Abstract
Association between Hydroxychloroquine (HCQ) and Azithromycin (AZT) is under evaluation for patients with lower respiratory tract infection (LRTI) caused by the Severe Acute Respiratory Syndrome (SARS‐CoV‐2). Both drugs have a known torsadogenic potential, but sparse data are available concerning QT prolongation induced by this association. Our objective was to assess for COVID‐19 LRTI variations of QT interval under HCQ/AZT in patients hospitalized, and to compare manual versus automated QT measurements. Before therapy initiation, a baseline 12 lead‐ECG was electronically sent to our cardiology department for automated and manual QT analysis (Bazett and Fridericia’s correction), repeated 2 days after initiation. According to our institutional protocol (Pasteur University Hospital), HCQ/AZT was initiated only if baseline QTc ≤ 480ms and potassium level> 4.0 mmol/L. From March 24th to April 20th 2020, 73 patients were included (mean age 62 ± 14 years, male 67%). Two patients out of 73 (2.7%) were not eligible for drug initiation (QTc ≥ 500 ms). Baseline average automated QTc was 415 ± 29 ms and lengthened to 438 ± 40 ms after 48 hours of combined therapy. The treatment had to be stopped because of significant QTc prolongation in two out of 71 patients (2.8%). No drug‐induced life‐threatening arrhythmia, nor death was observed. Automated QTc measurements revealed accurate in comparison with manual QTc measurements. In this specific population of inpatients with COVID‐19 LRTI, HCQ/AZT could not be initiated or had to be interrupted in less than 6% of the cases.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Few data are available concerning QT prolongation induced by HCQ/AZT association, and their torsadogenic potential, in the context of COVID‐19 disease. But safety concerns have been recently raised in recently published data (33% of extreme QT prolongation above 500 ms).
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Our objective was to assess in patients hospitalized for COVID‐19 LRTI variations of QT interval under HCQ/AZT, applying a strict protocol for QT monitoring. Secondly, we wanted to compare manual versus automated QT measurements.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Our study showed that the combined therapy HCQ/AZT (currently under evaluation for the treatment of COVID‐19 disease), could be administered in more than 94% of inpatients who presented LRTI, after careful clinical, ECG and biological assessment followed by ECG monitoring. The safety profile of this therapeutic association (delivered over a short period of time) was achieved provided a strict institutional protocol was followed.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ While relatively accurate, the automated QTc measurement moderately underestimates the QTc interval compared to manual measurement. Thus, confirmation of definitive QTc value by a cardiologist is still warranted.
Several therapeutic strategies are currently investigated for the management of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) pandemic. Among them, the association between Hydroxychloroquine (HCQ) and Azithromycin (AZT) is under evaluation, with contrasting results. 1 , 2 When used in established indications for well‐known diseases, both drugs have a reported torsadogenic potential. 3 , 4 In the context of COVID‐19 disease, very new data are being rapidly reported by different research teams worldwide, and raising some serious safety concerns about QT prolongation and potential arrhythmogenic effects with HCQ/AZT association. 5 , 6 Because of the severity of this disease, a significant proportion of critically‐ill patients (eventually mechanically‐ventilated, MV) has been evaluated in these recent studies, this population being particularly prone to experience drug‐induced QT prolongation. 7 Furthermore, it is well established that more severe forms of pneumonia could represent a risk factor for QT prolongation per se. 8 To avoid any life‐threatening drug‐induced event in the context of COVID‐19 disease, several national documents have been recently published to guide physicians for QT monitoring during this pandemic. 9 , 10 Previous studies reported the validation of automated QT measurement, i.e. generated by electrocardiographic (ECG) machines, in comparison with manual measurement of the QT interval; but most of them included healthy volunteers. 11
In the present study, we aimed to: (i) assess the proportion of inpatients (outside the critical care unit) suffering from Lower Respiratory Tract Infection (LRTI), and potentially eligible for a combined therapy HCQ/AZT; according to strict predetermined baseline clinical and ECG criteria; (ii) evaluate QT variation/prolongation 48 hours after initiation of HCQ/AZT association in this specific population of SARS‐CoV‐2 LRTI (safety profile with the use of a dedicated institutional protocol); (iii) assess the accuracy of automated corrected QT (QTc) measurement in patients hospitalized in this specific setting, in comparison with a manual method of QT measurement.
Methods
Patient population
After information on risk‐benefit ratio of this combined therapy HCQ/AZT, patients who gave their agreement and without contraindications were included in this prospective observational study. The study was done in accordance to the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The combined therapy (HCQ/AZT) was proposed to all the patients hospitalized with confirmed SARS‐CoV‐2 LRTI (by CT‐scan and positive COVID‐19 nasopharyngeal polymerase chain reaction test result), and/or oxygen requirement ≥ 2 L/minute.
