoid uninfected people contacting those with COVID‐19.
Procedures triage
We divided endoscopic procedures into three types: urgent, semi‐urgent, and elective, as defined in Table 1.
Table 1.
Peking Union Medical College Hospital (PUMCH) endoscopy procedure triage during COVID‐19 pandemic
| Procedure triage | Indications |
|---|---|
| Urgent |
Acute gastrointestinal bleeding Gastrointestinal foreign body Acute cholangitis and severe symptomatic obstructive jaundice due to gallstone or tumor Acute luminal obstruction requiring stenting |
| Semi‐urgent | Tumor diagnosis of highly suspicious cases and tumor staging |
| Elective | All other procedures, such as routine diagnostic EGD and routine screening colonoscopy |
COVID‐19, coronavirus disease 2019; EGD, esophagogastroduodenoscopy.
During the COVID‐19 outbreak, urgent cases would be finished whenever needed and immediately; semi‐urgent procedures on one fixed day in a week, and the necessity of each case would be discussed; elective procedures would be rescheduled until the outbreak is over.
Patient triage
For every patient needing an endoscopic procedure, we reviewed the COVID‐19‐related symptoms and obtained a detailed epidemiological history (Fever, Travel history to the pandemic area, Occupation, Cluster of cases, Contact of a suspected or confirmed case, FTOCC). A chest computerized tomography (CT) scan was ordered (Table S1). The presence of at least one criterion would be considered positive for FTOCC, and further COVID‐19 testing (reverse‐transcriptase polymerase chain reaction, RT‐PCR) should be completed.
The urgent cases with a positive RT‐PCR test or CT scan suggestive of COVID‐19 would receive the endoscopic procedures in the negative pressure room of the surgical operation room outside our endoscopy center. If adequate FTOCC history cannot be assessed, all urgent cases would be considered as “COVID‐19 positive” in terms of infection control. The other urgent patients would receive endoscopy in the standard endoscopy rooms.
For urgent cases with a positive RT‐PCR test or abnormal CT screening, their situations should be reevaluated post procedure: stable patients would be transferred to a designated hospital in Beijing for COVID‐19 management, while unstable patients would receive medical care in the emergency room equipped with a bio‐containment unit (Fig. 1).
Figure 1.
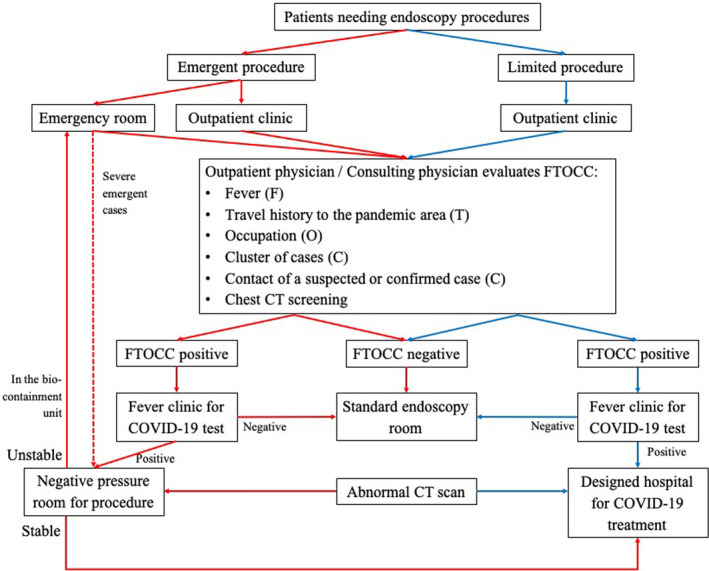
The triage of patients by the infection risk of COVID‐19. Red arrows indicated the flow of urgent cases and blue arrows indicated the flow of semi‐urgent cases. If adequate FTOCC history cannot be assessed for emergency, all urgent cases should be considered as “COVID‐19 positive” for infection control. Abnormal CT scan referred to the CT findings suggestive of COVID‐19. Abbreviations: CT, computerized tomography; COVID‐19, coronavirus disease 2019; FTOCC, fever, travel history to the pandemic area, occupation, cluster of cases, contact of a suspected or confirmed case.
