Editor
SARS‐CoV‐2 is a new coronavirus that causes COVID‐19, a disease associated with severe pneumonia. 1 Many other clinical manifestations have been associated with the disease (diarrhoea, anosmia, dysgeusia, etc.) and patients can be healthy carriers of the virus. 2 Several dermatologic manifestations associated with COVID‐19 have been reported. 3 Among them are numerous cases of chilblain‐like lesions (CBLLs) in young patients in good general condition, often not tested, or tested negative, for the SARS‐CoV‐2. 4 , 5 In a recent study on 375 Spanish patients with suspected or confirmed COVID‐19, these CBLL accounted for 19% of all cutaneous lesions;3 they were observed in young patients with mild systemic symptoms and seemed to appear late in the course of the disease. Of note, CBLL is rarely observed in hospitalized patients; 6 however, the causal association of these CBLL with SARS‐CoV‐2, although suspected, remains so far unproven.
We examined 45 outpatients referred to our department for CBLL, who were otherwise in good general condition. There were 26 men (58%) and 19 women (42%), of a mean age of 30.1 years. Twelve patients (27%) mentioned non‐specific systemic symptoms preceding the onset of CBLL. Fifteen patients (33.3%) considered a possible contamination from a family member. All patients presented red‐violaceous acral lesions (Fig. 1a) on the toes (82%), the fingers (4%) or on both sites (13%). Prior pruritus or pain was reported by 51% and 62% of patients, respectively.
Figure 1.
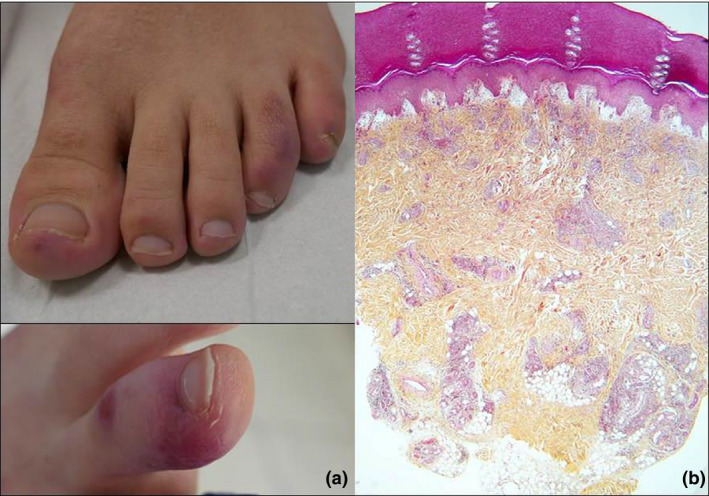
(a) Red‐violaceous acral lesions in two patients. (b) Histological examination of a skin biopsy shows severe subepidermal oedema and a heavy nodular lymphocytic infiltration throughout the dermis.
Seventeen of these patients benefited from detailed explorations.
Nasopharyngeal PCR test for SARS‐CoV‐2 was negative in 17/17. Laboratory work‐up showed no lymphopenia, inflammatory markers, D‐dimers or autoimmunity markers. An increased interferon score was detected in 6/15 patients (40%). Microscopic examination of skin biopsy (n = 17) (Fig. 1b) showed a hyperplastic epidermis containing scattered or confluent necrotic keratinocytes. The dermis contained extravasated red blood cells and a heavy lymphocytic infiltrate, occasionally also extending to the upper part of the hypodermis. Direct immunofluorescence examination often showed IgM, C3 and sometimes IgA deposits on skin vessels. Search for SARS‐Cov‐2 was performed with PCR in skin biopsies from 11 patients and proved invariably negative. Serological tests for anti‐SARS‐CoV‐2 antibodies performed after a median delay of 14 days (range 6–40) after the onset of symptoms were negative in all 17 patients.
Our virological (nasopharyngeal and in situ PCR) and serological findings do not allow to confirm a direct causal link between SARS‐CoV‐2 infection and the apparent epidemic of chilblains observed in the French population in the context of COVID‐19. However, such a link cannot be formally excluded. A particularly effective immune response against the infection could lead to a clinical presentation with very few symptoms, early negativity of nasopharyngeal PCR and delayed appearance of specific antibodies. Some studies have shown an impaired IFN‐type 1 activity associated with severe forms of COVID‐19 and suggest that patients with type 1 IFN deficiency could be a high‐risk population. 7 , 8 The increased interferon score found in 40% of our patients tested may reflect a specific type of immune response, as has been reported in chilblain lupus and chilblains associated with interferonopathies, such as the Aicardi‐Gouttières and SAVI syndromes. 9 , 10 We speculate that this intense IFN response could help the patients to contain the replication of SARS‐CoV‐2 and would explain why they usually remain asymptomatic and merely develop CBLL. This hypothesis should be confirmed by later serologies of these patients, who could become seropositive long after the onset of symptoms.
In conclusion, our findings do not demonstrate a formal causal relationship between these CBLL and SARS‐CoV‐2; however, we advocate that patients with such skin lesions be systematically screened for SARS‐CoV‐2 infection.
Ethical approval
This purely observational non‐interventional study based on a series of documented cases was approved by the Ethical Committee.
References
- 1. Zhu N, Zhang D, Wang W et al. A Novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 20: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu S, Lin J, Zhang Z et al. Alert for non‐respiratory symptoms of Coronavirus Disease 2019 (COVID‐19) patients in epidemic period: A case report of familial cluster with three asymptomatic COVID‐19 patients. J Med Virol 2020. 10.1002/jmv.25776 [DOI] [PubMed] [Google Scholar]
- 3. Casas CG, Català A, Hernández GC et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol [Internet]. 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramondetta A, Panzone M, Dapavo P et al. Chilblain acral lesions in the COVID‐19 era. Are they marker of infection in asymptomatic patients? J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piccolo V, Neri I, Filippeschi C et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Recalcati S, Barbagallo T, Frasin LA et al. Acral cutaneous lesions in the Time of COVID‐19. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid‐19 patients | medRxiv [Internet]. [WWW document]. URL https://www.medrxiv.org/content/10.1101/2020.04.19.20068015v1 (last accessed: 7 May 2020)
- 8. Trouillet‐Assant S, Viel S, Gaymard A et al. Type I IFN immunoprofiling in COVID‐19 patients. J Allergy Clin Immunol 2020; S0091 6749(20):30578–30579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crow YJ, Manel N. Aicardi‐Goutières syndrome and the type I interferonopathies. Nat Rev Immunol 2015; 15: 429–440. [DOI] [PubMed] [Google Scholar]
- 10. Rice GI, Forte GMA, Szynkiewicz M et al. Assessment of interferon‐related biomarkers in Aicardi‐Goutières syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case‐control study. Lancet Neurol 2013; 12: 1159–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgement
The patients in this manuscript have given written informed consent to the publication of their case details.