Detection of SARS‐CoV‐2 RNA
Total nucleic acids present within 200 μL of samples were extracted with automated system NucliSENS ® easyMAG ® (bioMérieux, Marcy‐l’Étoile, France) after proteinase K pretreatment and offboard lysis. Presence of SARS‐CoV‐2 specific RNA was assessed by reverse transcription‐PCR based on the protocol recommended by the French National Reference Center for Respiratory Viruses (Institut Pasteur, Paris, France).12 Primers and probes used in this protocol were designed to amplify two different targets (IP2 and IP4) in the RNA‐ dependent RNA polymerase (RdRp) SARS‐CoV‐2 specific gene. Reverse transcription‐PCR assays were realized by StepOnePlus Real‐Time PCR system (Applied Biosystems, Foster City, CA). The threshold cycle values (Ct) were collected from PCR assays as an indicator of viral load for positive samples.
Institutional protocol for HCQ/AZT therapy
The treatment consisted in 200 mg of oral HCQ sulfate, three times per day for 10 days, with AZT (500 mg on day one, followed by 250 mg per day for the next four days). They were then eligible for therapy initiation only after electrocardiogram (ECG) analysis by the Cardiology department. An institutional local protocol for ECG criteria was validated (Institutional Review Board) and approved within the University Hospital of Nice on March 24th. The protocol allowed HCQ/AZT initiation only if baseline QTc ≤ 480 ms and potassium level> 4.0 mmol/L. The treatment could not be initiated if QTc ≥ 500 ms. In case of QTc between 480 and 500 ms, the risk‐benefit ratio for the treatment was evaluated by the infectiologist. The treatment could not be started if any sign of inherited channelopathy was seen on the 12‐lead ECG (long QT syndrome, Brugada pattern, malignant early repolarization syndrome); 13 and in case of severe structural heart disease (left ventricular ejection fraction [LVEF] ≤ 30%, decompensated heart failure); or in the presence of other ECG abnormality such as left ventricular hypertrophy, long PR interval (≥ 200 ms) associated with bifascicular block, complete left bundle branch block, second or third degree atrio‐ventricular block, or pathological Q waves. Additionally, the physicians were advised to stop any other concomitant drug potentially lengthening QT interval (if possible) during the combined treatment by HCQ/AZT. Safety criterion for interrupting the therapy was a QTc prolongation ≥ 500 ms. The National Early Warning Score (NEWS) was calculated for every patient upon admission ward. The NEWS score was calculated based on the following parameters: age, respiratory rate, oxygen saturation, temperature, systolic blood pressure, pulse rate and level of consciousness. Three risk categories for clinical deterioration can be defined: low score (NEWS 0–4), medium score (NEWS 5–6), and high score (NEWS ≥ 7) for COVID patients.
QT measurement
Before therapy initiation, a baseline digital 12 lead‐ECG was electronically sent to our cardiology department for QT analysis. The ECGs were performed after 5 minutes supine rest, at a paper speed of 25 mm/second and amplification of 10 mm/mV, filter 40 Hz, with at least 10 second ECG at stable heart rate. Analysis of the digital ECG was performed on a computer screen with optimal resolution and manual calipers (magnifying or “zooming” by three to four folds). Heart rate (RR interval values) and uncorrected QT measurements were performed by one electrophysiologist in order to reduce any inter‐operator variability (S.‐S. B); 14 they were entered into a database and QT intervals were rate corrected using Bazett’s correction (heart rate < 90 beats per minute), and Fridericia’s correction (heart rate ≥ 90 beats per minute). For manual measurements, all 12 leads were visually analyzed; the lead where the QT interval was the most readily measured was selected for measurement (usually the precordial leads V2 or V3). The QT interval was measured at this lead, and thereafter compared to other leads to ensure that the longest QT interval of all leads was recorded according to the guidelines. 14 , 15 , 16 For each 12 lead‐ECG, automated QTc measurements, and manual measurements as described above, were compared. For automated ECG measurements, the proprietary QT algorithms contained in the ECG machines (General Electrics Marquette Mac 5500 HD and 1200 ST, GE Medical systems, Milwaukee, Wis.) were used.
Two days after initiation of combined therapy HCQ/AZT, 12‐lead ECG was repeated and sent for new analysis for each hospitalized patient.
Statistical analysis
The statistical analysis was completed using Excel (Microsoft, San Francisco, CA). Numerical variables are expressed as mean ± standard deviation (SD). Continuous variables were analyzed using the t‐test. The 5% level of significance (p < 0.05) was selected as representing a statistically significant difference.