For outpatients needing semi‐urgent procedures, we followed the same strategy. Patients with a positive RT‐PCR test or chest CT scan would be transferred to the designated hospital and endoscopic procedure would be rescheduled (Fig. 1).
All patients receiving procedures were followed up for 2 weeks to rule out COVID‐19.
Working team simplification
Only necessary endoscopy staff would come to work on a daily basis. All endoscopic procedures should be performed by senior endoscopists and hands‐on training should be suspended. Each day, only one endoscopist and one nurse were scheduled to stay in the hospital for urgent procedures. On the fixed day for semi‐urgent procedures, the number of endoscopists staffing the center depended on the type and number of procedures, as were the nurses and technicians.
Telehealth consultation
The physicians in our department would take turns to provide telehealth advice and explanation to reduce patients' concerns and anxiety.
Endoscopy center environmental control
Workspace management
The different routes of staff and patients in the endoscopy center were clearly marked. Workflow modification also required one‐way passages for the transportation of used/contaminated equipment (such as endoscope) to avoid cross‐contamination. At the patients’ entrance, a triage station was set and non‐contract thermometer and alcohol‐based sanitizer were provided. We assigned a room for each specific type of endoscopy (one room for upper GI endoscopy and another for colonoscopy). The staff would don personal protective equipment (PPE) in the change room before entering the procedure room and doff used PPE into the lidded medical trash bins and finish hand hygiene before getting back to the change room. We temporarily changed the endoscopy center outlay for maximal compliance with these requirements (Fig. 2).
Figure 2.
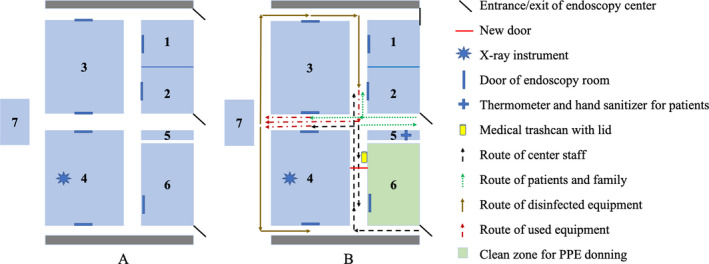
The outlay changes of the endoscopy center for the infection control strategy. Picture A: Rooms 1–4 (blue area) were procedural rooms before pandemic; Room 7 was the disinfection room. Picture B: During pandemic Room 2 and 4 were employed (Room 2 for colonoscopy, and Room 4 for upper GI endoscopy). With one entrance (black) closed and a new door (red) assembled, we partially separated the routes of center staff (black arrows with dotted line) and patients (green arrows with dotted line), and created a clean zone (green area) as the change room for the staff to put on PPE before entering procedural rooms. A triage station (cross) was set at the patient’s entrance and non‐contract thermometer and alcohol‐based sanitizer were provided. The workflow was also shown (brown arrows for disinfected equipment, and red arrows with dotted line for used equipment). Note the partial overlap of routes of staff, patients and used equipment. Abbreviations: GI, gastrointestinal; PPE, personal protective equipment.
Patient management
Besides the patient, only one family member was allowed into the center after the previous patient had left. When the patient and family member entered the center, their body temperature was taken and they were suggested to clean hands with sanitizer and wear masks throughout their stay.
Staff management
All staff should practice hand hygiene correctly and wear masks. On arriving, they changed into scrubs and work shoes and then PPE; and changed back after taking a shower before leaving the center. When in the office and dining room, a proper social distance would be kept (at least 1–1.8 m).
Procedure room decontamination
Air ventilation was enhanced via opening of the fresh air dampers. Mobile high efficiency particulate air (HEPA) units were added to augment the total air change rates in each procedure room.
After each case, a single‐use bed sheet was changed and decontamination measures with alcohol‐ or chlorine‐based solutions would be performed to clean the surface of bed rails, bedside tables, furniture and floor in frequent contact with patients, and also endoscopy instruments (Table 2). The next case should be scheduled after half an hour for full decontamination.
Table 2.
