Abstract
Background
The acute respiratory illness designated coronavirus disease 2019 (COVID‐19) was first reported in Wuhan, China, in December 2019 and caused a worldwide pandemic. Concerns arose about the impact of the COVID‐19 pandemic on blood donations and potential significant blood transfusion needs in severely ill COVID‐19 patients. Data on blood usage in hospitalized COVID‐19 patients are scarce.
Study Design and Methods
We performed a retrospective observational study of blood component transfusions in the first 4 weeks of COVID‐19 ward admissions. The study period began 14 days before the first COVID‐19 cohort wards opened in our hospital in March 2020 and ended 28 days afterward. The number of patients and blood components transfused in the COVID‐19 wards was tabulated. Transfusion rates of each blood component were compared in COVID‐19 wards versus all other inpatient wards.
Results
COVID‐19 wards opened with seven suspected patients and after 4 weeks had 305 cumulative COVID‐19 admissions. Forty‐one of 305 hospitalized COVID‐19 patients (13.4%) received transfusions with 11.1% receiving red blood cells (RBCs), 1.6% platelets (PLTs), 1.0% plasma, and 1.0% cryoprecipitate (cryo). COVID‐19 wards had significantly lower transfusion rates compared to non‐COVID wards for RBCs (0.03 vs 0.08 units/patient‐day), PLTs (0.003 vs 0.033), and plasma (0.002 vs 0.018; all p < 0.0001). Cryo rates were similar (0.008 vs 0.009, p = 0.6).
Conclusions
Hospitalized COVID‐19 patients required many fewer blood transfusions than other hospitalized patients. COVID‐19 transfusion data will inform planning and preparation of blood resource utilization during the pandemic.
Abbreviations
- cryo
cryoprecipitate
- DIC
disseminated intravascular coagulation
- ECMO
extracorporeal membrane oxygenation
- ICU
intensive care unit
The acute respiratory illness designated coronavirus disease 2019 (COVID‐19) was first reported on December 31, 2019, in Wuhan, China, and on January 7, 2020, the causal agent was identified as the novel severe respiratory distress syndrome coronavirus 2 (SARS‐CoV‐2). 1 Only 2 weeks later, the United States reported its first case of COVID‐19. 2 The disease spread rapidly leading to a worldwide pandemic and the declaration of a U.S. national emergency on March 13, 2020. 3 By April 15, 2020, there were 632,000 cases in the US, including over 25,000 in Illinois. 4 , 5 Concerns arose about a severe impact of the COVID‐19 pandemic on blood donations and potential significant blood transfusion needs in severely ill COVID‐19 patients. 6 , 7
Loss of blood collections due to societal closures as well as widespread illness caused significant drops in blood donations in countries with early severe outbreaks. 8 , 9 Washington state and other areas of the United States saw a temporary significant decrease in blood supply during the early period of the pandemic. 10 Hospitals and blood centers proactively implemented blood conservation strategies as well as efforts to maintain blood donations. 11 However, no studies to date have provided detailed blood usage in COVID‐19 patients. Better understanding of this aspect of COVID‐19 care can aid planning and preparation during the pandemic. Our brief report describes the blood transfusion needs of hospitalized COVID‐19 patients.
1. MATERIALS AND METHODS
We performed a retrospective observational study of blood component transfusions in the first 4 weeks of COVID‐19 admissions in our 900‐bed adult urban academic medical center. The study period started 14 days before (Weeks −2 and –1) the first COVID‐19‐cohort wards opened in our hospital on March 13, 2020, and ended 28 days afterward (Weeks 1‐4). All confirmed and clinically suspected COVID‐19 admissions were located on the COVID‐19 cohort wards. The beginning of Week 1 also marked the onset of in‐hospital patient testing to facilitate rapid rule‐out testing and avoidance or relocation of COVID‐19–negative patients from the COVID‐19 wards.
At the beginning of Week −1 due to projections for inadequate blood supplies and a request from our main blood supplier to reduce transfusion service blood inventories by 25%, our transfusion service notified all providers to adhere to hospital transfusion guidelines and expanded our prospective blood order reviews. Postponement of all elective surgeries was implemented during Week 1. The study period was prior to local availability of COVID‐19 convalescent plasma.
