Summary
Background
Acral lesions, mainly chilblains, are the most frequently reported cutaneous lesions associated with COVID‐19. In more than 80% of patients tested, nasopharyngeal swabs were negative on reverse transcription polymerase chain reaction (RT‐PCR) for SARS‐CoV‐2 when performed, and serology was generally not performed.
Methods
A national survey was launched on 30 March 2020 by the French Society of Dermatology asking physicians to report cases of skin manifestations in patients with suspected or confirmed COVID‐19 by using a standardized questionnaire. We report the results for acral manifestations.
Results
We collected 311 cases of acral manifestations [58.5% women, median age 25.7 years (range 18–39)]. The most frequent clinical presentation (65%) was typical chilblains. In total, 93 cases (30%) showed clinical suspicion of COVID‐19, 67 (22%) had only less specific infectious symptoms and 151 (49%) had no clinical signs preceding or during the course of acral lesions. Histology of skin biopsies was consistent with chilblains. Overall, 12 patients showed significant immunological abnormalities. Of the 150 (48%) patients who were tested, 10 patients were positive. Seven of 121 (6%) RT‐PCR‐tested patients were positive for SARS‐CoV‐2, and five of 75 (7%) serology‐tested patients had IgG anti‐SARS‐CoV‐2. Tested/untested patients or those with/without confirmed COVID‐19 did not differ in age, sex, history or acral lesion clinical characteristics.
Conclusions
The results of this survey do not rule out that SARS‐CoV‐2 could be directly responsible for some cases of chilblains, but we found no evidence of SARS‐CoV‐2 infection in the large majority of patients with acral lesions during the COVID‐19 lockdown period in France.
What is already known about this topic?
About 1000 cases of acral lesions, mainly chilblains, were reported during the COVID‐19 outbreak.
Chilblains were reported to occur in young people within 2 weeks of infectious signs, which were mild when present.
Most cases did not have COVID‐19 confirmed by reverse transcription polymerase chain reaction (RT‐PCR), and few serology results were available.
What does this study add?
Among 311 patients with acral lesions, mainly chilblains, during the COVID‐19 lockdown period in France, the majority of patients tested had no evidence of SARS‐CoV‐2 infection.
Overall, 70 of 75 patients were seronegative for SARS‐Cov‐2 serology and 114 of 121 patients were negative for SARS‐CoV‐2 RT‐PCR.
Coronavirus disease 2019 (COVID‐19) is a disease resulting from SARS‐CoV‐2 infection, which was first reported in Wuhan, China and has subsequently spread to the rest of the world.1, 2 The World Health Organization (WHO) declared a pandemic viral infection on 11 March 2020.
The first cutaneous manifestations were described in mid‐March 2020; since then, more than 50 studies reporting more than 1000 cases have been published, mainly from European countries. Cutaneous manifestations possibly associated with COVID‐19 are diverse and include different types of exanthema (maculopapular, urticarial and chickenpox‐like lesions).3, 4 Acral lesions, mainly chilblains, are the most frequently reported cutaneous manifestation.3, 5 The direct causal link between these skin manifestations and SARS‐CoV‐2 infection is suspected but remains uncertain.
Acral lesions are mainly observed in adolescents and young adults and are clinically described as pseudochilblains, sometimes with bullous lesions or pseudoerythema multiforme, which are two patterns that can overlap. Lesions resolved spontaneously in 1–4 weeks.3, 6
Owing to the high number of reported cases, media sources alerted that chilblains are a sign of COVID‐19 and that the appearance of such cutaneous manifestations should lead to measures of isolation and COVID‐19 testing.
The aim of our study was to describe the clinical characteristics of acral lesions at the time of the COVID‐19 outbreak in order to provide evidence of an association or lack of association between SARS‐CoV‐2 infection and acral manifestations.
Patients and methods
A national survey was launched on 30 March 2020 by the French Society of Dermatology, which asked physicians to report cases of skin manifestations in patients with clinically suspected COVID‐19 or COVID‐19 confirmed by reverse transcription polymerase chain reaction (RT‐PCR) and/or serology. Here, we report the survey results for acral manifestations, which closed on 4 May 2020. The number of confirmed cases of COVID‐19 registered in France increased from 39 642 to 131 863 from 30 March 2020 to 4 May 2020. Lockdown in France lasted from 17 March 2020 to 11 May 2020. The survey is ongoing for other cutaneous manifestations.
A standardized questionnaire was sent to society members on the mailing list and was accessible on the society website (www.sfdermato.org). In addition to completing the questionnaire, recipients were asked to send pictures, results of biological tests including nasopharyngeal swabs for RT‐PCR detection of SARS‐CoV‐2, serology for SARS‐CoV‐2 IgG detection, and histology of skin biopsies if available.
The following case data were collected using the questionnaire: month and year of birth; sex; previous cutaneous manifestation; known connective tissue disease; previous Raynaud syndrome; chilblains; any other diseases and current treatment; date of first general symptoms; presence or absence of fever, cough, dyspnoea, asthaenia, nausea/vomiting, diarrhoea, headache, anosmia/ageusia or pneumonia; date of first cutaneous manifestations; clinical characteristics; date of resolution of general symptoms; date of resolution of cutaneous manifestation; date and results of SARS‐CoV‐2 RT‐PCR testing and serology; date of hospitalization; date of intensive care unit (ICU) hospitalization; cutaneous manifestation treatment; histology and biological examinations.
According to the WHO definition (modified on 29 May 2020) we classified the patients as having no general symptoms; possible case (if the patient had at least one of the following symptoms: fever, cough, dyspnoea, anosmia, ageusia or dysgeusia) (https://www.ecdc.europa.eu/en/covid‐19/surveillance/case‐definition); or patients with less specific symptoms (if the patient had asthaenia, nausea/vomiting, diarrhoea, headache and none of the aforementioned symptoms). The flow of patients in the study is outlined in Figure 1.
Figure 1.