To compare the QT intervals generated by manual and machine methods in a pairwise fashion, the method proposed by Bland and Altman was employed. 17 In these plots, the difference between uncorrected QT intervals obtained on the same ECG by machine and manual measurements (machine minus manual) was plotted versus the mean of the 2 values. Limits of agreements (LOA) were defined as the mean ± 1.96 × SD of these differences.
Results
Eligibility and safety of HCQ/AZT treatment
The clinical characteristics of the patients are reported in Table 1 . All the patients had a SARS‐CoV‐2 LRTI confirmed on CT scanner (and positive PCR), with a mean NEWS score calculated at 6.4 ± 2.5. None of the patients from this cohort was hospitalized in critical care unit or undergoing MV at the time he/she was proposed to receive HCQ/AZT association. From March 24th to April 20th, 73 patients were included, male 67%. Ages ranged between 29 and 92 years, with an average age of 62 ± 14 years. Two patients out of 73 (2.7%) were not eligible for drug initiation: the oldest patient in our population was a 92‐years‐old male patient with a history of myocardial infarction in 2018, and a QTc (Bazett’s correction) calculated at 490 ms. It was estimated that the risk‐benefit ratio was not favorable for starting the combined therapy for this patient. The second was a 66‐years‐old male patient with dilated cardiomyopathy (LVEF = 30%), chronic dialysis, and treated with amiodarone for persistent left atrial tachycardia, with a baseline QTc (Bazett’s correction) calculated at 502 ms. He experienced a symptomatic sustained ventricular flutter, spontaneously resolutive, despite amiodarone. He did not receive the combined HCQ/AZT therapy.
Table 1.
Patients characteristics
| N = 73 | |
|---|---|
| Mean age (years) | 62 ± 14 |
| Sex ratio | Male 49; 67% |
| BMI (kg/m2) | 28.3 ± 5.6 |
| Mean NEWS Score | 6.4 ± 2.5 |
| Mean heart rate (beats per minute) | 83 ± 15; Range 47–129 |
| Hypertension, n (%) | 33 (44.6%) |
| Diabetes, n (%) | 19 (25.7%) |
| Stroke, n (%) | 6 (8.1%) |
| Congestive heart failure, n (%) | 7 (9.5%) |
| Coronary artery disease, n (%) | 9 (12.3%) |
| Coronary artery bypass graft | 2 (2.7%) |
| Previous coronary artery stenting | 8 (10.9%) |
| Chronic renal failure, n (%) | 5 (6.7%) |
| Chronic Obstructive Pulmonary Disease, n (%) | 12 (16.2%) |
| Previous history of malignancy, n (%) | 16 (21.9%) |
| Active malignancy, n (%) | 4 (5.4%) |
| Usual treatments favoring prolonged QT, n (%) a | 19 (26.0%) |
| Treatments favoring prolonged QT introduced during hospitalization, n (%) b | 5 (6.8%) |
Alimemazine, alprazolam, amiodarone, amphotericin B, escitalopram, fluconazole, furosemide, hydrochlorothiazide, levomepromazine, mianserin, olanzapine, oxaliplatin, paroxetine, pantoprazole, risperidone, sotalol, tacrolimus, zuclopenthixol (Table S1).
Amphotericin B, fluconazole, furosemide, pantoprazole, tazocyn.
In 2 out of 71 patients (2.8%), the treatment had to be stopped because of significant QTc prolongation (≥ 500 ms): concurrent QT‐prolonging medication polypharmacy in both patients. The first patient was a 55 years‐old female patient who was receiving an association of alimemazine/levomepromazine/zuclopenthixol (Figures 1 and 2). The second was a 59 years‐old male patient, treated with sotalol (interrupted 24 hours before HCQ/AZT initiation) for paroxysmal atrial fibrillation. On day two, he presented a non‐documented syncopal episode, with a significantly prolonged QTc calculated at 503 ms (Bazett’s correction).
Figure 1.
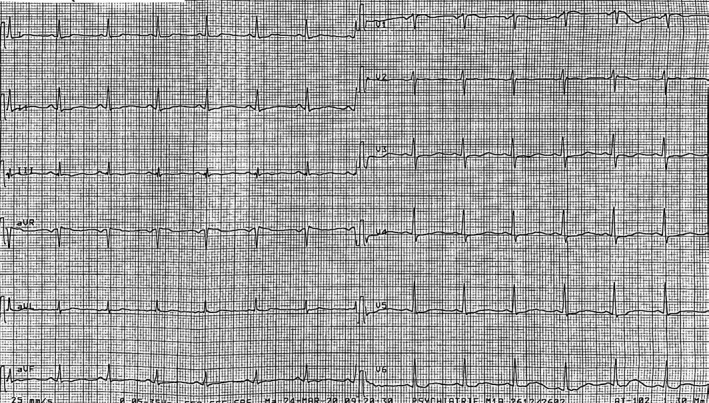
Twelve‐lead electrocardiogram of a 55 years‐old female patient presenting a significantly prolonged QTc interval (590 ms), under current psychotropics medication.
