Abstract
At the end of 2019, a novel flu‐like coronavirus named COVID‐19 (coronavirus disease 2019) was recognized by World Health Organization. No specific treatments exist for COVID‐19 at this time. New evidence suggests that therapeutic options focusing on antiviral agents may alleviate COVID‐19 symptoms as well as those that lead to the decrease in the inflammatory responses. Flavonoids, as phenolic compounds, have attracted considerable attention due to their various biological properties. In this review, the promising effects and possible mechanisms of action of naringenin, a citrus‐derived flavonoid, against COVID‐19 were discussed. We searched PubMed/Medline, Science direct, Scopus, and Google Scholar databases up to March 2020 using the definitive keywords. The evidence reviewed here indicates that naringenin might exert therapeutic effects against COVID‐19 through the inhibition of COVID‐19 main protease, 3‐chymotrypsin‐like protease (3CLpro), and reduction of angiotensin converting enzyme receptors activity. One of the other mechanisms by which naringenin might exert therapeutic effects against COVID‐19 is, at least partly, by attenuating inflammatory responses. The antiviral activity of the flavanone naringenin against some viruses has also been reported. On the whole, the favorable effects of naringenin lead to a conclusion that naringenin may be a promising treatment strategy against COVID‐19.
Keywords: ACE2 receptors, antiviral effects, coronavirus main protease, COVID‐19, naringenin
Abbreviations
- 3CLpro
3‐chymotrypsin‐like protease
- ACE2
angiotensin converting enzyme
- ALT
alanine transaminase
- AMPK
AMP‐activated protein kinase
- ApoB100
apolipoprotein B100
- ARDS
acute respiratory distress syndrome
- Asp187
aspartic acid
- AST
aspartate transaminase
- BHK‐21
Baby hamster kidney‐21
- CAT
catalase
- CHIKV
chikungunya virus
- COVID‐19
coronavirus disease 2019
- CoVs
Coronaviruses
- COX‐2
cyclooxygenase‐2
- DENV
dengue virus
- Glu
glutamic acid
- GPx
glutathione peroxidase
- GR
glutathione reductase
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- His
histidine
- IFNγ
interferon γ
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- LEU
leucine
- LOOH
lipid hydroperoxides
- LYS
lysine
- MAPK
mitogen‐activated protein kinase
- MCP‐1
monocyte chemoattractant protein‐1
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- NF‐κB
nuclear factor kappa B
- NOX2
NADPH oxidase‐2
- NSV
sindbis neurovirulent strain
- PDB
protein data bank
- PGE2
prostaglandin E2
- PLpro
papain‐like protease
- PPARα
peroxisome proliferator‐activated receptor α
- PRO
proline
- RAS
renin‐angiotensin system
- RBD
receptor binding domain
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SARS
severe acute respiratory syndrome
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substances
- Thr
threonine
- TLR4
toll like receptor 4
- TNF‐α
tumor necrosis factor‐alpha
- VLDL
very low‐density lipoprotein
- WHO
World Health Organization
- ZIKV
Zika virus
1. INTRODUCTION
Coronaviruses (CoVs) are the well‐known cause of severe respiratory, enteric, and systemic infections in both animals and humans (Malik et al., 2020). CoVs are the subfamily Orthocoronavirinae in the family of Coronaviridae, and this subfamily includes alpha‐, beta‐, gamma‐, and delta‐CoVs (Shereen, Khan, Kazmi, Bashir, & Siddique, 2020; Woo & Lau, 2019). At the end of 2019, a novel flu‐like coronavirus named COVID‐19 (coronavirus disease 2019) was recognized by World Health Organization (WHO) and there was an evidence of the sustained human‐to‐human transmission by close contacts (Peng et al., 2020). Nowadays, the global prevalence of COVID‐19 has reached pandemic proportions, which resulted in a great concern of public health worldwide (Sehn, 2020). Currently, no specific treatments exist for COVID‐19 (Guo et al., 2020), and up to April 7, 2020, more than 1,279,000 cases of COVID‐19 have been reported by WHO. Severe acute respiratory syndrome (SARS‐CoV‐2) has affected 211 countries worldwide (WHO, 2020). The acute respiratory distress syndrome (ARDS) is the most prevalent cause of death among the patients with COVID‐19 that finally leads to multiple organ failure and sepsis (Wang, Zhao, Xu, & Gu, 2020). The groups who are at the highest risk of COVID‐19 are older adults and subjects with major chronic diseases such as cancers, diabetes, and hypertension (The, 2020). The outbreak of SARS in 2002 in China's Guangdong province, and the prevalence of Middle East respiratory syndrome coronavirus (MERS‐CoV) in 2012 in the Kingdom of Saudi Arabia have demonstrated the lethality of CoVs when they cross the species barrier and then infect humans (Schoeman & Fielding, 2019; Zhong et al., 2003).