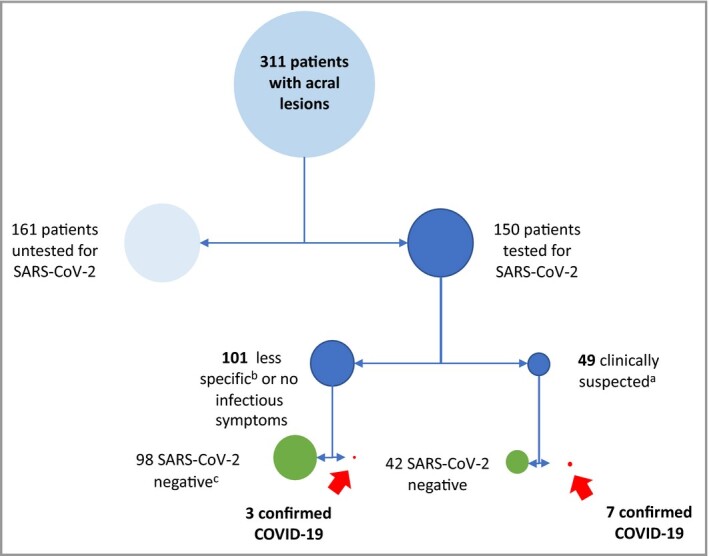
Flow of patients in the study.
The size of each circle is proportional to the number of patients. aFever and/or cough and/or dyspnoea and/or anosmia/ageusia. bAsthaenia, nausea/vomiting, diarrhoea, headache. cReverse transcription polymerase chain reaction and/or serology.
Photographs were reviewed consensually by three experienced dermatologists (M.B.‐B., L.L.C., H.A.) in order to classify clinical characteristics. We defined typical chilblains as single or multiple lesions, maculopapular, oedematous and erythematous to violaceous (Figure 2).
Figure 2.
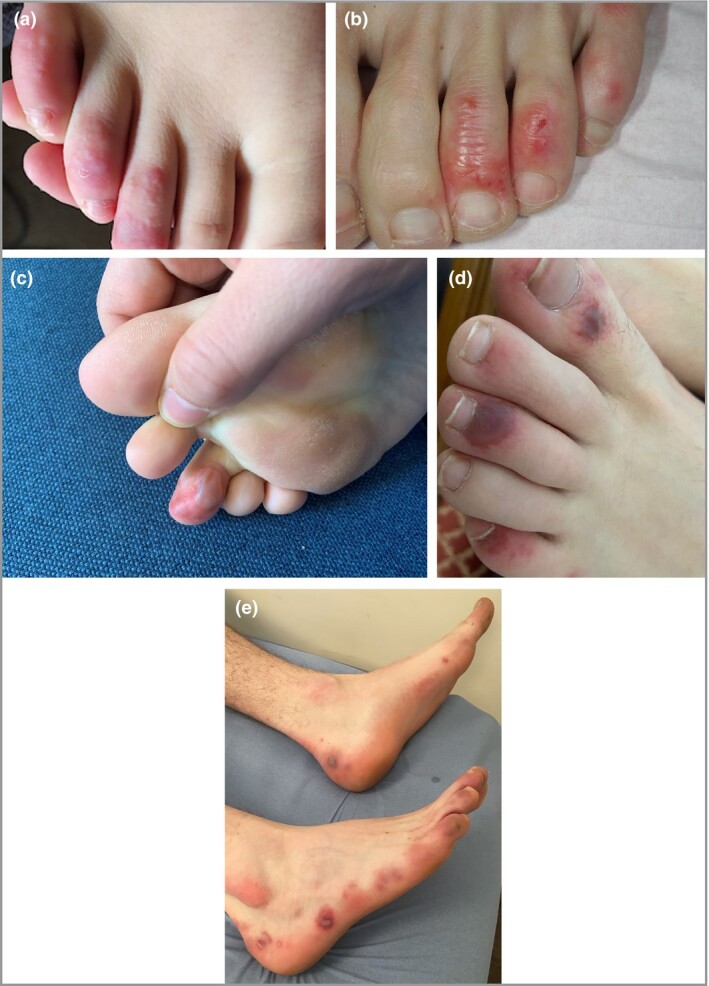
Typical chilblains. (a, b) Typical lesions observed in a majority of patients. (c) Typical chilblains with bullae. (d) More severe lesions with purpuric aspect. (e) Chilblains on the toes and lateral side of feet.
We classified other lesions into the following three categories:
Erythema multiforme (EM)‐like, characterized by round maculopapular lesions including target lesions (Figure 3).
Punctiform purpuric lesions (Figure 4).
Diffuse vascular erythema and oedema of dorsum or sole of foot and/or palms (Figure 5).
Figure 3.
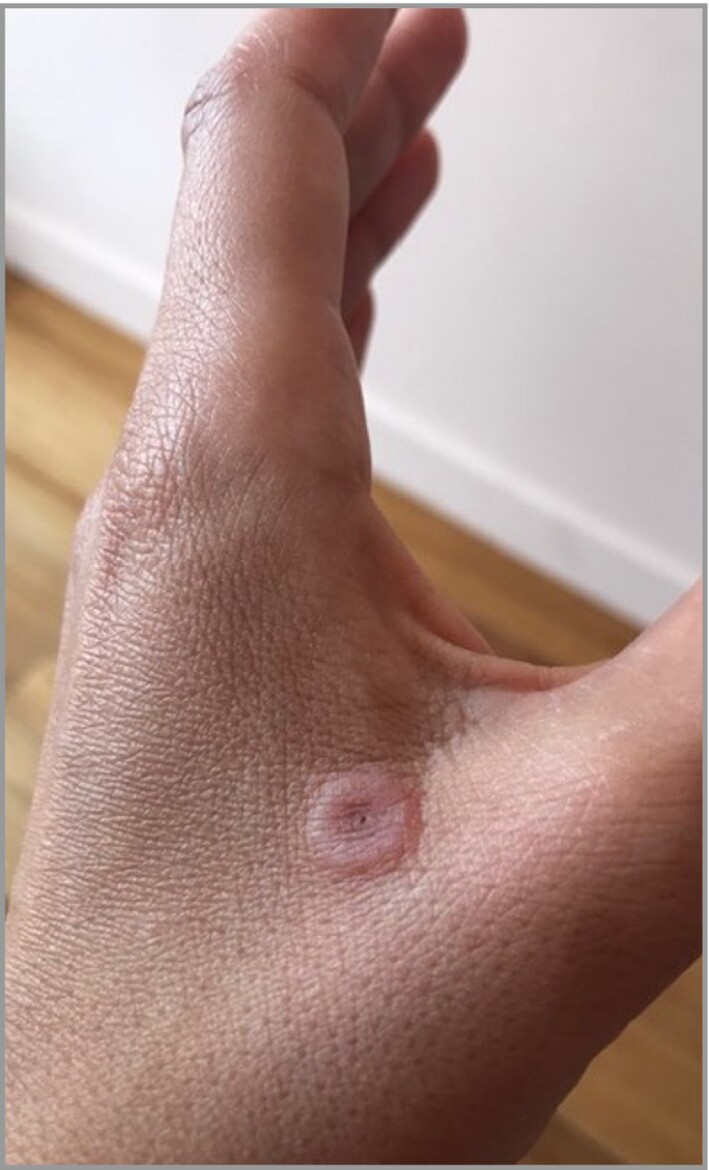
Erythema multiforme‐like lesion on the hands.