Figure 2.
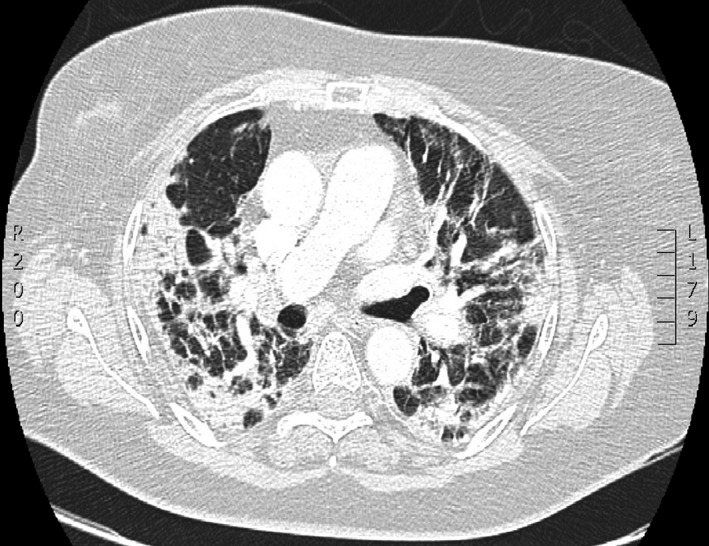
Corresponding CT‐scan image of the same patient with typical pulmonary lesions of COVID‐19 lower respiratory tract infection with a NEWS score initially calculated at 9 on admission.
During the hospitalization, no patient (but one) presented syncope, torsade de pointe (TdP), or cardiac arrest under treatment (HCQ/AZT). One inpatient experienced a persistent counterclockwise atrial flutter, treated with rate‐control agent and anticoagulation. He is planned for a cavotricuspid isthmus radiofrequency ablation.
QTc measurements
Manual QT measurements with rate correction were performed in all 73 patients without exclusion: baseline average QTc measurement with Bazett’s and Fridericia’s correction were respectively 436 ± 44 ms and 413 ± 42 ms. Automated QTc measurements were available for 48 patients: baseline average automated QTc measurement was 415 ± 29 ms.
After two days of combined therapy, average QTc values measured manually were prolonged with both correction methods (from 436 ± 44 to 460 ± 39 ms with Bazett’s correction; from 413 ± 42 to 430 ± 30 ms with Fridericia’s correction). Average automated QTc values lengthened to 438 ± 40 ms (Figure 3 ). Of note, nine out of 71 (12.6%) patients showed a delta QTc prolongation above 60 ms after two days of combined therapy. No further QTc prolongation above 500 ms was observed at the end of the therapy in these 9 patients. The delta QTc variations are provided in Figure 4 .
Figure 3.
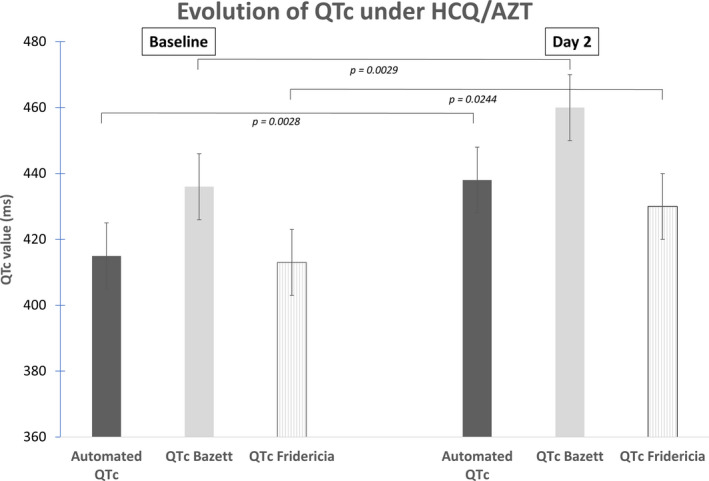
Graph showing the evolution of the QTc from baseline to day 2, with the combined therapy (Hydroxychloroquine/Azithromycin).
Figure 4.