The most important reason for the high transmission rate of COVID‐19 is genetic recombination event at S protein in the receptor binding domain (RBD) region of this virus (Ghaffari, Roshanravan, Tutunchi, Ostadrahimi, & Kafil, 2020; Zhu et al., 2020). SARS‐CoV‐2 RNA sequence has almost 30,000 bases in length (Xiao et al., 2020), and the analysis of whole‐genome sequencing data has exhibited a strong similarity between SARS‐CoV‐2 and SARS‐CoV in the RBD (Zhang & Holmes, 2020). SARS‐CoV‐2 RBD has a great binding affinity to the human angiotensin‐converting enzyme 2 (ACE2) receptors, which are widely expressed in various cells belonged to kidney, lung, brain, and digestive tract. ACE2 can negatively modulate the renin‐angiotensin system (RAS) through the degradation of angiotensin II, and can play a protective role against the progression of acute lung failure (Madjid, Safavi‐Naeini, Solomon, & Vardeny, 2020). SARS‐CoV‐2 seems to infect host cells through ACE2 (Diaz, 2020). It is supposed that, the decreased ACE2 activity in host‐cell membranes may reduce the ability of SARS‐CoV‐2 to enter cells (Letko, Marzi, & Munster, 2020). According to the current theory, the entrance of the virus into cells increases the inflammatory activity, which leads to serious damages, especially in the respiratory tract. Collectively, COVID‐19 seems to be the fifth endemic CoV worldwide. Since an established vaccine or medication is yet to be discovered against this viral infection, preventive policies are very crucial at this time. New evidence suggests that therapeutic options focusing on antiviral agents may alleviate COVID‐19 symptoms as well as those that lead to the decrease in the inflammatory responses (Monteleone & Ardizzone, 2020; Stebbing et al., 2020). Various important biological activities of flavonoids, as phenolic compounds, have been reported that include antiviral, anti‐inflammatory, therapeutic, antibacterial, and other properties in nature (Tapas, Sakarkar, & Kakde, 2008). Among the naturally occurring flavonoids, naringenin, due to its potent biological roles, is one of the most important flavonoids (Den Hartogh & Tsiani, 2019). Therefore, in the present study, we aimed to discuss the promising effects and possible mechanisms of action of naringenin, which is a flavonoid with antiviral and anti‐inflammatory activities, against COVID‐19.
2. METHODS
2.1. Search strategy
We investigated four most popular search engines PubMed/Medline, Science direct, Scopus, and Google Scholar using the following keywords: “naringenin” or “naringenin7‐sulfate” or “4′ 5 7‐trihydroxyflavanone” in the title and “viral diseases” or “virus‐related diseases” or “infectious disease” or “inflammatory lung diseases” or “lung injury” or “inflammation” or “COVID‐19″ or “coronaviruses” in the title or abstract. Relevant studies published in the English language up to March 2020 were eligible. All articles evaluating the effects of naringenin on viral diseases, infectious disease, and inflammatory lung diseases were included. Studies with insufficient information were excluded from the review. To minimize the loss of studies, the reference lists of articles that were included were also reviewed to identify additional studies. The articles identified in the search were saved in an EndNote software file and sorted to remove duplicate reports. The remaining studies were examined for choosing eligible articles. Then, the full texts of the screened articles were critically analyzed for extraction of data.
2.2. Results
In total, 103 potentially title/abstract were retrieved by the search strategy, of which 71 were considered after removal of duplicate articles. Of these, 58 articles were excluded because of not providing the inclusion criteria. Finally, 13 articles were included in the present study based on the research topic. Figure 1 presents the diagram for the search and selection process of the present review. Details of the selected studies are presented in Table 1.
FIGURE 1.
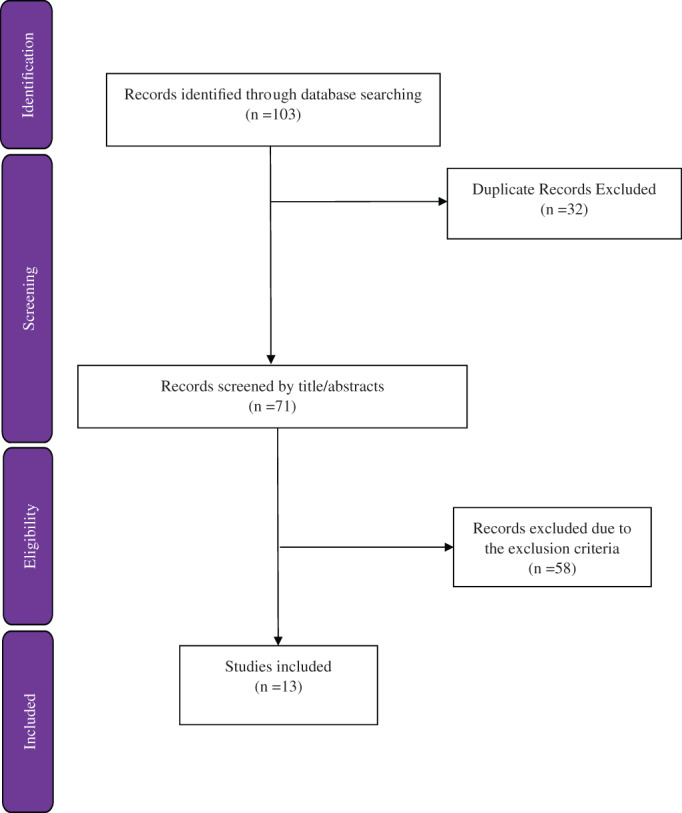
Flow diagram of the literature search and study selection process [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 1.