Figure 4.
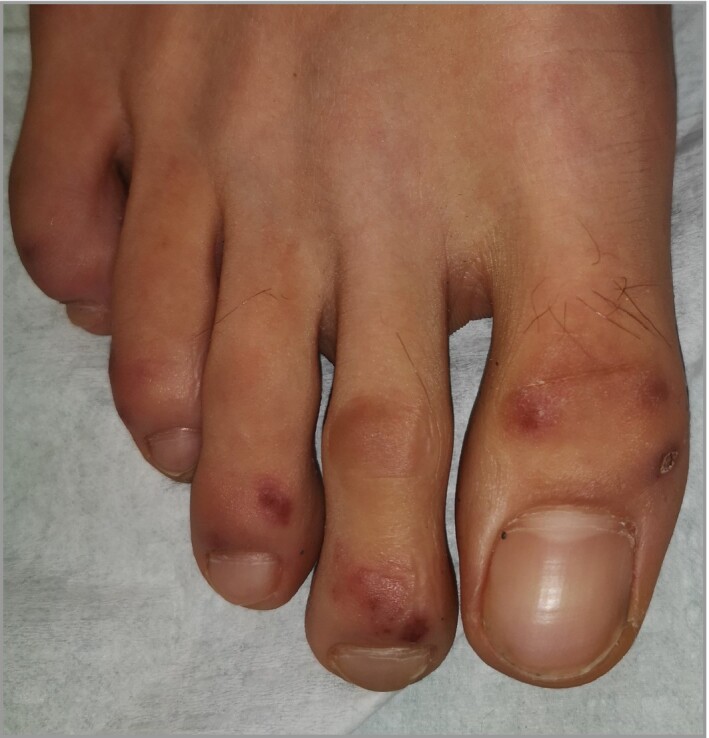
Punctiform purpuric lesions on the toes and feet.
Figure 5.
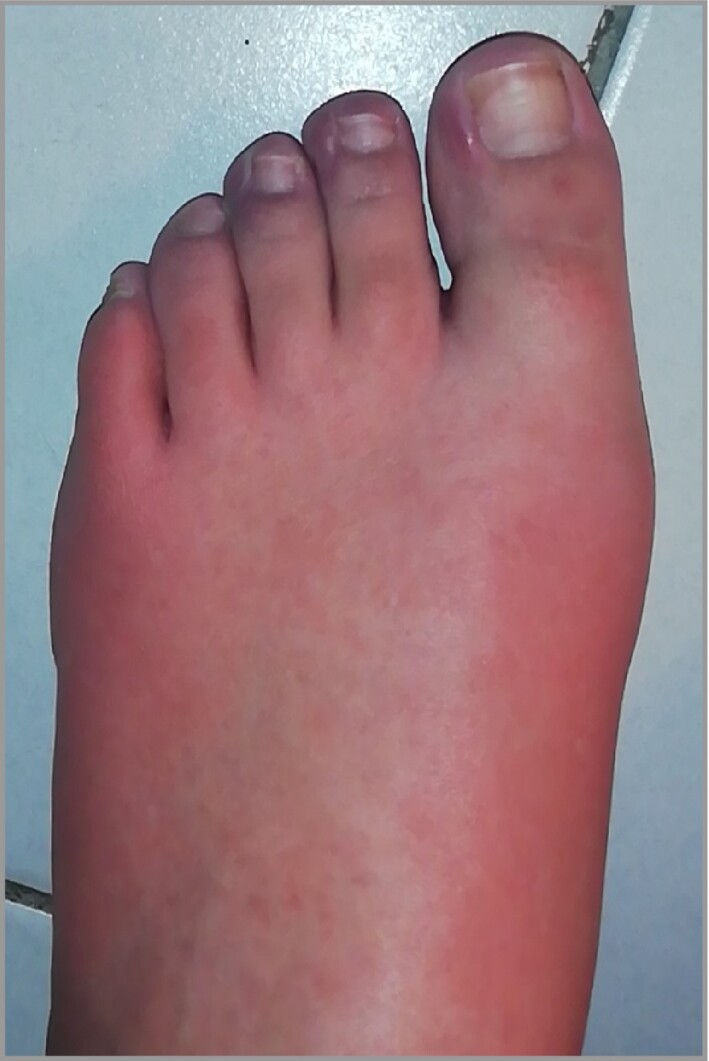
Diffuse vascular erythema and oedema of dorsum of feet.
Patients (or legal representative for minors) indicated their nonopposition to participation in the survey. A signed authorization for use of photographs was also requested. Information was sent with anonymization. The study was authorized by the Henri Mondor Hospital ethics committee (Créteil, France) and declared to the Commission Nationale de l'Informatique et des Libertés (no. 2217623).
For serology, serum samples were processed for 60 patients on an Abbott Architect instrument using the Abbott SARS‐CoV‐2 IgG assay following the manufacturer’s instructions. The assay is a chemiluminescent microparticle immunoassay for qualitative detection of IgG against the SARS‐CoV‐2 nucleoprotein in human serum. Qualitative results and index values reported by the instrument, associated with a 99.9% specificity and 100% sensitivity, were used in the analysis.7 For the 10 other patients, the kit used for serology testing was not specified.