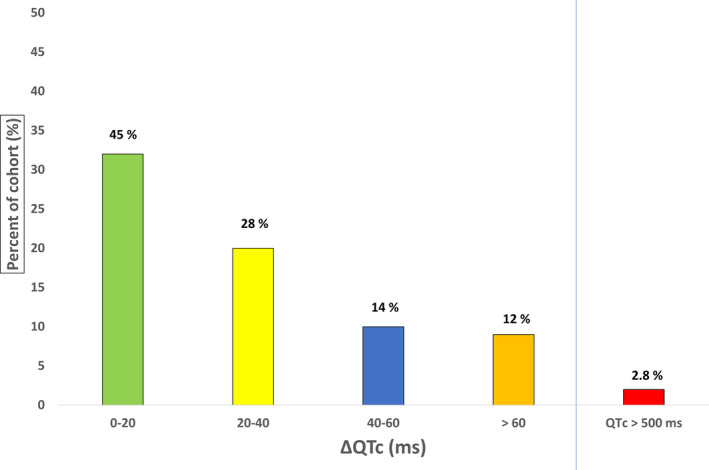
Graph showing delta QTc variations in the 71 patients treated with the HCQ/AZT association (manual measurements with Bazett’s correction).
The pairwise comparison between methods (48 patients) showed a good agreement between automated and manual QTc measurements: bias = 29 ms and standard deviation of bias = 43 ms for Bazett’s correction; even better for Fridericia’s correction with bias = 4 ms and standard deviation of bias = 39 ms. (Figures 5 and 6).
Figure 5.
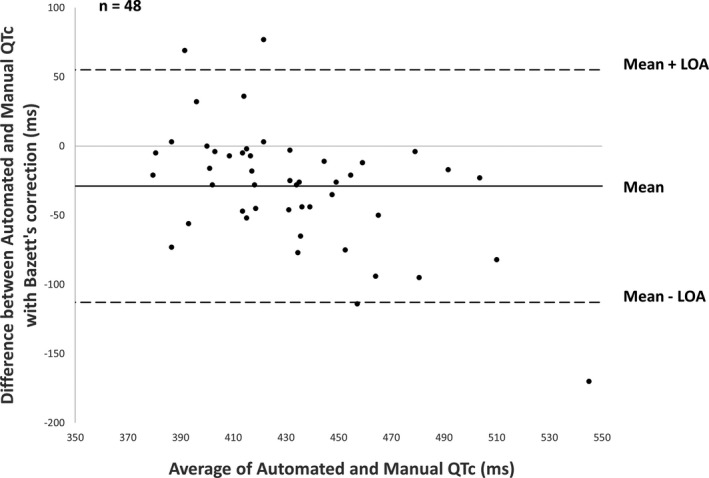
Bland‐Altman plot comparing automated corrected QT generated by ECG machine and manually (Bazett’s correction). LOA, limits of agreement.
Figure 6.
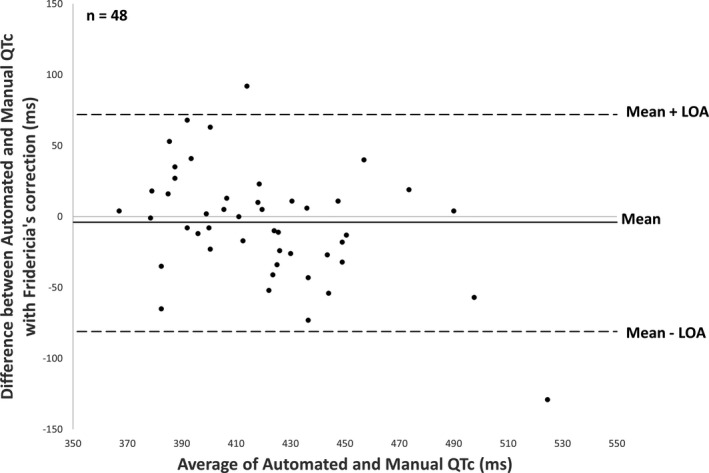
Bland‐Altman plot comparing automated corrected QT generated by ECG machine and manually (Fridericia’s correction). LOA, limits of agreement.
Discussion
Safety profile of HCQ/AZT association
Our study reported a good safety profile among inpatients treated with the association of HCQ/AZT for the management of SARS‐CoV‐2 LRTI. A majority of inpatients were eligible for the initiation of the combined therapy (only 2.7% contra‐indicated according to ECG and/or structural heart disease), while the therapy had to be interrupted in only 2.8% of the patients in this study. This is in contrast with a recent publication concerning 22 patients, and showing 33% of QT prolongation exceeding 500 ms with combined drug therapy, and a necessity to interrupt the antiviral treatment for 17.5% of them. 5 This initial population of 40 patients included a majority of critically ill patients (75% requiring invasive MV and 63% receiving vasoactive drugs). Of note, half of the patients were under concomitant QT prolongating drug. Our results may not be extrapolated to critically ill patients. Mercuro et al have just reported that 11 out of 53 patients (21%) with combined HCQ/AZT experienced QT prolongation above 500 ms, with necessity to stop the dual therapy for 11% of them. 6 A third of their population was critically ill, with one fourth under MV. Half of the population was composed by women, with a higher BMI (31.5 versus 28.3 in our cohort), and of note, 43.3% of the patients were taking loop diuretics, with one third of the cohort having a serum potassium level below 4 mmol/L. So, major differences can be seen with our homogeneous population of inpatients (none in critical care unit when receiving HCQ/AZT) without MV. The New York experience just showed a 10.7% of severe increase of QTc above 500 ms in 84 patients treated with HCQ/AZT. 18 A lower incidence of extreme QT prolongation was seen in our experience for several reasons. Firstly, a strict protocol was applied in our institution which recommended to stop the combined therapy early (from day 2), if a severe QTc prolongation was to be observed (while continued until day 5 in other studies). This measure may explain a lower number of extreme QT prolongation in our hospitalized population. The other major differences concern the methods of QT measurement (automated and manual, with Bazett and Fridericia’s correction in our study); in comparison with a “manual‐only” QT determination (Bazett’s correction in the Lyon experience). 5 Nevertheless, the exact similar proportion of patients with a delta QTc prolongation above 60 ms was found in all centers (12.6% in our study, 12% in the experience from New York, 13% in Boston).