Summary of studies evaluating antiviral and anti‐inflammatory effects of naringenin
| Articles | Type of study | Reference | Samples | Study design | Main results |
|---|---|---|---|---|---|
| Viral‐related articles | In vitro | Paredes, Alzuru, Mendez, & Rodriguez‐Ortega, 2003 | BHK‐21 were infected with NSV | Administration of naringenin: 25 μg/ml | Inhibition of viral replication up to 80% |
| In vitro, In vivo | Nahmias et al., 2008 | Huh7.5.1human hepatoma cell line were infected with HCV; male mice were infected with HCV | Administration of naringenin: 200, 1,000, and 5,000 μM | Silencing apoB100 messenger RNA, a 70% reduction in the secretion of both apoB100 and HCV, a 80% reduction in HCV secretion in infected cells, a decrease in TG levels following injection | |
| In vitro | Goldwasser et al., 2011 | Huh7.5.1 human hepatoma cell line infected with HCV | Administration of naringenin: 200 μM | A dose‐dependent inhibition of HCV production without affecting intracellular levels of the viral RNA or protein, inhibition of the assembly of intracellular infectious viral particles, a rapid 1.4 log reduction in HCV similar to 1,000 U of interferon | |
| In vitro | Zandi et al., 2011 | Vero cells infected with DENV‐2 | Administration of Naringenin: 50 μg/ml | A decrease in the DENV‐2 RNA level by 50% with naringenin compared to the non‐treated virus inoculum | |
| In vitro | Ahmadi et al., 2016 | BHK‐21 were infected with CHIKV; Vero cells were infected with CHIKV | Administration of naringenin: Up to 500 μM | Reduction of the CHIKV intracellular replication efficiency and downregulation of the production of viral proteins involved in replication | |
| In vitro | Frabasile et al., 2017 | Huh7.5 cells were infected with DENV; primary human monocytes were infected with DENV | Administration of naringenin: 250 μM | Inhibition of DENV replication with an efficiency similar to IFN‐α 2A and ribavirin, a reduction in the number of DENV‐infected cells | |
| In vitro | Cataneo et al., 2019 | Human A549 cells were infected with ZIKV; primary human monocyte‐derived dendritic cells were infected by ZIKV | Administration of naringenin: 15.6, 31.25, 62.5 and 125 μM | Inhibition of viral replication or assembly of viral particles | |
| Human | Goncalves et al., 2017 | 43 adult patients with chronic HCV | Supplementation with 500 ml/day orange juice containing 2.7 mg naringenin | Protection against harmful effects of HCV by an increase in antioxidant capacity and a decrease in inflammation | |
| Quantitative analysis | Alam, Parvez, Arbab, & Al‐Dosari, 2017 | Guiera senegalensis | Sensitive RP‐/NP‐HPTLC methods | Exhibiting anti‐HBV activity of Guiera senegalensis including naringenin | |
| Inflammation‐related articles | In vivo | Fouad, Albuali, & Jresat, 2016 | Induced‐lung injury rats | Administration of naringenin: 50, 100 mg/kg | Attenuating the production of inflammatory cytokines, pulmonary edema, neutrophil recruitment, myeloperoxidase activity, and reduction of oxidative/nitrosative stress markers |
| In vivo | Ali et al., 2017 | Rats with lung damage | Administration of naringenin: 100 mg/kg | Downregulation of the expression of NF‐κB and COX2 | |
| COVID‐19 related articles | Molecular docking analysis | Cheng et al., 2020 | Citrus fruit flavonoids including naringenin | Docking analysis | Reduction of ACE2 receptor activity through the binding of naringenin to ACE2 with binding site proline, leucine, and lysine |
| Molecular docking analysis | Khaerunnisa, Kurniawan, Awaluddin, Suhartati, & Soetjipto, 2020 | 6 LU7 and native ligands including naringenin | Docking analysis | Inhibition of COVID‐19 main protease through the interaction of naringenin with amino acids histidine, glutamic acid, aspartic acid, and threonine in the CoV main protease active site |
Abbreviations: ACE2, angiotensin‐converting enzyme 2; apoB100, apolipoprotein B100; BHK‐21, baby hamster cells 21 clone 15; CHIKV, chikungunya virus; DENV, dengue virus; HBV, hepatitis B virus; HCV, hepatitis C virus; IFN‐α 2A, interferon‐alpha 2A; NSV, sindbis neurovirulent strain; PR‐/NP‐HPTLC, reverse phase−/normal phase‐high performance; TG, triglyceride; Vero cells, african green monkey kidney cells; ZIKV, zika virus.