The numbers of patients and blood components transfused in the COVID‐19 wards and all other inpatient wards were obtained from transfusion locations recorded in our laboratory information system (Cerner PathNet, North Kansas City, MO). Cryoprecipitate (cryo) was expressed as pools. Total weekly inpatient and outpatient transfusions were also compiled from the laboratory information system. The daily numbers of total hospital inpatients and COVID‐19 ward inpatients were obtained from hospital patient census records. Total numbers of COVID‐19 admissions during the study period were obtained from hospital data provided to the Illinois Department of Public Health. Transfusion rates were expressed as blood components per patient per day to control for the rapidly changing numbers of COVID‐19 patients. Transfusion rates were calculated for the pre–COVID‐19 period (all wards, Weeks −2 and –1), the non–COVID‐19 wards during the COVID‐19period, and the COVID‐19 wards during the COVID‐19 period (both wards, Weeks 1‐4). Statistical comparisons of transfusion rates employed 95% confidence intervals (CIs) and two‐tailed Fisher's exact tests seeking p < 0.05 (Prism 8.0.3, GraphPad Software, San Diego, CA).
2. RESULTS
Our COVID‐19 wards opened with seven suspected patients, less than 1% of 852 inpatients. After 4 weeks, we had 305 cumulative COVID‐19 admissions, of which 139 were still admitted (23% of 607 inpatients), 160 were discharged and six had died. Table 1 shows the proportions of COVID‐19‐ward patients transfused, and the numbers of units they received. Eleven percent of patients received red blood cells (RBCs), but less than 2% received each of the other components. Platelet (PLT) and plasma transfusions were especially low. The COVID‐19 wards accounted for 12.3% of all inpatient‐days during Weeks 1 through 4, but received only 4% of all inpatient blood components during that time. The COVID‐19 intensive care unit (ICU) wards, which accounted for 34% of the COVID‐19 ward patient‐days, transfused 51% to 62% of the COVID‐19‐ward RBCs, PLTs, and cryo and all COVID‐19 ward plasma units (data not shown).
TABLE 1.
COVID‐19‐ward transfusions a
| Transfused patients | % of COVID‐19 ward admissions transfused | Transfused units: COVID‐19 wards/total inpatient (%) | |
|---|---|---|---|
| Any transfusion | 41 | 13.4 | 90/2122 (4.2%) |
| RBC | 34 | 11.1 | 63/1192 (5.3%) |
| PLTs | 5 | 1.6 | 7/500 (1.4%) |
| Plasma | 3 | 1.0 | 4/280 (1.4%) |
| Cryo | 3 | 1.0 | 16/150 (10.7%) |
Transfusions in the first 4 weeks of COVID‐19 wards. Cryo units are expressed as pools.
Two of the three patients receiving COVID‐19 ward cryo (six and nine pools, respectively) had disseminated intravascular coagulation (DIC), a recognized complication of severe COVID‐19. 12 , 13 The DIC case who received nine cryo pools was one of five COVID‐19 patients on extracorporeal membrane oxygenation (ECMO) during the study period.
COVID‐19 wards had significantly lower transfusion rates compared to concurrent non–COVID‐19 wards for RBCs (0.030 vs 0.075 units/patient‐day), PLTs (0.003 vs 0.033), and plasma (0.002 vs 0.018; all p < 0.0001; (Fig. 1). Expressed as percentages, these COVID‐19 ward transfusion rates were 40, 9, and 11% of the non–COVID‐19 rates, respectively. The cryo usage rate on COVID‐19 wards was not significantly different than on non–COVID‐19 wards. COVID‐19 ICU wards had significantly higher rates than COVID‐19 non‐ICU wards for RBCs (0.045 vs 0.022 units/patient‐day, p < 0.01) and plasma (0.006 vs 0.000, p < 0.05), but not for PLTs (0.006 vs 0.002, p = 0.24) or cryo (0.014 vs 0.004, p = 0.30).
FIGURE 1.
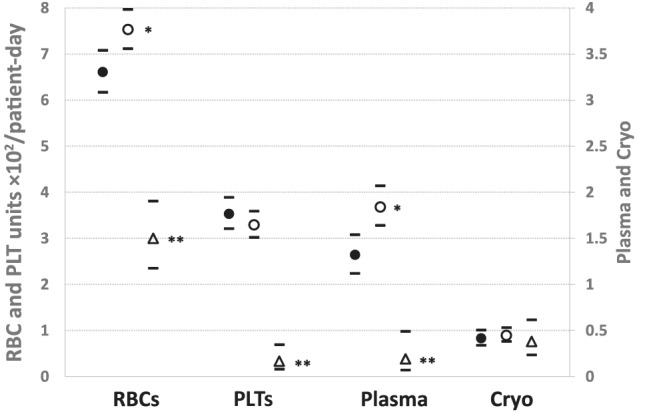
Inpatient transfusion rates before and after COVID‐19. *p < 0.01 versus pre–COVID‐19 period. **p < 0.0001 versus all wards before COVID‐19 and non–COVID‐19 wards during COVID‐19. Bars: 95% CIs. Transfusion rates: units × 102/patient‐day. •, All inpatient wards before COVID‐19: 2‐week baseline period before COVID‐19 wards opened. ○, Non–COVID‐19 wards during COVID‐19 period. Δ, COVID‐19 wards: 4‐week period after COVID‐19 wards opened
After elective surgeries were postponed and the COVID‐19 wards opened, the patients who still needed hospitalization in the non–COVID‐19 wards had higher RBC and plasma transfusion rates than inpatients in the pre–COVID‐19 period (Fig. 1). However, Fig. 2 shows the marked decline in overall weekly transfusions of all blood components in the study period, associated with enhanced blood conservation measures, canceled elective surgeries, the decrease in total inpatients as wards were cleared for future COVID‐19 patients, and the increasing numbers of COVID‐19 ward patients receiving RBCs, plasma, and PLTs at significantly lower rates. By Week 4 our blood supplier reported increased blood collections (including numerous extra blood drives in our hospital system) and improved supplies, permitting transfusion service inventories to return to normal. RBC and plasma transfusions increased somewhat in this period but PLT and cryo usage continued to decline.