This safety profile could be achieved thanks to the validation of an institutional protocol combining strict clinical, biological (potassium level above 4.0 mmol/L), and ECG criteria. Of note, our protocol was in line with the guidance document edited from the Heart Rhythm Society, and the protocol just released by the Mayo Clinic. 19 , 20
QT prolongation and TdP risk
HCQ is known for blocking the KCNH2‐encoded hERG/Kv11.1 ether‐a‐go‐go potassium channel. 21 Both drugs (HCQ/AZT) are potentially prolonging QT interval, but no clinical arrhythmic toxicity (syncope or TdP) was reported in our study (one TdP described in the series from Mercuro et al). 6 This discrepancy between increased proportion of extreme QT prolongation reported in the recent experiences with HCQ and the relative low number of clinical events, 22 confirms that QT prolongation is a sensitive, but not specific surrogate for TdP risk. 23 This relationship between QT prolongation and TdP is not linear as drugs that prolong the QT have not consistently been associated with cardiac arrythmias (“balanced blockers”). The CiPA initiative demonstrated the importance of the QTc and J‐Tpeak prolongation as a robust predictor of the torsadogenic potential in case of a predominant hERG blocker, in contrast with a balanced blocker, i.e. QTc prolongation without corrected J‐Tpeak prolongation. In this study, chloroquine was finally demonstrated to be an unbalanced hERG blocker at clinical concentrations, thus justifying a close ECG monitoring (even more when associated with AZT), and confirming the interest of our study.
One has to remember that HCQ has been well tolerated in large relatively young populations (and without severe comorbidities), as an antimalarial therapy, and safely used in the rheumatoid arthritis and systemic lupus erythematosus populations without specific ECG monitoring. 24 Of note, in France, HCQ does not have any authorization for malaria prophylaxis registered by the National Drug Agency (ANSM). Actually, the well‐established risk of arrhythmic toxicity with HCQ with chronic use (long half‐life of 40 days), 25 should contrast with a theoretically limited risk in the context of COVID‐19 therapy under strict cardiologic evaluation and monitoring, because the current scheme for treatment duration is short (between 5 to 10 days), even if associated with AZT. A simulation study reported that the predicted risk of QTc prolongation was minimal with an average baseline QTc around 420 ms, even with a high dose of HCQ. 26 Noteworthily, our results are in line with another recent experience from three New York centers including 119 patients under dual therapy, and reporting with 3.5% of drug interruption, without any TdP recorded in a total of 201 patients treated. 27
Conflicting results may be observed concerning the potential for QT prolongation with AZT. While several cases reported the occurrence TdP with AZT, either in the presence of congenital long QT syndrome or without. 28 , 29 In contrast, another study showed the absence of QTc prolongation in hospitalized patients treated with AZT. 8 The authors showed that community‐acquired pneumonia by itself creates an arrhythmogenic situation (with a higher pneumonia score representing a risk factor for QT prolongation). More recently, the odds ratio for AZT‐induced severe QT prolongation was calculated to 1.43 in a Korean study, with a higher risk in subgroup analysis in patients aged between 60 and 80 years. 30 Finally, AZT was shown to be a weak hERG blocker agent. 31
Comparison between methods for QT measurements
One other important finding of our study is the accuracy of the automated QT measurements with ECG machines, in comparison with manual measurements, in this specific population of inpatients treated for a COVID‐19 infection. Actually, we observed an average −20 ms difference between average baseline automated QTc and manual QT measurements (Bazett’s correction). The agreement was perfect when using Fridericia’s correction. This is in line with previous studies that found average 19–20 ms shorter uncorrected QT values between automated and manual measurements in healthy volunteers. 32 , 33 This variability is considered to be within acceptable ranges. 6 Nevertheless, these shorter automated QTc values may have a clinical implication when considering borderline QTc values (between 480 and 500 ms). This relative underestimation may decrease the safety profile of the HCQ/AZT treatment. This explains why the ECG monitoring after two days of treatment is critical. On the other hand, an overestimation of QTc values by the ECG machine could have represented a loss of chance for patients excluded from the combined therapy if clear effectiveness is demonstrated.