3. FLAVONOIDS AND COVID‐19
Based on the available information, 3‐chymotrypsin‐like protease (3CLpro) and papain‐like protease (PLpro), as CoV‐encoded proteins, play crucial roles in CoV replication, and also exert essential roles in the inhibition of host innate immune responses (Baez‐Santos, St John, & Mesecar, 2015). Therefore, targeting these proteases appears to be pivotal for the treatment of COVID‐19 (Tahir Ul Qamar, Alqahtani, Alamri, & Chen, 2020). Recent data suggests that some metabolites from a group called flavonoids can inhibit the activity of these proteins. In fact, flavonoid compounds attach to the active site of proteins, and then inactivate them (Sawikowska, 2020). Moreover, flavonoids are a large class of plant pigments by having many subgroups including chalcones, flavones, flavonols, and isoflavones (Panche, Diwan, & Chandra, 2016). Flavonoids have been shown to have multiple functions including antioxidant activity, free radical scavenging capacity, hepatoprotective, anti‐inflammatory, anticancer, and antibacterial effects, as well as the potential antiviral activities (Pietta, 2000). In this regard, Shimizu et al. (2017) demonstrated that flavonoids could act against hepatitis C virus (HCV) infection by blocking the entry stage of HCV cycle. As a citrus flavonoid, naringenin could alleviate HCV infection by causing a reduction in apolipoprotein B100 (apoB100) secretion, which is required for HCV infection (Hernandez‐Aquino & Muriel, 2018). Mechanistically, it is documented that the antiviral activity of some flavonoids against SARS‐CoV‐2 can be mediated through their ability in blocking 3CLpro (Jo, Kim, Shin, & Kim, 2020).
4. NARINGENIN
Among flavonoids, naringenin with the chemical name of 4′ 5 7‐trihydroxyflavanone, is considered as one of the most important flavonoids, mainly a flavanone, due to its potential biological activities such as antioxidant, anti‐inflammatory, and antiviral properties (Den Hartogh & Tsiani, 2019). Naringenin is the aglycone of naringin, known as the bitter component of citrus fruits (Ameer, Weintraub, Johnson, Yost, & Rouseff, 1996). This flavanone is widely distributed in a variety of fruits and vegetables such as grapefruit, lemon, oranges, bergamot, and tomatoes; and thus, its consumption from the diet can be relatively high (Manchope, Casagrande, & Verri Jr., 2017). To date, oral bioavailability rate of naringenin is almost 5.81% and its absorption occurs through both passive diffusion and active transport in gastrointestinal tract (Kanaze, Bounartzi, Georgarakis, & Niopas, 2007). Moreover, Xu et al. (2009) demonstrated that the absorption rate of naringenin was 47, 42, and 39% in deudenum, terminal illeum, and jejunum, respectively. After absorption, naringenin binds to albumin and is then distributed in the highly perfused organs such as the liver, cerebrum, kidney, spleen, and heart (Erlund, Meririnne, Alfthan, & Aro, 2001). Finally, metabolites of naringenin can be excreted through biliary and urinary pathways (Barreca et al., 2017). Due to having several beneficial health effects, naringenin can be used in different pharmaceutical formulations to improve human health (Salehi et al., 2019). However, because of its limited bioavailability, some formulations like naringenin‐loaded nanoparticles have been developed to resolve the limited bioavailability of naringenin (Zobeiri et al., 2018). In 1996, the first study was conducted on the toxicity of naringenin. In a model system of the isolated rat liver nuclei, naringenin induced a concentration‐dependent peroxidation of nuclear membrane lipids along with DNA strand breaks (Sahu & Gray, 1997). In addition, naringenin can be oxidized to generate naringenin phenoxyl radicals (Galati, Moridani, Chan, & O'Brien, 2001) and it also exhibited a medium lethal dose LD(50) > 5,000 mg/kg (Ortiz‐Andrade et al., 2008). However, due to the relatively low bioavailability and the rapid metabolism and elimination of most of the flavonoids like naringenin, no side effects have been reported by consuming them (Clark, Zahradka, & Taylor, 2015). In this regard, a pharmacokinetic study exhibited no undesirable or adverse effects after the oral administration of naringenin in the human subjects (Kanaze et al., 2007). Similarly, no side effects were observed in a clinical study that assessed the efficacy and safety of polyphenolic citrus dry extract including naringenin among the healthy overweight subjects (Dallas et al., 2014). However, due to the lack of adequate studies performed on the safety and toxicity of naringenin, this flavanone should be cautiously used in clinical settings (Hernandez‐Aquino & Muriel, 2018).