Statistical analyses
Continuous data are described using mean ± SD or median [interquartile range (IQR)] depending on whether there was a normal distribution. Categorical data are described as n (%). Our main analysis was descriptive, assessing characteristics of patients with acral manifestations. The characteristics of patients with/without testing and positive/negative on testing were compared using the χ2‐test or Fisher’s exact test for categorical data and the Mann–Whitney U‐test for continuous variables. All tests were two‐tailed, and the threshold for statistical significance was set to P < 0.05. All statistical analyses were performed using Stata version 14.1 (StataCorp LP, College Station, TX, USA).
Results
Population characteristics
We collected 481 cases of cutaneous manifestations including 311 cases of acral manifestations between 30 March 2020 and 4 May 2020 (Figure 1). Most patients were female (n = 182, 58.5%) and the median age was 25.7 years (IQR 18–38.3). A total of 150 patients underwent nasopharyngeal‐swab RT‐PCR and/or serology for SARS‐CoV‐2 (69 patients had RT‐PCR and serology, 58 patients had RT‐PCR only and 23 patients had serology only). Six (2%) patients had a history of autoimmune disease, 32 (10%) patients had a history of chilblains and 31 (10%) patients had a history of Raynaud syndrome; six of these patients had both Raynaud syndrome and chilblains.
Overall, 93 (30%) patients had fever and/or cough and/or dyspnoea and/or anosmia/ageusia and were classified as suspected COVID‐19; three patients had pneumonia, none was admitted to an ICU and none died. A total of 67 (22%) patients had only less specific infectious symptoms (nausea, vomiting and/or diarrhoea and/or odynophagia and/or asthaenia and/or myalgia and/or headache) and 151 (49%) patients had no clinical signs preceding or during the course of acral lesions. In most cases, no specific treatment was applied, and topical corticosteroids were prescribed for 54 (17%) patients.
Clinical and histological characteristics
Clinical presentation was classified into four categories after photographic review. Classification was feasible for 245 (79%) patients, for whom photographs were available. The most frequent clinical presentation was typical chilblains (202 of 245, 82%) located mainly on the dorsum of the toes (Figure 2a–e); 22 cases had a severe form with bullae (Figure 2c). Other forms were EM‐like lesions, mainly on the lateral part of the feet (Figure 3), in 15 (6%) patients; punctiform purpuric lesions on the dorsum and/or pulps in 11 (4.5%) patients (Figure 4) and diffuse vascular erythema and oedema of the dorsum or soles of the feet and/or palms in 17 (7%) patients (Figure 5).
Acral lesions were localized on the feet in 236 (76%) cases, on the hands in 36 of 311 (12%) cases and on both in 37 (12%) cases. The median time between infectious symptoms and the appearance of acral lesions was 10.5 days (IQR 1–16) (data available for 148 patients with infectious symptoms) and acral lesions preceded infectious symptoms in 23 cases.
Skin biopsies were obtained for 29 patients (17 patients typical chilblains, two with EM‐like lesions, one with punctiform purpuric lesions, nine without photographs). All but three cases (superficial biopsy or unspecific findings) exhibited vacuolization or apoptosis of keratinocytes, superficial and deep infiltrates, mainly of lymphocytes with a perieccrine and perivascular reinforcement in most cases and, in some cases, superficial capillary thrombosis and different degrees of dermal oedema.
Testing for SARS‐CoV‐2
Among the 311 included patients, 150 had RT‐PCR testing or serology for SARS‐CoV‐2. Seven of 121 patients tested using RT‐PCR were positive and five of 75 serology‐tested patients were positive (IgG). Of the 69 patients who underwent RT‐PCR and serology, results were concordant for 58 patients (56 negatives for both, two positives for both), discordant for two (negative PCR and positive serology) and for nine patients only RT‐PCR results (negative) were available (serologies were taken but not processed).
Thus, 10 of 150 (7%) tested patients had confirmed COVID‐19 (Figure 1). Serology was performed 21 days (median) (IQR 12–30) after the beginning of infectious symptoms (n = 45 patients with symptoms). Results for the following tests were normal or negative in all investigated cases: C‐reactive protein level (n = 20), activated partial thromboplastin time (n = 61), complement (n = 14), cold agglutinins (n = 34), cryofibrinogen (n = 30), coxsackie and parvovirus (n = 57). Blood cell count was normal in 57 of 66 patients, with no significant abnormalities.
Overall, 12 patients exhibited significant immunological abnormalities. Detailed characteristics are presented in Table S1 (see Supporting Information).
Comparison between tested and untested patients
Tested and untested patients did not differ in age, sex, history or acral lesion clinical characteristics, nor did they differ in rate of clinically suspected COVID‐19 with only nonspecific symptoms and no symptoms (Table 1). We observed that headache was significantly more frequent in tested patients compared with untested patients [32% (48 of 150) vs. 19% (30 of 161), P < 0.009].
Table 1.
Comparison between patients with and without RT‐PCR testing or serology for SARS‐Cov‐2
| Characteristics | Total population | With PCR testing or serology | Without PCR testing or serology | P‐valuesa |
| Total | 311 (100) | 150 (48) | 161 (52) | |
| Sex (female) | 182 (59) | 85 (57) | 97 (60) | 0.6 |
| Age, years, median (IQR) | 25.7 (18–39) | 27 (20–38) | 24.5 (17–39) | 0.1 |
| History | ||||
| Connective tissue diseases | 6 (2) | 2 (1) | 4 (2) | 0.7 |
| Chilblains | 32 (10) | 21 (14) | 11 (7) | 0.06 |
| Raynaud syndrome | 31(10) | 15 (10) | 16 (10) | 1 |
| Extracutaneous clinical manifestations | ||||
| Fever | 42 (13.5) | 21 (14) | 21 (13) | 0.9 |
| Cough | 54 (17) | 30 (20) | 26 (16) | 0.4 |
| Dyspnoea | 33 (11) | 21 (14) | 12 (7) | 0.07 |
| Asthaenia | 91 (29) | 46 (31) | 45 (28) | 0.6 |
| Myalgia | 31 (10) | 18 (12) | 13 (8) | 0.3 |
| Headache | 78 (25) | 48 (32) | 30 (19) | 0.009 |
| Odynophagia | 34 (11) | 21 (14) | 13 (8) | 0.1 |
| Anosmia/ageusia | 17 (5) | 13 (9) | 4 (2) | 0.02 |
| Fever and/or cough and/or dyspnoea and/or anosmia/ageusia | 93 (30) | 49 (33) | 44 (27.5) | |
| Asthaenia and/or myalgia and/or headache and/or odynophagia | 67 (22) | 28 (19) | 39 (24) | 0.4 |
| None | 151 (49) | 73 (49) | 78 (48) | |
| Acral lesion characteristics | ||||
| Typical chilblains | 201/245 (82) | 100/124 (81) | 101/121 (83) | 0.6 |
RT‐PCR, reverse transcription polymerase chain reaction; IQR, interquartile range.
a P‐values from χ2‐test or Fisher’s exact test for categorial variables or Mann–Whitney U‐test for continuous variables. Data are presented as n (%) unless otherwise indicated.