ECG monitoring for hospitalized patients is challenging. 12‐lead ECG acquisition for each COVID‐19 infected patient faces significant limitation because it implies an additional exposure for the nurses, and the necessity to repeat the ECG increases exposure of complex equipment (multiple ECG wires). It would have been advantageous to use mobile (or handheld) ECG devices for monitoring (and even tele‐monitoring) QTc, in the context of a contagious disease. 3435 Nevertheless, 12‐lead ECG acquisition still allowed standardized analysis and follow‐up of the QTc.
Limitations
Our study is monocentric and included a limited number of inpatients with COVID‐19 LRTI. Nevertheless, the study was performed at the initial phase of the SARS‐CoV‐2 pandemic in France and excluded outpatients. According to the decree from the French Health Ministry published on March 26th 2020, administration of HCQ was exclusively restricted to hospitalized patients with severe/highly symptomatic COVID‐19 infection, explaining why outpatients were excluded from this study.
Funding
No funding was received for this work.
Conflicts of Interest
The authors declared no competing interests for this work.
Authors’ Contributions
S.‐S.B., P.T., S.‐S.B. and E.F. wrote the manuscript; S.‐S.B., P.T., and E.F. designed the research; S.‐S.B., J.C., F.S., D.S., G.T., D.B., B.S., M.L., J.D., D.D., C.‐H.M., J.L., and V.E. performed the research; S.‐S.B. analyzed the data.
Supporting information
Table S1
Acknowledgements
We thank all the teams (medical and paramedical staffs) involved in the care of inpatients and facing the SARS‐CoV‐2 pandemic every day.
References
- 1. Gautret, P. et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int. J. Antimicrob. Agents. 105949 (2020). 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2. Mehra, M.R. , Desai, S.S. , Ruschitzka, F. & Patel, A.N. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID‐ 19: a multinational registry analysis. Lancet 31180–31186 (2020). 10.1016/S0140-6736(20)31180-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. O’Laughlin, J.P. , Mehta, P.H. & Wong, B.C. Life threatening severe QTc prolongation in patient with systemic lupus erythematosus due to hydroxychloroquine. Case Rep. Cardiol. 2016, 4626279 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Howard, P.A. Azithromycin‐induced proarrhythmia and cardiovascular death. Ann Pharmacother. 47, 1547–1551 (2013). [DOI] [PubMed] [Google Scholar]
- 5. Bessière, F. et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID‐19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. (2020). 10.1001/jamacardio.2020.1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mercuro, N.J. et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. (2020). 10.1001/jamacardio.2020.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Etchegoyen, C.V. , Keller, G.A. , Mrad, S. , Cheng, S. & Di Girolamo, G. Drug‐induced QT Interval Prolongation in the Intensive Care Unit. Curr Clin Pharmacol. 12, 210–222 (2017). [DOI] [PubMed] [Google Scholar]
- 8. Goldstein, L.H. et al. Azithromycin is not associated with QT prolongation in hospitalized patients with community acquired pneumonia. Pharmacoepidemiol Drug Saf. 10, 1042–1048 (2015). [DOI] [PubMed] [Google Scholar]
- 9. Sacher, F. et al. Working group of pacing, electrophysiology of the french society of cardiology, the board of the French Society of Cardiology. Use of drugs with potential cardiac effect in the setting of SARS‐CoV‐2 infection. Arch Cardiovasc. Dis. 113, 293–296 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sapp, J.L. et al. Guidance on minimizing risk of drug‐induced ventricular arrhythmia during treatment of COVID‐19: a statement from the canadian heart rhythm society. Can J. Cardiol. 36, 948–951 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Azie, N.E. et al. Comparing methods of measurement for detecting drug‐induced changes in the QT interval: implications for thoroughly conducted ECG studies. Ann. Noninvasive Electrocardiol. 2, 166–174 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Centre for Disease Prevention and Control (ECDC) . European surveillance for human infection with novel coronavirus (2019‐nCoV), (2020). Stockholm: ECDC; <https://www.ecdc.europa.eu/en/european‐surveillance‐human‐infection‐novel‐coronavirus‐2019‐ncov>. Accessed Jan 30, 2020. [Google Scholar]
- 13. Wu, C.I. et al. SARS‐CoV‐2, COVID‐19, and inherited arrhythmia syndromes. Heart Rhythm. 31, 1547–5271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hnatkova, K. & Malik, M. Sources of QTc variability: Implications for effective ECG monitoring in clinical practice. Ann Noninvasive Electrocardiol. 25, e12730 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rautaharju, P.M. et al. American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 53, 982–991 (2009). [DOI] [PubMed] [Google Scholar]