FIGURE 2.

COVID‐19 impact on numbers of inpatients and overall transfusions. Total weekly blood component transfusions are shown for 2 weeks before and 4 weeks after our first COVID‐19 cohort hospital wards opened. Outpatient transfusions are included to reflect overall trends in blood usage for our transfusion service. Total inpatients and COVID‐19 ward inpatients are shown as of the last day of each week
3. DISCUSSION
Our study demonstrates low blood product utilization in patients hospitalized on COVID‐19 wards during the pandemic. Thirteen percent of hospitalized COVID‐19 patients required transfusions, which comprised only 4% of total inpatient blood transfusions during the study period. Hospitalized COVID‐19 ward patients had significantly lower transfusion rates of RBCS, PLTs, and plasma than concurrent hospitalized patients without COVID‐19. COVID‐19 ICUs had higher rates of RBC and plasma transfusion than COVID‐19 ward patients who were not in ICUs, but the overall blood usage in both groups remained low.
Low blood usage in hospitalized COVID‐19 patients may lessen the impact of the COVID‐19 pandemic on the overall blood supply. Research on blood usage in hospitalized COVID‐19 patients is limited. One Chinese hospital reported low numbers of blood components transfused, but did not provide the numbers of patients. 14 Published hematologic variables in COVID‐19 patients demonstrate most patients have normal or mildly decreased hemoglobin (Hb)and PLT counts with median Hb nadir of 13.3 g/dL and median PLT count nadir of 185 × 109/L. 15 These hematologic indices are similar to results from the 2005 SARS pandemic where decreases in PLT and Hb counts were mild and rarely required blood transfusion. 16 , 17 , 18
Disease severity factors such as organ system failure and coagulopathy including DIC have been noted in COVID‐19 patients and may impact transfusion needs. 7 , 12 , 13 Hyperfibrinogenemia and hypercoagulability in thromboelastography have also been described in COVID‐19 patients. 19 , 20 We had two patients with severe DIC who received multiple cryo doses. However, our hospital adopted expert recommendations for anticoagulation in COVID‐19 patients, which may have reduced the prevalence of this complication. 13 Procedures performed on patients such as ECMO may increase blood transfusion needs, although in our hospital avoidance of systemic anticoagulation during ECMO was recently shown to minimize transfusion needs. 21 , 22
In this preliminary study, we used COVID‐19 ward locations as a rapid means to estimate transfusion rates in a large number of patients. This included some patients who were assigned there briefly for clinical suspicion before their tests were negative. However, in‐house SARS‐CoV‐2 testing was initiated early in Week 1, so ruled‐out patients were transferred to non–COVID‐19 wards promptly. Detailed analysis of patient‐specific transfusion indications was beyond the scope of this study.
Patient blood management and efforts to stabilize and conserve the blood supply are applicable during pandemics and normal times. Our blood conservation recommendations may have influenced reduced transfusions. Proactive implementation of blood shortage management strategies may limit blood usage. In the COVID‐19 pandemic, cancellation of elective surgeries at our hospital was associated with a further significant decrease in blood usage (Fig. 2). Initial decreases in blood donation due to donor safety concerns and canceled blood collection events can be lessened with increased donor outreach and implementation of social distancing strategies in blood donation settings. 10
While pandemics may reduce blood supply, our study demonstrated that hospitalized COVID‐19 patients had low blood usage. Future studies examining the impact of patient factors may help further elucidate mechanisms affecting blood utilization in hospitalized COVID‐19 populations.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Barriteau CM, Bochey P, Lindholm PF, Hartman K, Sumugod R, Ramsey G. Blood transfusion utilization in hospitalized COVID‐19 patients. Transfusion. 2020;60:1919–1923. 10.1111/trf.15947
REFERENCES
- 1. Patel A, Jernigan DB, 2019. ‐nCoV CDC Response Team. Initial public health response and interim clinical guidance for the 2019 novel coronavirus outbreak ‐ United States, December 31, 2019‐February 4, 2020. Morb Mortal Wkly Rep. 2020;69:140‐6. [DOI] [PMC free article] [PubMed]
- 2. 2019 Novel Coronavirus Outbreak (COVID‐19). Tumwater, WA: Washington State Department of Health; 2020. https://www.doh.wa.gov/Emergencies/Coronavirus. Accessed April 27, 2020.