Comparison between patients with positive reverse transcription polymerase chain reaction and/or serology results and patients with negative tests
Patients with/without confirmed COVID‐19 did not differ in age, sex, history or acral lesion clinical characteristics (Table 2). Headache and anosmia/ageusia were significantly more frequent in confirmed cases [70% (seven of 10) and 50% (five of 10), respectively] than in unconfirmed cases [29% (41 of 140) and 8% (six of 140), respectively] (P < 0.013 and 0.001).
Table 2.
Comparisons between patients positive on RT‐PCR and/or serology and negative on RT‐PCR and/or serology for SARS‐Cov‐2 infection
| Characteristics | Positive RT‐PCR and/or serology (n =150 tested) | Negative RT‐PCR and/or serology (n = 150 tested) | P‐valuesa |
| Total | 10 (7) | 140 (93) | |
| Sex (female) | 7 (78) | 78 (56) | 0.3 |
| Age, years, median (IQR) | 34 (26–38) | 27 (20–39) | 0.3 |
| History | |||
| Connective tissue diseases | 0 | 2 (1) | 1 |
| Chilblains | 1 (10) | 20 (14) | 1 |
| Raynaud syndrome | 2 (20) | 13 (9) | 0.3 |
| Extracutaneous clinical manifestations | |||
| Fever | 2 (20) | 19 (14) | 0.6 |
| Cough | 2 (20) | 28 (20) | 1 |
| Dyspnoea | 3 (30) | 18 (13) | 0.1 |
| Asthaenia | 5 (50) | 41 (29) | 0.2 |
| Myalgia | 3 (30) | 15 (11) | 0.1 |
| Headache | 7 (70) | 41 (29) | 0.013 |
| Odynophagia | 3 (30) | 18 (13) | 0.15 |
| Anosmia/ageusia | 5 (50) | 8 (6) | 0.001 |
| Fever and/or cough and/or dyspnoea and/or anosmia/ageusia | 7 (70) | 42 (30) | |
| Asthaenia and/or myalgia and/or headache and/or odynophagia | 0 | 28 (20) | |
| None | 3 (30) | 70 (50) | 0.048 |
| Acral lesion characteristics | |||
| Typical chilblains | 5/8 (63) | 95/116 (81) | 0.2 |
RT‐PCR, reverse transcription polymerase chain reaction; IQR, interquartile range.
a P‐values from χ2‐test or Fisher’s exact test for categorial variables or Mann–Whitney U‐test for continuous variables. Data are presented as n (%) unless otherwise indicated.
Discussion
We report 311 cases of acral lesions, mainly chilblains, occurring during the COVID‐19 outbreak in France. Among the 150 patients who underwent RT‐PCR testing and/or serology, only 10 had confirmed COVID‐19. Among 75 patients with serology, five (7%) were positive.
The characteristics of our population are comparable with those of the 995 patients with acral lesions reported during the COVID‐19 outbreak in 14 previously published series or studies (reporting more than 10 patients) (Table S2; see Supporting Information). These series came from four countries (Italy, Spain, France and USA). It is possible that some cases were reported in more than one series. With regard to the 311 patients in our population, to our knowledge, the histological characteristics of 17 cases had already been reported.8 As in our study, in which the median age was 25.7 years (IQR 18–38.3), most patients who were reported in these previous studies were children or young adults (the mean age was 10–20 years in 10 series, 32.5 years in one study and the median age varied from 14 years to 27 years in three studies). The rate of patients who had no general symptoms preceding or concomitant with skin lesions was 30% (207 of 678) based on 10 previously published series (data were missing for four other series), compared with 49% (151 of 311) in our study. When present, symptoms were mild. Skin lesions were generally described as chilblains or pseudochilblains and more rarely as EM‐like lesions. Overall, 300 patients had laboratory confirmation by RT‐PCR using a nasopharyngeal swab, which was positive in 54 (18%) patients based on 13 series. In one study3 the proportion was higher [41% (29 of 71)] than in the 12 others [11% (25 of 229)], which varied from 0% to 21%. By comparison, in our study the proportion of positive RT‐PCR results in patients who were tested was 6% (seven of 121). Serology was rarely performed and was positive for IgG in seven of 39 cases and positive for IgA in six of 19 cases in the previously published series, compared with five of 75 (7%) cases positive for IgG in our study.3,5,9–20
Serology was not available at the time of publication of these previous series. Also, RT‐PCR negativity was to be expected because chilblains were considered a late manifestation of the infection, occurring 1–5 weeks after signs of infection, with the disappearance of detectable virus after a brief or paucisymptomatic infection in young healthy individuals, possibly owing to an immunological response targeting the cutaneous vessels. Indeed, firstly, the sensitivity of RT‐PCR varied from 71% to 98% based on negative RT‐PCR tests that were positive on repeat testing. Secondly, the median time from the onset of symptoms to negative SARS‐CoV‐2 RT‐PCR test result in hospitalized patients was 20 days.21, 22
Thus, it is possible that in these studies negative RT‐PCR test results in some patients could be false‐negatives. By contrast, in our study, the absence of SARS‐CoV‐2 IgG antibodies in 65 of 70 patients tested using a highly sensitive and specific test, 21 days (median) (IQR 12–30) after the onset of symptoms, is strong evidence against a direct causal link between COVID‐19 and chilblains. Indeed, performance assessment of the Abbott Architect serology instrument (used in 60 of 75 patients in our study) has found that all patients with positive SARS‐CoV‐2 RT‐PCR had positive serology 17 days after the beginning of symptoms. In a series reporting 19 cases of chilblains in adolescents, serology was negative for IgG using the same kit used in our study (Abbott) and were positive for IgG in one patient, positive for IgA in six patients and were borderline for three patients using another kit (Euroimmun, Lübeck, Germany).19 The authors suggested that in asymptomatic children or those who had mild infection, the humoral response could be IgA rather than IgG. However, as the Euroimmun kit is known to provide false‐positive results, these results should be interpreted with caution.22–24
Four studies reported frequency of cutaneous manifestations in patients with confirmed SARS‐Cov‐2 infection in 0.2% (N = 1099 patients), 4.9% (N = 103), 20% (N = 88), 10% (N = 125).2, 18, 25, 26 Among these patients, only one had chilblains. However, the mean age of these patients was higher.