4.1. Antiviral effect of naringenin
Flavonoids have been reported to have some biological activities against several types of viruses (Cataneo et al., 2019). The antiviral activity of the flavanone naringenin against some viruses such as HCV, Chikungunya virus (CHIKV), Dengue virus (DENV), and Zika virus (ZIKV) has been tested. Also, it was demonstrated that HCV production by human hepatocytes is dependent on the expression of apoB100 and the assembly of very low‐density lipoprotein (VLDL) (Huang, Sun et al., 2007). Administration of 200 μM naringenin to the Huh7.5.1 human hepatoma cell infected by HCV, led to the inhibition of apoB100‐dependent HCV secretion. In addition, it was reported that naringenin resulted in silencing of the apoB mRNA in the infected cells and also caused a 70% reduction in the release of both apoB100 and HCV (Nahmias et al., 2008). In another study, naringenin administration inhibited HCV secretion with no effect on intracellular viral RNA or on protein levels. The assembly of infectious virus particles was also blocked by naringenin. Moreover, by activating peroxisome proliferator‐activated receptor α (PPARα), naringenin led to a reduction in VLDL production, which is necessary for secretion of HCV particles (Goldwasser et al., 2011). HCV infection leads to oxidative stress due to the stimulation of cell metabolism, with a decrease in the activity of antioxidant enzymes and an increase in the activity of liver enzymes alanine transaminase (ALT) and aspartate transaminase (AST) (Goncalves et al., 2017). Goncalves et al. (Goncalves et al., 2017) demonstrated that intake of 2.7 mg naringenin in the patients with hepatitis C results in a great reduction in lipid profile and liver enzyme AST. Therefore, naringenin can improve HCV infection, and in a dose‐dependent manner, its administration can inhibit the post entry stages of CHIKV replication activity by downregulating the production of the viral proteins involved in replication. Prevention of CHIKV intracellular replication in CHIKV infected hamster kidney cells was caused by the administration of 6.818 μM naringenin (Ahmadi et al., 2016). Additionally, naringenin (250 μM) showed a direct virucidal activity against DENV type‐2 (DENV‐2) infection, while it did not inhibit viral replication (Zandi et al., 2011). However, in another study, naringenin administration to infected Huh7.5 cells could inhibit all of the DENV serotypes replication (Frabasile et al., 2017). In a study by Cataneo et al. (2019) ZIKV infection in human A549 cells was inhibited by naringenin administration in a concentration‐dependent manner. The antiviral activity of naringenin was also found when the primary human monocyte‐derived dendritic cells were treated after infection. Accordingly, this finding suggests that naringenin can inhibit viral replication or assembly of viral particles. In addition, an interaction between the protease domain of the NS2B‐NS3 protein of ZIKV and naringenin can explain the anti‐ZIKV activity of naringenin (Cataneo et al., 2019). Also it was shown that, in baby hamster cells 21 clone 15 (BHK‐21), administration of 25 μg/ml naringenin blocked sindbis neurovirulent strain (NSV) replication up to 80% (Paredes et al., 2003). As an antiviral biomarker in Guiera senegalensis, a traditional medicinal plant, naringenin administration (0.14 μg/mg) could inhibit hepatitis B virus (HBV) life cycle either by targeting viral envelopes or by reverse‐transcriptases (Alam et al., 2017). The summary of the studies demonstrating antiviral effects of naringenin is presented in Table 1.
4.2. Antioxidant and anti‐inflammatory activities of naringenin
The antioxidant activity of naringenin is attributed to hydroxyl substituents (OH) in its structure. These hydroxyl groups have high reactivity against reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Hernandez‐Aquino & Muriel, 2018; Zaidun, Thent, & Latiff, 2018). Naringenin exerts its antioxidant activity by scavenging of free radicals, and by preventing lipid peroxidation‐mediated oxidative DNA damage in a dose‐dependent manner (Cavia‐Saiz et al., 2010; Da Pozzo et al., 2017; Rashmi, Bojan Magesh, Mohanram Ramkumar, Suryanarayanan, & Venkata SubbaRao, 2018). In an experimental study, naringenin treatment was reported to decrease the levels of thiobarbituric acid reactive substances (TBARS), conjugated dienes, and lipid hydroperoxides (LOOH) (Chtourou et al., 2015). Furthermore, it could promote the activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) (Cavia‐Saiz et al., 2010).
In addition to playing an antioxidant role, naringenin has been reported to exert a potent anti‐inflammatory activity through the inhibition of nuclear factor kappa B (NF‐κB) signaling pathway (Jayaraman, Jesudoss, Menon, & Namasivayam, 2012). NF‐κB stimulates the expression of several important inflammatory proteins such as tumor necrosis factor‐alpha (TNF‐α), interleukin‐6 (IL‐6), cyclooxygenase‐2 (COX‐2), interleukin‐1 (IL‐1), and inducible nitric oxide synthase (iNOS) (Hernandez‐Aquino & Muriel, 2018). Also, the results obtained from in vitro studies and in vivo animal models indicate that naringenin can downregulate the expression of several inflammatory markers such as toll like receptor 4 (TLR4), TNF‐α, IL‐1β, IL‐6, iNOS, and COX‐2 through the attenuation of the NF‐κB pathway and the activation of the AMP‐activated protein kinase (AMPK), which is associated with the inhibition of multiple pro‐inflammatory signaling pathways (Yoshida et al., 2010; Zobeiri et al., 2018).