Peking Union Medical College Hospital (PUMCH) endoscopy room environmental decontamination during COVID‐19 pandemic
| Work area | Decontamination method | Decontamination frequency |
|---|---|---|
| Endoscopy instruments, operating table, electrosurgical workstation, nursing trolley, computer table | 1000 mg/L chlorine‐containing disinfectant, keep for 30 min and then wipe with clean water; 75% alcohol for computer monitor and metal parts. | After each case |
| Room wall and floor |
1000 mg/L chlorine‐containing disinfectant, then mop with clean water after the ground is dry; Or use ultraviolet light to sterilize for 1 h; Finally open the window for ventilation. |
After each case |
| Room air | Mobile HEPA units, and automatic air sterilizer (with in‐unit ultraviolet). | Continuously working |
COVID‐19, coronavirus disease 2019; HEPA, high efficiency particulate air.
Use of personal protective equipment
In our center, full PPE includes disposable work cap, surgical masks/N95 respirators or equivalent, face shield/goggles, disposable protective clothing/gown, gloves and shoe covers. We suggested graded protection strategy in our center (Table 3). The endoscopists, nurses and anesthetists in the endoscopy rooms should wear full PPE (the Fig. S1). We used double gloving and changed N95 respirator masks regularly (every 4 h) or if visibly polluted. We also kept fine records of the stock and usage of PPE to guarantee supply for our staff.
Table 3.
Peking Union Medical College Hospital (PUMCH) endoscopy center graded personal protection standards during COVID‐19 pandemic
| Work areas | Protection level | Personal protection equipment |
|---|---|---|
| Reservation area (behind window) | Level 1 | Work cap, surgical mask, glove (when necessary) |
| Triage station area (open) and nurse station | Level 1 | Work cap, surgical mask, glove, grown (when necessary; AAMI level 1) |
| Procedural room | Level 3 | Work cap, N95 respirators or equivalent, face shield/goggles, impermeable protective clothing (AAMI level 3), double gloves and shoe cover |
| Negative operation room (for confirmed or suspected cases) | Level 3 | Work cap, N95 respirators or equivalent, face shield/goggles, impermeable protective clothing (AAMI level 3), double gloves and shoe cover |
| Scope disinfection room | Level 3 | Work cap, N95 respirators or equivalent, face shield/goggles, impermeable protective clothing (AAMI level 3), double gloves and shoe cover |
| Patients and their family | Level 1 | Surgical masks, hands hygiene with sanitizer when entry and exit |
AAMI, Association for the Advancement of Medical Instrumentation; COVID‐19, coronavirus disease 2019.
Note that the protection level provided here was advised by the hospital infection control office of the PUMCH based on the guideline of National Health Commission and local PPE resource: level 1 for general clinic and ward, level 2 for fever clinic, and level 3 for isolation ward for confirmed or suspected COVID‐19 cases, endoscopy center, and operation room.
Proper donning and doffing of PPE is extremely important for maximum protection (refer to https://www.cdc.gov/coronavirus/2019‐ncov/hcp/using‐ppe.html for detailed and graphic instructions; Table S2). All staff should be trained by infection control experts and should supervise each other.
Scope disinfection
After each procedure, all endoscopes and reusable accessories would be placed in the yellow‐color plastic bag immediately and sent to the disinfection room, where it would receive the standard cleaning, disinfection and reprocessing protocols as usual, during which the scopes were manually cleaned under water firstly and then transferred to the automatic cleaning machine (Siemens, Endoclens‐NSX; using ortho‐phthalaldehyde as the disinfectant). The technicians in the disinfection room should also wear PPE properly due to the possibility of aerosolization.
All endoscopic procedures accomplished during the COVID‐19 outbreak are retrospectively reviewed and the indications are listed. The progress of resumption of elective endoscopy service is also recorded.
Results
Procedures postponed during COVID‐19 outbreak
There were about 3, 128 endoscopic procedures postponed from January 24 to March 20, 2020, among which there were 1, 803 screening esophagogastroduodenoscopy (EGD), 1, 221 screening colonoscopy and 104 endoscopic ultrasound for submucosal lesions. Since resumption of elective service, we have finished 1, 822 postponed cases, with others rescheduled in the list.
Procedures accomplished during COVID‐19 outbreak
From January 24, 2020, to March 30, 2020, we accomplished 127 cases following the aforementioned ICS, among which there were 92 urgent cases, mainly due to GI bleeding, GI tract foreign bodies, and acute cholangitis due to gallstone, and 35 cases of semi‐urgent procedures (Table 4).