The lack of difference in sex, age, history of connective tissue disease, and acral lesion characteristics between patients with/without confirmed COVID‐19 could also provide evidence against the role of viral infection in chilblains.
Our study has some limitations, mainly that all patients did not undergo the same investigation. However, the characteristics of untested patients did not differ significantly from those of tested patients. We hypothesized that these results could reasonably be extrapolated to the whole population. Photographs were not available for 66 of 311 patients, who could not be classified more precisely other than having chilblains. The strengths of this survey are the large number of included patients and that almost half of the population had a test to confirm COVID‐19, including serology for 75 patients. Our study had a different methodology from the study of Galván Casas et al.,3 in which 41% of patients with acral lesions had a positive RT‐PCR test and in which criteria for inclusion required a specific clinical sign or RT‐PCR confirmation.
The characteristics of our population were similar to those previously reported in patients with chilblains, namely female predominance and adolescents or young adults, with immunological abnormalities found in few patients.27, 28 Histological findings, when available (deep infiltrates, mainly of lymphocytes with a perieccrine reinforcement associated with dermal oedema and necrotic keratinocytes), were consistent with the classical description of the histological features of chilblains.29
These benign acral chilblain‐type lesions occurring in outpatients without severe manifestations of COVID‐19 must be distinguished from the acral ischaemic lesions described in seven severely ill patients with COVID‐19.30 These severe acroischaemia cases were associated with elevated D‐dimer level and fibrinogen degradation products and prolonged prothrombin time with disseminated intravascular coagulation. These thrombotic complications associated with COVID‐19 resemble other systemic coagulopathies during severe infections.31 When investigated, none of our patients had anomalies of haemostatic markers. Similarly, chilblain‐type lesions were not associated with antiphospholipid antibodies, which may be transiently observed in patients with critical infectious diseases.
If chilblains are not directly related to COVID‐19, how can we explain the numerous reported cases? Most reported cases occurred between March and April 2020 in Southern Europe. The incidence of chilblains is not known, so affirming an increase is difficult. However, many dermatologists confirmed that this chief complaint was more frequent than in previous years. This situation could be due to the media stating that chilblains were caused by SARS‐CoV‐2 infection, leading to a higher rate of consultation for benign cutaneous manifestations, or the lockdown leading to increased inactivity and long periods at home barefoot on a cold floor. For March and April, the mean temperature in France was 9 °C (range −5.8–25.2) and 13.7 °C (−6.3–27.1), respectively (http://www.terre‐net.fr).
The results of this survey did not rule out that SARS‐CoV‐2 infection could be directly responsible for some cases of chilblains, but we found no evidence of SARS‐CoV‐2 infection in the large majority of patients with acral lesions during the COVID‐19 lockdown period in France. From our results, this usually benign cutaneous manifestation resolved spontaneously in a few weeks and has neither diagnostic nor prognostic value for COVID‐19.
Acknowledgments
The authors would like to thank the following people for their contribution to this research: Ivana Abdo Morales, Safia Abed, Caroline Accary, Karim Aissat, Florence Amelot, Philippe Assouly, Geneviève Aubrun, Bertrand Bachollet, Guillaume Baille, Fabienne Ballanger‐Desolneux, Corinne Balloy, Annick Barbaud, Inès Baros, Coralie Becquart, Clarence de Belilovsky, Nabil Belfeki, Zoe Bhujoo, Estelle Blanchard, Gerome Bohelay, Vincent Bonneterre, Isabelle Bonte, Christian Bordier, Perrine Brun‐Leveque, Charlotte Borocco, Marie‐Laure Bouyssou‐Gautier, Clement Braesch, Nesrine Brahimi, Alice Brehon, Agnès Cabarrot, Vincent Cante, Clemence Capelle, Sylvie Cararo, Delphine Carre‐Gislard, Jacqueline Castany, Céline Cazorla, Mélanie Chamaillard, Gérard Cipriano, Paul Cirotteau, Emilie Clément, Caroline Jade‐Clerc, Antoine Cleys, Hester Colboc, Marie‐Cécile Comby‐Viallard, Benoit Comet, Christelle Comte, Mariane Cony, Florence Corgibet, Marie‐Anne Cosson, Caroline Cotten, Jean‐Charles Crépin, Jean‐Bernard Damiens, Juliana Darasteanu, Gabriela Deda Erbenova, Elodie Delas, Céline Desvignes, Mathilde Devaux, Martin‐Xavier Doré, Valérie Dorizy‐Vuong, Brigitte Drouet, Margaux Dubus, Pierre Duffau, Eugénie Dulbecco, Vincent Duliège, Aurore Dupont, François Durupt, Xavier Duthil, Claudine Duvivier, Sophie Duvert‐Lehembre, Sylvie Etesse, Alice Eynard, Marion Fenot, Christine Fernandez, Catherine Figoni Laugel, Juliette Fontaine, Marien Fournet, Mariem Fourati, Marion Fradet, Adrienne Francopoulo, Elodie Gable, Olivier Gaudin, Aurélia Gey, Sophie Gibert, Elisa Goujon, Laurence Goyat‐Labbe, Bérangère Grenier, Robin Guelimi, Catherine Guichard, Marine Guignant, François Guilbert, Claire‐Estelle Guillaume, Stéphanie Guillet, Patrick Guillot, Constance