- 16. Cheung, C.C. , Davies, B. , Gibbs, K. , Laksman, Z.W. & Krahn, A.D. Multi‐lead QT screening is necessary for QT measurement: implications for management of patients in the COVID‐19 era. J. Am. Coll. Cardiol. (2020). 10.1016/j.jacep.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bland, J.M. & Altman, D.G. Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 346, 1085–1087 (1995). [DOI] [PubMed] [Google Scholar]
- 18. Chorin, E. et al. The QT interval in patients with COVID‐19 treated with hydroxychloroquine and azithromycin. Nat. Med. (2020). 10.1038/s41591-020-0888-2 [DOI] [PubMed] [Google Scholar]
- 19. Lakkireddy, D.R. et al Guidance for cardiac electrophysiology during the coronavirus (COVID‐19) Pandemic from the Heart Rhythm Society COVID‐19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. (2020). 10.1016/j.hrthm.2020.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giudicessi, J.R. , Noseworthy, P.A. , Friedman, P.A. & Ackerman, M.J. Urgent guidance for navigating and circumventing the QTc prolonging and torsadogenic potential of possible pharmacotherapies for COVID‐19. Mayo Clin. Proc. 95, (2020). 10.1016/j.mayocp.2020.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Traebert, M. , Dumotier, B. , Meister, L. , Hoffmann, P. , Dominguez‐Estevez, M. & Suter, W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol. 484, 41–48 (2004). [DOI] [PubMed] [Google Scholar]
- 22. Jankelson, L. , Karam, G. , Becker, M.L. , Chinitz, L.A. & Tsai, M.C. QT prolongation, torsades de pointes and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID‐19: a systematic review. Heart Rhythm. (2020). 10.1016/j.hrthm.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vicente, J. et al. Assessment of multi‐ion channel block in a phase I randomized study design: results of the CiPA phase I ECG biomarker validation study. Clin. Pharmacol. Ther. 105, 943–953 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haeusler, I.L. , Chan, X.H.S. , Guérin, P.J. & White, N.J. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs: a systematic review. BMC Med. 16, 200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen, C.Y. , Wang, F.L. & Lin, C.C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila). 44, 173–175 (2006). [DOI] [PubMed] [Google Scholar]
- 26. Garcia‐Cremades, M. et al. Optimizing hydroxychloroquine dosing for patients with COVID‐19: an integrative modeling approach for effective drug repurposing. Clin Pharmacol Ther. (2020). 10.1002/cpt.1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saleh, M. et al. The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS‐CoV‐2 infection. Circ. Arrhythm. Electrophysiol. (2020). 10.1161/CIRCEP.120.008662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arellano‐Rodrigo, E. , García, A. , Mont, L. & Roqué, M. Torsade de pointes and cardiorespiratory arrest induced by azithromycin in a patient with congenital long QT syndrome. Med. Clin. (Barc) 117, 118–119 (2001). Spanish. [DOI] [PubMed] [Google Scholar]
- 29. Kezerashvili, A. , Khattak, H. , Barsky, A. , Nazari, R. & Fisher, J.D. Azithromycin as a cause of QT‐interval prolongation and torsade de pointes in the absence of other known precipitating factors. J Interv Card Electrophysiol. 18, 243–246 (2007). [DOI] [PubMed] [Google Scholar]
- 30. Choi, Y. , Lim, H.S. , Chung, D. , Choi, J.G. & Yoon, D. Risk evaluation of azithromycin‐induced QT prolongation in real‐world practice. Biomed Res Int. 2018, 1574806 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blinova, K. et al. Comprehensive translational assessment of human‐induced pluripotent stem cell derived cardiomyocytes for evaluating drug‐induced arrhythmias. Toxicol Sci. 155, 234–247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darpo, B. et al. Man versus machine: is there an optimal method for QT measurements in thorough QT studies? J. Clin. Pharmacol. 46, 598–612 (2006). [DOI] [PubMed] [Google Scholar]
- 33. Fosser, C. , Duczynski, G. , Agin, M. , Wicker, P. & Darpo, B. Comparison of manual and automated measurements of the QT interval in healthy volunteers: an analysis of five thorough QT studies. Clin. Pharmacol. Ther. 86, 503–506 (2009). [DOI] [PubMed] [Google Scholar]
- 34. Garabelli, P. et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12‐lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J. Cardiovasc. Electrophysiol. 27, 827–832 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