Table 4.
Peking Union Medical College Hospital (PUMCH) endoscopy procedures during COVID‐19 pandemic between January 24 and March 30, 2020
| 2020 | 2019 | |||
|---|---|---|---|---|
|
Urgent procedures (N = 92) |
Semi‐urgent procedures (N = 35) |
Urgent procedures (N = 105) |
Non‐urgent procedures (N = 5006) |
|
| Male (%) | 62 (67.4) | 23 (65.7) | 61 (58.1) | 2663 (53.2) |
| Age (years) | 62.6 ± 14.6 | 60.8 ± 12.4 | 53.3 ± 13.4 | 63.3 ± 14.0 |
| Procedure type | ||||
| EGD | 66 | 9 | 83 | 2542 |
| Colonoscopy | 9 | 9 | 14 | 2036 |
| EUS | 0 | 17 | 0 | 264 |
| ERCP | 17 | / | 8 | 109 |
| Double‐balloon enteroscopy | / | / | / | 8 |
| Capsule endoscopy | / | / | / | 47 |
| Indications | ||||
| GI bleeding | 61 | / | 86 | 0 |
| Foreign body | 11 | / | 11 | 0 |
| Acute cholangitis | 9 | / | 7 | 0 |
| Severe symptomatic obstructive jaundice | 8 | / | 1 | 0 |
| Acute sigmoid volvulus | 4 | / | 0 | 0 |
| Tumor staging | / | 12 | / | 44 |
| Tumor diagnosis | / | 23 | / | 219 |
COVID‐19, coronavirus disease 2019; EGD, esophagogastroduodenoscopy; ERCP, endoscopic retrograde cholangiopancreatography; EUS, endoscopic ultrasound; GI, gastrointestinal.
Note that the endoscopic procedures of the same period in 2019 were also shown as urgent or non‐urgent procedures for comparison.
All urgent cases received initial treatment in the emergency room where the FTOCC history, chest CT and/or RT‐PCR test were finished. Among the urgent cases, 19 cases underwent both chest CT and RT‐PCR test because of positive FTOCC (18 cases for fever, and one case for traveling history), but none were found to be COVID‐19 cases. All semi‐urgent patients received chest CT screening (no positive FTOCC cases) without positive results.
Resumption of daily endoscopy service
With no new local COVID‐19 cases reported for about 4 weeks in Beijing, we have begun the elective endoscopy service since March 30, 2020, with a modified patients triage strategy: patients of urgent procedure undergo both chest CT and RT‐PCR test to determine whether to receive procedure in the negative pressure room; patients of semi‐urgent or elective procedure undergo RT‐PCR test within one week before the procedure, and they will receive endoscopy after staying in Beijing for 14 days with negative RT‐PCR results.
Other items of strategy (working team simplification, center environmental control, use of personal protective equipment, scope disinfection) are still followed in our center probably until the response mechanism for public health emergencies is terminated.
With limited supplies of PPE, initially only 25% of the normal capacity compared to pre‐COVID‐19 levels was restored and only three procedure rooms opened for half a day, and we aimed at a stepwise manner. In late April, after the first‐level response mechanism for public health emergencies was adjusted to the second‐level and with more stable PPE supplies, we have increased to 50% of normal capacity.
Discussion
The SARS‐CoV‐2 is spreading rapidly throughout the world. The GI endoscopists face great challenges and risks: high viral loads in the aerosols generated from the upper airway and the fecal materials. There is significant and unrecognized exposure of the endoscopist’s face to potentially infectious samples during procedures. 29 During the SARS outbreak, droplets from infected patients could reach persons located 6 feet or more. 30 So, the ICS must be established and followed in the endoscopy centers to avoid nosocomial transmissions.
We set a procedures triage system to sort endoscopic procedures into three categories: urgent, semi‐urgent, and elective. The urgent cases, like acute GI bleeding and foreign body, even for confirmed COVID‐19 cases, should not be delayed. Semi‐urgent cases, such as potential or confirmed cancer cases, may be difficult to reschedule. We scheduled a fixed day of the week (every Monday) to manage these cases after careful review and thorough screening for COVID‐19. For elective procedures, there is a broad consensus that they should be rescheduled (Table S3). 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 But the risk of progression to advanced disease is indeterminate for early‐stage GI cancer if delayed up to months; 31 , 32 while colonic polyps of different size will probably not progress in months. 33 , 34 So, we started endoscopic treatment for early‐stage GI cancer/colonic polyps after resumption of elective service.