Hazen, Diane Heron‐Mermin, Gaelle Hirsch, Claudine Hocke, Heiko Hortig, Flavien Huet, Emilie Imbert, Mathilde Jardri ,Marie‐Hélène Jegou, Géraldine Jeudy, Stéphanie Jobard, Denis Jullien, Ludovic Karkowski, Ulker Kilic Huck, Caroline Klene, Nicolas Kluger, Emeline Kubica, Eric Lachiver, Pierre‐Philippe Laget, Aurélien Lannot, Maiana Larsabal, Anais Lasserre, Nicolas Le Caruyer, Marie Therese Leccia, Oriane Lefer, Xavier Legendre, Laurent Le Lièvre, Christine Lieb‐Bazile, Noémie Litrowski, Mathilde Lombard de Buffières de Rambuteau, Sandra Ly, Laurent Machet, Emmanuel Mahé, Annabel Maruani, Laurence Maulin, François Maurier, Marine‐Emeline Marniquet, Sonia Marquez Briand, Karine Marrou, Laurine Marty, Etienne Meriglier, Mohammed Midhat, Brigitte Milpied, Philippe Moguelet, Marie Moncourier, Delphine Monnier, Laurie Monitor, Claudie Mourier, Françoise Morin, Sandrine Napias, Cristele Nicolas, Marion Oberlé, Jonathan Olry, Virginie Papillon, Patricia Pavese, Camille Payet Revest, Raphaelle Pellegrin, Anne Perrin, Emilia Pires, Florian Prost, Stéphanie Pujol, Bruno Ranchin, Alexis Redor, Valérie Remy‐Leroux, Roxane Reviron, Laurence Riffaud, Marie‐Caroline Riglet, Marine Robert, Frédérique Roca, Hughes Roger, Pauline Rosier, Jacques Rouanet, Christelle Rousseau, Sylvie Roy, Camille Salzes, Frédérique Sarazin, Patricia Senet, Dominique Serraf Tircazes, Amandine Servy, Nathalie Schwald Adan, Emilie Shipley, Claire Sibon, Christelle Silva, Carole Sin, Angèle Soria, Marina Soufi, Isabelle Templier, Florence Tholliez, Domitille Thomas Baulieu, Sabiha Trabelsi, François Truchetet, Julie Versapuech, Estelle Viala, Eric Villaret, Béatrice Walls, Frédérique Weber‐Muller, Nicolas Winter and Gaethane Wirth.
Author Contribution
Laurence Le Cleach: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing‐original draft (lead). Lea Dousset: Data curation (equal); Validation (equal). Haudrey Assier: Data curation (equal); Investigation (equal); Validation (equal). Slim Fourati: Formal analysis (equal); Methodology (equal); Resources (equal); Validation (equal); Writing‐original draft (supporting). Sebastien Barbarot: Data curation (equal); Investigation (equal); Validation (equal); Writing‐original draft (supporting). Claire Boulard: Data curation (equal); Investigation (equal); Validation (equal); Writing‐original draft (supporting). Catherine Bourseau‐Quetier: Investigation (equal); Validation (equal). Laurent Cambon: Investigation (equal); Validation (equal). Charles Cazanave: Data curation (equal); Investigation (equal); Validation (equal). Audrey Colin: Data curation (equal); Project administration (equal). Elise Kostrzewa: Investigation (equal); Validation (equal). Cecile Lesort: Data curation (equal); Validation (equal); Writing‐original draft (supporting). Annabelle Levy Roy: Data curation (equal); Investigation (equal); Validation (equal). Florian Lombart: Data curation (equal); Investigation (equal); Validation (equal). Josephina Marco Bonnet: Data curation (equal); Investigation (equal); Validation (equal). Jean‐Benoit Monfort: Data curation (equal); Investigation (equal); Writing‐original draft (supporting). Mahtab Samimi: Data curation (equal); Investigation (equal); Validation (equal); Writing‐original draft (supporting). Mathilde Tardieu: Data curation (equal); Investigation (equal); Validation (equal). Pierre Wolkenstein: Conceptualization (equal); Methodology (equal); Validation (equal). Emilie Sbidian: Formal analysis (equal); Methodology (equal); Writing‐original draft (supporting). Marie Beylot‐Barry: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Methodology (equal); Supervision (equal); Validation (equal); Writing‐original draft (supporting).
Supplementary Material
Table S1 Characteristics of the 12 patients with immunological abnormalities.
Table S2 Summary of 14 series (> 10 cases) reporting acral lesion during COVID‐19 outbreak published on 6 June 2020.
Powerpoint S1 Journal Club Slide Set.
Contributor Information
L. Le Cleach, Dermatology Department Hôpital Henri Mondor Créteil France; EA 7379 EpiDermEUPEC Créteil France.
L. Dousset, Dermatology Department University Hospital Bordeaux Bordeaux France
H. Assier, Dermatology Department Hôpital Henri Mondor Créteil France
S. Fourati, Department of Virology Hôpital Henri MondorUniversité Paris‐Est Créteil France
S. Barbarot, Department of Dermatology Nantes Université, CHU NantesUMR 1280 PhAN, INRAE F‐44000Nantes France
C. Boulard, Le Havre Hospital, Department of Dermatology 76600Le Havre France
C. Bourseau Quetier, Private Practice rue Jules Ferry Blanquefort France
L. Cambon, Private Practice rue de la Balance Toulouse France
C Cazanave, Infectious Diseases University Hospital Bordeaux Bordeaux France.
A. Colin, Dermatology Department Hôpital Henri Mondor Créteil France
E. Kostrzewa, Dermatology Department Hôpital Robert Boulin Libourne France
C. Lesort, Department of Dermatology Edouard Herriot Hospital, Hospices Civils de Lyon Lyon France
A. Levy Roy, Private PracticeAvenue du Général de Gaulle 13410Lambesc France
F. Lombart, Dermatology Amiens University Hospital Centre Amiens France
J. Marco‐Bonnet, Private practice Avenue Pierre Brossolette 92120 Montrouge France
J.‐B. Monfort, Sorbonne Université Paris France Dermatology and Allergology Department AP‐HPHôpital Tenon F‐75020Paris France.