- 3. Trump DJ. Proclamation on Declaring a National Emergency Concerning the Novel Coronavirus Disease (COVID‐19) Outbreak. Washington, DC: The White House; 2020. https://www.whitehouse.gov/presidential‐actions/proclamation‐declaring‐national‐emergency‐concerning‐novel‐coronavirus‐disease‐covid‐19‐outbreak/. [Google Scholar]
- 4. Coronavirus Disease 2019 (COVID‐19). Springfield: Illinois Department of Public Health; 2020. http://www.dph.illinois.gov/covid19. Accessed April 16, 2020.
- 5. Coronavirus Disease 2019 (COVID‐19): Cases in the U.S. Atlanta: Centers for Disease Control and Prevention; 2002. https://www.cdc.gov/coronavirus/2019‐ncov/cases‐updates/cases‐in‐us.html. Accessed April 16, 2020.
- 6. Updated Information for Blood Establishments Regarding the Novel Coronavirus (COVID‐19) Outbreak. 2020. Silver Spring, MD: Food and Drug Administration; 2020 https://www.fda.gov/vaccines‐blood‐biologics/safety‐availability‐biologics/updated‐information‐blood‐establishments‐regarding‐novel‐coronavirus‐covid‐19‐outbreak
- 7. Cai X, Ren M, Chen F, et al. Blood transfusion during the COVID‐19 outbreak. Blood Transfus. 2020;18:79‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Franchini M, Farrugia A, Velati C, et al. The impact of the SARS‐CoV‐2 outbreak on the safety and availability of blood transfusions in Italy. Vox Sang. 2020. 10.1111/vox.12928. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohammadi S, Tabatabaei Yazdi SM, Eshghi P, et al. Coronavirus‐19 disease (COVID‐19) and decrease in blood donation: experience of Iranian Blood Transfusion Organization (IBTO). Vox Sang. 2020. 10.1111/vox.12930. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pagano MB, Hess JR, Tsang HC, et al. Prepare to adapt: blood supply and transfusion support during the first 2 weeks of the 2019 novel coronavirus (COVID‐19) pandemic affecting Washington State. Transfusion. 2020;60:908‐11. [DOI] [PubMed] [Google Scholar]
- 11. Shander A, Goobie SM, Warner MA, et al. The essential role of patient blood management in a pandemic: a call for action. Anesth Analg. 2020;131:74‐85. 10.1213/ane.0000000000004844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee AY, Connors JM, Kreuziger LB, et al. COVID‐19 and Coagulopathy: Frequently Asked Questions. Version 3.0. Washington, DC: American Society of Hematology; 2020. https://www.hematology.org/covid-19/covid-19-and-coagulopathy. [Google Scholar]
- 13. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18:1023‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Han W, Pan L, et al. Impact of COVID‐19 on blood centres in Zhejiang province China. Vox Sang. 2020. 10.1111/vox.12931. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan BE, Chong VC, Chan SS, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95:E131‐4. [DOI] [PubMed] [Google Scholar]
- 16. Chng WJ, Lai HC, Earnest A, et al. Haematological parameters in severe acute respiratory syndrome. Clin Lab Haematol. 2005;27:15‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986‐94. [DOI] [PubMed] [Google Scholar]
- 18. Wong RS, Wu A, To KF , et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326:1358‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID‐19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Hemostas. 2020;00:1‐5. 10.1111/jth.14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spiezia L, Boscolo A, Poletto F, et al. COVID‐19‐related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemostas. 2020;120:998‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Esper SA, Welsby IJ, Subramaniam K, et al. Adult extracorporeal membrane oxygenation: an international survey of transfusion and anticoagulation techniques. Vox Sang. 2017;112:443‐52. [DOI] [PubMed] [Google Scholar]
- 22. Kurihara C, Walter JM, Karim A, et al. Feasibility of venovenous extracorporeal membrane oxygenation without systemic anticoagulation. Ann Thorac Surg. 2020. 10.1016/j.athoracsur.2020.02.011. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