Assessment of infection risk before endoscopy is important. We suggested that all patients requiring endoscopy should be evaluated for FTOCC. As was suggested in the CSDE guidance, we routinely ordered screening chest CT scan prior to endoscopy, which was a fast and effective screening tool. 35 , 36 , 37 , 38 Considering the possibility of initial false negative result of RT‐PCR test (within 1014 patients undergoing both chest CT and RT‐PCR test, 413 patients had negative RT‐PCR results, 74.6% (308/413) among whom had positive chest CT findings), 36 , 39 we suggest patients with suggestive CT scan results should be treated as COVID‐19 cases in the pandemic areas. But this strategy may be difficult to generalize due to issues relating to cost, resources, and accessibility.
It should be also noted that the patient triage strategy based on the infection risk is ever‐changing according to the epidemic condition. For the estimated low local prevalence of COVID‐19 after no new local case for four weeks, we turned to a new strategy to triage patients of elective and semi‐urgent procedures based on RT‐PCR results together with the 14‐day quarantine, which would minimize the possibility of nosocomial transmission caused by the COVID‐19 patients with a false negative result. With the negative predictive value increasing with decreasing prevalence, one negative result of RT‐PCR can probably exclude COVID‐19 in the majority of cases. 39 , 40 A recent guidance recommends a molecule test for COVID‐19 within 48 h of a scheduled procedure, 41 which is reasonable for reducing the infection risk between the test and procedure, especially for areas with a relatively high prevalence. With enough availability of RT‐PCR test, our practice in PUMCH could be improved in this way (shorten the period between test and procedure to 2–3 days).
Not every endoscopy center is built with the principles of maximal infection control. In our center, changes were made for maximal compliance with these requirements: to separate the entrance/exit of staff and patients; to assign room for specific endoscopic procedures; to replace the change room to ensure all staff entering endoscopy room with PPE; to separate gown‐up and gown‐down areas; to provide one‐way passages for the transportation of used/contaminated endoscopes. We suggested a triage station at the patients’ entrance to monitor body temperature and sanitize their hands. All patients and family members were suggested to wear masks, which was proven effective for reducing transmission risk. 42
All endoscopic procedures are AGPs. The upper GI endoscopy carries a risk for aerosolization for patient’s cough and vomiting, while possible aerosolization happens when passing instruments through the working channel of colonoscopy. Meanwhile, virus shedding is very high in the upper airway, which meant high viral loads are likely in the aerosol. 43 So, AGPs could possibly lead to airborne transmission of SARS‐CoV‐2. 44 Almost all guidance suggests proper PPE usage during procedures, with N95 respirators or equivalent recommended; a meta‐analysis also showed a benefit from N95 respirators over standard masks in SARS. 45 But other evidence showed that surgical masks were also effective. 46 So, we suggested graded PPE usage (Table 3); in certain situations a surgical mask was enough, and full PPE usage in procedures, irrespective of upper or lower GI tract endoscopy, was necessary. The N95 respirator should be changed every 4 h. 23 The US CDC has issued recommendations allowing for extended use and limited re‐use of N95 respirators if supplies become depleted. 47 We believed that extended use or re‐use of N95 respirators should be handled with great care for difficulty in maintaining structural integrity and risk of contamination. 48 , 49
Patient’s discomfort and retching may be minimized by adequate sedation to reduce aerosol generation in the upper GI endoscopy. But as a high‐risk AGP, intubation should be handled with great care by experienced anesthetists after airway assessment, during which modified rapid sequence induction is strongly recommended and proper PPE like N95 respirator, or powered air‐purifying respirator, is obligatory. 50 Only the anesthesia team would remain in the procedural room during intubation if possible.