M. Samimi, Dermatology Department University Hospital of ToursISP1282 INRA‐University of Tours Tours France
M. Tardieu, Dermatology Department Centre Hospitalier Universitaire Grenoble Alpes 38700La Tronche France
P. Wolkenstein, Dermatology Department Hôpital Henri Mondor Créteil France
E. Sbidian, Dermatology Department Hôpital Henri Mondor Créteil France EA 7379 EpiDermEUPEC Créteil France.
M. Beylot‐Barry, Dermatology Department University Hospital Bordeaux Bordeaux France French Society of Dermatology Paris France.
References
- Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W, Ni Z, Hu Y et al. Clinical Characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván Casas C, Català A, Carretero Hernández G et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia JL, Kamceva M, Rao SA, Linos E. Cutaneous manifestations of COVID‐19: a preliminary review. J Am Acad Dermatol 2020; 83:687–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Nieto D, Jimenez‐Cauhe J, Suarez‐Valle A et al. Characterization of acute acro‐ischemic lesions in non‐hospitalized patients: a case series of 132 patients during the COVID‐19 outbreak. J Am Acad Dermatol 2020; 83:e61–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janah H, Zinebi A, Elbenaye J. Atypical erythema multiforme palmar plaques lesions due to Sars‐Cov‐2. J Eur Acad Dermatol Venereol 2020; 10.1111/jdv.16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan A, Pepper G, Wener MH et al. Performance characteristics of the Abbott Architect SARS‐CoV‐2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol 2020; 58:e00941–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanitakis J, Lesort C, Danset M, Jullien D. Chilblain‐like acral lesions during the COVID‐19 pandemic (“COVID toes”): histologic, immunofluorescence and immunohistochemical study of 17 cases. J Am Acad Dermatol 2020; 10.1016/j.jaad.2020.05.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalcati S, Barbagallo T, Frasin LA et al. Acral cutaneous lesions in the time of COVID‐19. J Eur Acad Dermatol Venereol 2020; 10.1111/jdv.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo V, Neri I, Filippeschi C et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol 2020; 34:e291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaní J, Baselga E, Mitjà O et al. [Chilblain and acral purpuric lesions in Spain during COVID confinement: retrospective analysis of 12 cases]. Actas Dermosifiliogr 2020; 111:426–9 (in Spanish). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andina D, Noguera‐Morel L, Bascuas‐Arribas M et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol 2020; 37:406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Masson A, Bouaziz J‐D, Sulimovic L et al. Chilblains are a common cutaneous finding during the COVID‐19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol 2020; 83:667–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Lara G, Linares‐González L, Ródenas‐Herranz T, Ruiz‐Villaverde R. Chilblain‐like lesions in pediatrics dermatological outpatients during the COVID‐19 outbreak. Dermatol Ther 2020; 10.1111/dth.13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Robles J, de la Hera I, Pardo J et al. Chilblain‐like lesions: a case series of 41 patients during the COVID‐19 pandemic. Clin Exp Dermatol 2020; 10.1111/ced.14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrolonardo M, Romita P, Bonifazi E et al. The management of the outbreak of acral skin manifestations in asymptomatic children during COVID ‐19 era. Dermatol Ther 2020; 10.1111/dth.13617. [DOI] [PubMed] [Google Scholar]
- Saenz Aguirre A, De la Torre Gomar FJ, Rosés‐Gibert P et al. Novel outbreak of acral lesions in times of COVID‐19: a description of 74 cases from a tertiary university hospital in Spain. Clin Exp Dermatol 2020; 10.1111/ced.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneri C, Venanzi Rullo E, Gallizzi R et al. Diversity of clinical appearance of cutaneous manifestations in the course of COVID‐19. J Eur Acad Dermatol Venereol 2020; 10.1111/jdv.16669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hachem M, Diociaiuti A, Concato C et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain‐like lesions: lights and shadows on the relationship with COVID‐19 infection. J Eur Acad Dermatol Venereol 2020; 10.1111/jdv.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EE, McMahon DE, Lipoff JB et al. Pernio‐like skin lesions associated with COVID‐19: a case series of 318 patients from 8 countries. J Am Acad Dermatol 2020; 83:486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao AT, Tong YX, Gao C et al. Dynamic profile of RT‐PCR findings from 301 COVID‐19 patients in Wuhan, China: a descriptive study. J Clin Virol 2020; 127:104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J, Whiting PF, Brush JE. Interpreting a covid‐19 test result. BMJ 2020; 369:m1808. [DOI] [PubMed] [Google Scholar]
- Lassaunière R, Frische A, Harboe ZB. et al. Evaluation of nine commercial SARS‐CoV‐2 immunoassays. Available at: https://www.medrxiv.org/content/10.1101/2020.04.09.20056325v1 (last accessed 30 June 2020).
- Beavis KG, Matushek SM, Abeleda APF et al. Evaluation of the EUROIMMUN Anti‐SARS‐CoV‐2 ELISA Assay for detection of IgA and IgG antibodies. J Clin Virol 2020; 129:104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34:e212–13. [DOI] [PubMed] [Google Scholar]
- Hedou M, Carsuzaa F, Chary E et al. Comment on “Cutaneous manifestations in COVID‐19: a first perspective” by Recalcati S. J Eur Acad Dermatol Venereol 2020; 34:e299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkurt ZM, Ucmak D, Yildiz K et al. Chilblains in Turkey: a case‐control study. An Bras Dermatol 2014; 89:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takci Z, Vahaboglu G, Eksioglu H. Epidemiological patterns of perniosis, and its association with systemic disorder. Clin Exp Dermatol 2012; 37:844–9. [DOI] [PubMed] [Google Scholar]
- Cribier B, Djeridi N, Peltre B, Grosshans E. A histologic and immunohistochemical study of chilblains. J Am Acad Dermatol 2001; 45:924–9. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao W, Xiao M et al. [Clinical and coagulation characteristics in 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia]. Zhonghua Xue Ye Xue Za Zhi 2020; 41:302–7 (in Chinese). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID‐19. Lancet Haematol 2020; 7:e438–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Characteristics of the 12 patients with immunological abnormalities.
Table S2 Summary of 14 series (> 10 cases) reporting acral lesion during COVID‐19 outbreak published on 6 June 2020.
Powerpoint S1 Journal Club Slide Set.