5. ADVANTAGES OF NARINGENIN COMPARED TO OTHER IMPORTANT NATURAL ANTI‐INFLAMMATORY AGENTS
Curcumin has been shown to regulate many transcription factors, cytokines, adhesion molecules, and enzymes associated with inflammation. In this regard, numerous studies have revealed the potential role of curcumin in the prevention and treatment of various pro‐inflammatory diseases (Moballegh Nasery et al., 2020). Zerumbone, which is isolated from the tropical plant Zingiber zerumbet Smith, has been reported to have anti‐inflammatory effects by inhibiting iNOS, COX‐2 expressions, and prostaglandin E2 (PGE2) production (Prasannan et al., 2012). Thymoquinone is considered as the main active component in Nigella sativa that has been found to exert anti‐inflammatory activities by reducing the expression of iNOS protein (Siveen, Ahn et al., 2014; Siveen, Mustafa et al., 2014). The NF‐κB inhibition activity of honokiol, as a phenolic compound (Rajendran et al., 2012); escin, as the main active ingredient of Aesculus hippocastanum seed extract (Tan et al., 2010); pinitol, which is isolated from Abies pindrow leaves (Sethi, Ahn, Sung, & Aggarwal, 2008); and tocotrienols, which are the members of vitamin E family (Siveen, Ahn et al., 2014; Siveen, Mustafa et al., 2014), have also been reported.
Due to the dose‐related side effects of curcumin and thymoquinone at the dosages of 12 g/day (Hewlings & Kalman, 2017) and 2–3 g/day (Goyal et al., 2017), respectively, the use of some novel agents like naringenin with no undesirable side effects, can be considered. In comparison to naringenin, zerumbone has shown antimicrobial effects against fungi and bacteria as well as some anti‐inflammatory activities (Nagaraj, Shridhar, Nirguna Babu, & Gowrishankar, 2012). However, antiviral effects of zerumbone have not been reported yet. Although escin, pinitol, and tocotrienols exhibit anti‐inflammatory and antiviral activities, the beneficial biological effects of naringenin on human health appear to be more extensive (Michelini, Alche, & Bueno, 2018; Mileva & Galabov, 2018; Sethi et al., 2008). In fact, flavonoid compounds have received much attention due to having many types of pharmacological activities including antioxidative, anti‐inflammatory, anti‐mutagenic, antimicrobial, hepatoprotective, and anti‐carcinogenic effects (Panche et al., 2016). To the best of our knowledge, all the natural agents with the above‐mentioned anti‐inflammatory activities, except curcumin and thymoquinone, showed no inhibitory effect on COVID‐19 main protease. However, naringenin is capable of inhibiting the enzymatic activity of CoV 3CLpro (Khaerunnisa et al., 2020).
6. PROTECTIVE EFFECTS AND POSSIBLE MECHANISMS OF ACTION OF NARINGENIN AGAINST COVID‐19
A recent study suggests that some flavonoids such as naringenin, kaempferol, quercetin, and apigenin are the most recommended compounds that may act as the potential inhibitors of SARS‐CoV‐2 main protease (Khaerunnisa et al., 2020). These agents share a pharmacophore similar to nelfinavir (Dabeek & Marra, 2019; Salehi et al., 2019). Nelfinavir is a protease inhibitor used in patients infected by the human immunodeficiency virus (HIV) (Yamamoto et al., 2004). Because proteases play essential roles in viral replication of different types of viruses, they may be considered as potential pharmacological targets for preventing CoV replication (Chang, Kim, Lovell, Rathnayake, & Groutas, 2019; Xin Liu & Wang, 2020). The COVID‐19 main protease, Protein Data Bank (PDB) ID 6 LU7, is pivotal for the proteolytic maturation of CoV (Xin Liu & Wang, 2020). SARS‐CoV‐2 main protease or 3CLpro contains two chains, which make a homodimer (Berman et al., 2002). The molecular docking analysis study demonstrated that naringenin binds to 3CLpro chains as a ligand and blocks its activity. The binding energy obtained from docking 6 LU7 with naringenin was −7.99 kcal/mol, which was associated with the number of H‐bonds formed with 6 LU7. Naringenin H‐bonds interact with amino acids histidine (His164), glutamic acid (Glu166), aspartic acid (Asp187), threonine (Thr190) in the CoV main protease active site. Altogether, due to the lower binding energy of naringenin and the presence of H‐bonds, the affinity of naringenin bonds is high and this flavanone is capable of inhibiting the enzymatic activity of CoV 3CLpro (Khaerunnisa et al., 2020).