Ideally, negative pressure rooms should be used for the endoscopic procedures of confirmed or suspected COVID‐19 cases. 8 If negative pressure rooms are unavailable, portable industrial‐grade HEPA filters may be a reasonable alternative to enhance air filtration during intubation and extubation. 51 , 52
SARS‐CoV‐2 is shed to the environment as expired particles, during toileting and through contact with fomites, and survival of virus on surfaces can last for hours to days. 8 , 53 So, each endoscopy center should have a detailed plan for standard room disinfection with routine disinfectants effective against SARS‐CoV‐2. 54 Standard hand hygiene procedures before and after each case should be practiced, which was proven to be beneficial during SARS. 55
The scope disinfection should follow the standard cleaning endoscopic disinfection and reprocessing protocols. 56 , 57 Evidence has shown that SARS‐CoV‐2 was readily inactivated by commonly used disinfectants. 54 To reduce aerosol formation, scopes should be manually cleaned under water surface. 23 Technicians in the disinfection room should wear N95 respirators when reprocessing equipment used in COVID‐19 cases.
Finally, stepwise resumption of elective endoscopic procedures will always be a concern. Under the modified ICS, we have begun our elective procedures after no new local COVID‐19 case for about 4 weeks, although being only 25% of the normal capacity initially. The resumption of endoscopy service depended on the following factors: (i) the number and epidemic curve of confirmed COVID‐19 cases locally; (ii) the availability of medical equipment, especially PPE; and (iii) the accumulated volume of postponed endoscopy cases (Table S3). 22 The administrators of endoscopy centers should monitor the pandemic closely and modify the ICS accordingly to keep a sustainable and effective service.
In conclusion, it is imperative that all endoscopy centers establish standard infection control strategies to ensure that proper measures are in place to protect patients and staff during COVID‐19 pandemic. We suggest consulting local hospital infection control experts for detailed risk assessment before implementing the infection control measures.
Conflict of Interests
Authors declare no conflict of interests for this article.
Funding information
This study was supported by the National key scientific instrument and equipment development project (2013YQ160439).
Supporting information
Figure S1 Personal protective equipment with N95 or equivalent in the procedural room. Note the impermeable protective clothing (AAMI level 3) and double gloves. Abbreviations: AAMI, Association for the Advancement of Medical Instrumentation.
Table S1 Patient checklist for COVID‐19 suggested by Chinese Society of Digestive Endoscopy.
Table S2 The whole progress of donning and doffing of personal protective equipment.
Table S3 Summary of guidance for endoscopy during the COVID‐19 pandemic from International/National Societies of Gastroenterology/Gastrointestinal Endoscopy.
Acknowledgements
All authors would thank all the staff in the endoscopy center for their dedicated work in COVID‐19 pandemic.
The authors Shengyu Zhang, Xi Wu and Hui Pan contributed equally to this work.
References
- 1. Huang C, Wang Y, Li X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Coronavirus disease 2019 (COVID-19): Situation Report - 51 [Internet]. Geneva: World Health Organization; 2020. Available from https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200311‐sitrep‐51‐covid‐19.pdf?sfvrsn=1ba62e57_10.
- 4. World Health Organization . Coronavirus disease 2019 (COVID-19): Situation Report - 75 [Internet]. Geneva: World Health Organization; 2020. Available from https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200404‐sitrep‐75‐covid‐19.pdf?sfvrsn=99251b2b_4.
- 5. Tian Y, Rong L, Nian W, He Y. Review article: Gastrointestinal features in COVID‐19 and the possibility of faecal transmission. Aliment Pharmacol Ther 2020; 51: 843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Doremalen N, Bushmaker T, Morris DH et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med 2020; 382: 1564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holshue ML, DeBolt C, Lindquist S et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382: 929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gu J, Han B, Wang J. COVID‐19: Gastrointestinal manifestations and potential fecal‐oral transmission. Gastroenterology 2020; 158: 1518–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology 2020; 158: 1831–3.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus‐infected pneumonia. J Med Virol 2020; 92: 680–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 2002; 532: 107–10. [DOI] [PubMed] [Google Scholar]
- 14. Hoffmann M, Kleine‐Weber H, Schroeder S et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang H, Kang Z, Gong H et al. Digestive system is a potential route of COVID‐19: An analysis of single‐cell coexpression pattern of key proteins in viral entry process. Gut 2020; 69: 1010–8. [Google Scholar]
- 16. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–42. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization . Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) [Internet]. Geneva: World Health Organization; 2020. Available from: https://www.who.int/docs/default‐source/coronaviruse/who‐china‐joint‐mission‐oncovid‐19‐final‐report.pdf. [Google Scholar]
- 18. Remuzzi A, Remuzzi G. COVID‐19 and Italy: What next? Lancet 2020; 395: 1225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soetikno R, Teoh AY, Kaltenbach T et al. Considerations in performing endoscopy during the COVID‐19 pandemic. Gastrointest Endosc 2020; 92: 176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sultan S, Lim JK, Altayar O et al. AGA rapid recommendations for gastrointestinal procedures during the COVID‐19 pandemic. Gastroenterology 2020; 159: 739–58.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Repici A, Maselli R, Colombo M et al. Coronavirus (COVID‐19) outbreak: What the department of endoscopy should know. Gastrointest Endosc 2020; 92: 192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiu PWY, Ng SC, Inoue H et al. Practice of endoscopy during COVID‐19 pandemic: Position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE‐COVID statements). Gut 2020; 69: 991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gralnek IM, Hassan C, Beilenhoff U et al. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID‐19 pandemic. Endoscopy 2020; 52: 483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. British Society of Gastroenterology . Endoscopy activity and COVID-19: BSG and JAG guidance [Internet] . London: British Society of Gastroenterology; 2020. Available from: https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/.
- 25. Zhang Y, Zhang X, Liu L, Wang H, Zhao Q. Suggestions for infection prevention and control in digestive endoscopy during current 2019‐nCoV pneumonia outbreak in Wuhan, Hubei province, China. Endoscopy 2020; 52: 312–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chinese Society of Digestive Endoscopy . Digestive endoscopy during the COVID-19 pandemic: Chinese Society of Digestive Endoscopy guidance [Internet]. Chinese Society of Digestive Endoscopy; 2020. Available from http://www.csde.org.cn/news/detail.aspx?article_id=2883. Chinese.
- 27. Irisawa A, Furuta T, Matsumoto T et al. Gastrointestinal endoscopy in the era of the acute pandemic of COVID‐19: Recommendations by Japan Gastroenterological Endoscopy Society. Dig Endosc 2020; 32: 648–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chinese Society of Digestive Endoscopy . Cleaning and disinfection of digestive endoscopy center during the COVID‐19 pandemic: Chinese Society of Digestive Endoscopy guidance [Internet]. Chinese Society of Digestive Endoscopy; 2020. Available from: http://www.csde.org.cn/news/detail.aspx?article_id=2884. Chinese. [Google Scholar]
- 29. Johnston ER, Habib‐Bein N, Dueker JM et al. Risk of bacterial exposure to the endoscopist's face during endoscopy. Gastrointest Endosc 2019; 89: 818–24. [DOI] [PubMed] [Google Scholar]
- 30. Wong TW, Lee CK, Tam W et al. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerg Infect Dis 2004; 10: 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park SY, Jeon SW, Jung MK et al. Long‐term follow‐up study of gastric intraepithelial neoplasias: Progression from low‐grade dysplasia to invasive carcinoma. Eur J Gastroenterol Hepatol 2008; 20: 966–70. [DOI] [PubMed] [Google Scholar]
- 32. Tsukuma H, Oshima A, Narahara H, Morii T. Natural history of early gastric cancer: A non‐concurrent, long term, follow up study. Gut 2000; 47: 618–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mizuno K, Suzuki Y, Takeuchi M, Kobayashi M, Aoyagi Y. Natural history of diminutive colorectal polyps: Long‐term prospective observation by colonoscopy. Dig Endosc 2014; 26(Suppl 2): 84–9. [DOI] [PubMed] [Google Scholar]