As it was mentioned earlier, it has also been indicated that CoVs use the ACE2 receptors to enter the host cells; thus, compounds that can reduce the ACE2 activity may be useful in the treatment of the patients with COVID‐19 (Letko et al., 2020; Lu et al., 2020). A recent study has investigated the immunoregulatory effects of citrus flavonoids as well as their impacts on ACE2 activity. The binding affinity of these compounds to ACE2 was assessed by molecular docking. The interaction between naringenin and ACE2 was also examined by binding energy. The docking findings demonstrate that naringenin is able to bind to ACE2 with docking energy of −6.05 kcal/mol, with binding site proline (PRO‐146), leucine (LEU‐143), and lysine (LYS‐131). On the whole, the findings of the recent study showed that the energy required for the binding between naringenin and ACE2 was low, so it could easily bind to ACE2. The authors concluded that naringenin might be a promising treatment strategy for COVID‐19 (Cheng et al., 2020).
An emerging evidence demonstrates that the patients infected with SARS‐CoV‐2 have high levels of cytokines including TNF‐α, IL‐1β, IL‐10, interferon γ (IFNγ), and monocyte chemoattractant protein‐1 (MCP‐1). These findings indicate that the cytokine storm contributes to disease severity (Huang, Wang et al., 2020). To date, corticosteroids may be beneficial if utilized at the early acute stages of infection (Russell, Moss, Rigg, & Van Hemelrijck, 2020). Therefore, the use of anti‐inflammatory agents may be considered as a promising treatment approach to relieve COVID‐19 symptoms (Stebbing et al., 2020). As it was mentioned earlier, naringenin exhibits a potent anti‐inflammatory activity and can be recognized as an option to decrease cytokine levels of inflammatory markers including TNF‐α, IL‐1β, IL‐10, and IFNγ in the patients with COVID‐19 (Cheng et al., 2020). Also, a protective effect of naringenin against lipopolysaccharide‐induced acute lung injury was reported in rats. Naringenin at two doses (50 and 100 mg/kg/day), significantly attenuated the production of inflammatory cytokines, pulmonary edema, neutrophil recruitment, myeloperoxidase activity, and decreased the markers of oxidative/nitrosative stress in lungs of LPS‐challenged rats (Fouad et al., 2016). Moreover, administration of 100 mg/kg naringenin to rats with lung damage led to the down regulation of the expressions of NF‐κB and COX2 (Ali et al., 2017). Fan, Pan, Zhu, and Zhang (2017) also found that administration of 5–20 mg/kg naringenin to Wistar rats with arthritic inflammation resulted in a decrease in TNF‐α and NF‐κB mRNA levels. Furthermore, an in vivo study demonstrated a reduction in NF‐κB, TNF‐α, IL‐6, and IL‐1β after naringenin administration (Hua et al., 2016). Besides, in the mice treated with 10 mg/kg naringenin, inflammatory markers including TNF‐α, IL‐6, TLR4, iNOS, COX2, NADPH oxidase‐2 (NOX2), NF‐κB, and mitogen‐activated protein kinase (MAPK) were suppressed (X. Liu et al., 2016). A post‐translational inhibition of TNF‐α and IL‐6 was also observed in an in vitro study that assessed the effects of naringenin on murine macrophage cell line RAW264.7 (Jin, Zeng, Zhang, Zhang, & Liang, 2017). In addition, mRNA and protein expression levels of TNF‐α, IL‐6, and IL‐1β were attenuated by naringenin administration in female mice with hypertrophic scars (Shan et al., 2017). Collectively, a large amount of evidence supports the notion that naringenin represents a potential therapeutic agent to control the inflammation‐related diseases. Therefore, one of the other mechanisms by which naringenin might exert therapeutic effects against COVID‐19 is, at least partly, by attenuating inflammatory responses. The summary of the possible mechanisms for the protective effects of naringenin against COVID‐19 is presented in Figure 2.
FIGURE 2.