- 34. Pickhardt PJ, Pooler BD, Kim DH, Hassan C, Matkowskyj KA, Halberg RB. The natural history of colorectal polyps: Overview of predictive static and dynamic features. Gastroenterol Clin North Am 2018; 47: 515–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shi H, Han X, Jiang N et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis 2020; 20: 425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ai T, Yang Z, Hou H et al. Correlation of chest CT and RT‐PCR testing for coronavirus disease 2019 (COVID‐19) in China: A report of 1014 cases. Radiology 2020; 296: E32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Long C, Xu H, Shen Q et al. Diagnosis of the coronavirus disease (COVID‐19): rRT‐PCR or CT? Eur J Radiol 2020; 126: 108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sinonquel P, Roelandt P, Demedts I et al. COVID‐19 and gastrointestinal endoscopy: What should be taken into account? Dig Endosc 2020; 32: 723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kokkinakis I, Selby K, Favrat B, Genton B, Cornuz J. Covid‐19 diagnosis: Clinical recommendations and performance of nasopharyngeal swab‐PCR. Rev Med Suisse 2020; 16: 699–701. [PubMed] [Google Scholar]
- 40. Gupta S, Shahidi N, Gilroy N, Rex DK, Burgess NG, Bourke MJ. A proposal for the return to routine endoscopy during the COVID‐19 pandemic. Gastrointest Endosc 2020; 92: 735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hennessy B, Vicari J, Bernstein B et al. Guidance for resuming GI endoscopy and practice operations after the Covid‐19 pandemic. Gastrointest Endosc 2020; 92: 743–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wolfel R, Corman VM, Guggemos W et al. Virological assessment of hospitalized patients with COVID‐2019. Nature 2020; 581: 465–9. [DOI] [PubMed] [Google Scholar]
- 43. Leung NHL, Chu DKW, Shiu EYC et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med 2020; 26: 676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu IT, Li Y, Wong TW et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med 2004; 350: 1731–9. [DOI] [PubMed] [Google Scholar]
- 45. Offeddu V, Yung CF, Low MSF, Tam CC. Effectiveness of masks and respirators against respiratory infections in healthcare workers: A systematic review and meta‐analysis. Clin Infect Dis 2017; 65: 1934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang X, Pan Z, Cheng Z. Association between 2019‐nCoV transmission and N95 respirator use. J Hosp Infect 2020; 105: 104–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Centers for Disease Control and Prevention . CDC recommended guidance for extended use and limited reuse of N95 filtering facepiece respirators in healthcare settings [Internet]. Atlanta: Centers for Disease Control and Prevention; 2020. Available from https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html.
- 48. Nathan N. Waste not, want not: The re‐usability of N95 masks. Anest Analg 2020; 131: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mills D, Harnish DA, Lawrence C, Sandoval‐Powers M, Heimbuch BK. Ultraviolet germicidal irradiation of influenza‐contaminated N95 filtering facepiece respirators. Am J Infect Control 2018; 46: e49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zuo MZ, Huang YG, Ma WH et al. Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chin Med Sci J 2020; 35: 105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rutala WA, Jones SM, Worthington JM, Reist PC, Weber DJ. Efficacy of portable filtration units in reducing aerosolized particles in the size range of Mycobacterium tuberculosis . Infect Control Hosp Epidemiol 1995; 16: 391–8. [DOI] [PubMed] [Google Scholar]
- 52. Sehulster L, Chinn RY. Guidelines for environmental infection control in health‐care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 2003; 52: 1–42. [PubMed] [Google Scholar]
- 53. Santarpia JL, Rivera DN, Herrera V et al. Aerosol and surface transmission potential of SARS‐CoV‐2. medRxiv. Published online: 3 Jun 2020; DOI: 10.1101/2020.03.23.20039446. [DOI] [Google Scholar]
- 54. Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 2020; 104: 246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wong TW, Tam WW. Handwashing practice and the use of personal protective equipment among medical students after the SARS epidemic in Hong Kong. Am J Infect Control 2005; 33: 580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Calderwood AH, Day LW, Muthusamy VR et al. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc 2018; 87: 1167–79. [DOI] [PubMed] [Google Scholar]
- 57. Beilenhoff U, Biering H, Blum R et al. Reprocessing of flexible endoscopes and endoscopic accessories used in gastrointestinal endoscopy: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) ‐ Update 2018. Endoscopy 2018; 50: 1205–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Personal protective equipment with N95 or equivalent in the procedural room. Note the impermeable protective clothing (AAMI level 3) and double gloves. Abbreviations: AAMI, Association for the Advancement of Medical Instrumentation.
Table S1 Patient checklist for COVID‐19 suggested by Chinese Society of Digestive Endoscopy.
Table S2 The whole progress of donning and doffing of personal protective equipment.
Table S3 Summary of guidance for endoscopy during the COVID‐19 pandemic from International/National Societies of Gastroenterology/Gastrointestinal Endoscopy.