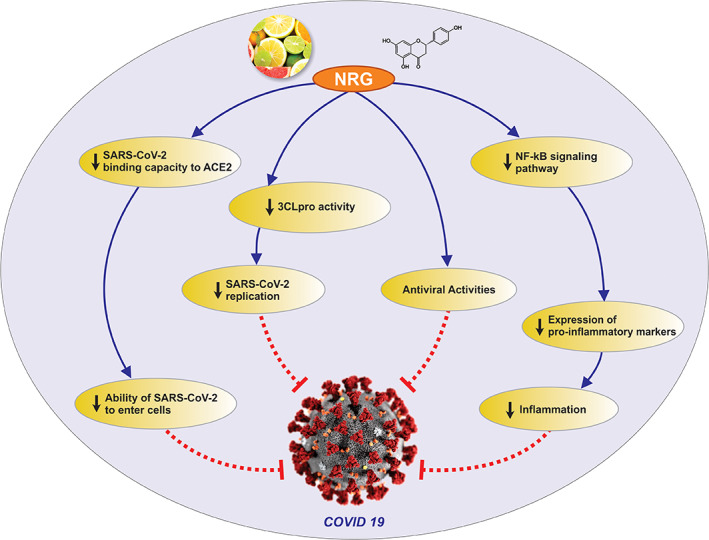
Possible mechanisms for the actions of naringenin against COVID‐19. ACE2, angiotensin‐converting enzyme 2; NRG, naringenin; NF‐κB, nuclear factor kappa B; 3CLpro, 3‐Chymotrypsin‐like protease; SARS, Severe acute respiratory syndrome [Colour figure can be viewed at wileyonlinelibrary.com]
7. CONCLUSIONS
In conclusion, the evidence reviewed here indicates that naringenin might exert therapeutic effects against COVID‐19 through the inhibition of COVID‐19 main protease, 3CLpro, and reduction of ACE2 receptors activity. One of the other mechanisms by which naringenin might exert therapeutic effects against COVID‐19 is, at least partly, by attenuating inflammatory responses. The antiviral activity of the flavanone naringenin has also been reported against some viruses. On the whole, the favorable effects of naringenin lead to a conclusion that naringenin may be considered as a promising treatment strategy against COVID‐19. However, the beneficial effects of naringenin still need to be confirmed in clinical trials.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Tutunchi H, Naeini F, Ostadrahimi A, Hosseinzadeh‐Attar MJ. Naringenin, a flavanone with antiviral and anti‐inflammatory effects: A promising treatment strategy against COVID‐19. Phytotherapy Research. 2020;34:3137–3147. 10.1002/ptr.6781
Contributor Information
Alireza Ostadrahimi, Email: ostadrahimi@tbzmed.ac.ir.
Mohammad Javad Hosseinzadeh‐Attar, Email: mhosseinzadeh@tums.ac.ir.
REFERENCES
- Ahmadi, A. , Hassandarvish, P. , Lani, R. , Yadollahi, P. , Jokar, A. , Bakar, S. A. , & Zandi, K. (2016). Inhibition of chikungunya virus replication by hesperetin and naringenin. RSC Advances, 6(73), 69421–69430. 10.1039/C6RA16640G [DOI] [Google Scholar]
- Alam, P. , Parvez, K. , Arbab, A. , & Al‐Dosari, M. (2017). Quantitative analysis of rutin, quercetin, naringenin, and gallic acid by validated RP‐ and NP‐HPTLC methods for quality control of anti‐HBV active extract of Guiera senegalensis. Pharmaceutical Biology, 55, 1317–1323. 10.1080/13880209.2017.1300175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, R. , Shahid, A. , Ali, N. , Hasan, S. K. , Majed, F. , & Sultana, S. (2017). Amelioration of benzo[a]pyrene‐induced oxidative stress and pulmonary toxicity by Naringenin in Wistar rats: A plausible role of COX‐2 and NF‐kappaB. Human & Experimental Toxicology, 36(4), 349–364. 10.1177/0960327116650009 [DOI] [PubMed] [Google Scholar]
- Ameer, B. , Weintraub, R. A. , Johnson, J. V. , Yost, R. A. , & Rouseff, R. L. (1996). Flavanone absorption after naringin, hesperidin, and citrus administration. Clinical Pharmacology & Therapeutics, 60(1), 34–40. 10.1016/S0009-9236(96)90164-2 [DOI] [PubMed] [Google Scholar]
- Baez‐Santos, Y. M. , St John, S. E. , & Mesecar, A. D. (2015). The SARS‐coronavirus papain‐like protease: Structure, function and inhibition by designed antiviral compounds. Antiviral Research, 115, 21–38. 10.1016/j.antiviral.2014.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreca, D. , Gattuso, G. , Bellocco, E. , Calderaro, A. , Trombetta, D. , Smeriglio, A. , … Nabavi, S. M. (2017). Flavanones: Citrus phytochemical with health‐promoting properties. BioFactors, 43(4), 495–506. 10.1002/biof.1363 [DOI] [PubMed] [Google Scholar]
- Berman, H. M. , Battistuz, T. , Bhat, T. N. , Bluhm, W. F. , Bourne, P. E. , Burkhardt, K. , … Zardecki, C. (2002). The Protein Data Bank. Acta Crystallographica Section D, 58(1), 899–907. 10.1107/S0907444902003451 [DOI] [PubMed] [Google Scholar]
- Cataneo, A. , Kuczera, D. , Koishi, A. , Zanluca, C. , Silveira, G. , Arruda, T. , … Bordignon, J. (2019). The citrus flavonoid naringenin impairs the in vitro infection of human cells by Zika virus. Scientific Reports, 9, 1–15. 10.1038/s41598-019-52626-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavia‐Saiz, M. , Busto, M. D. , Pilar‐Izquierdo, M. C. , Ortega, N. , Perez‐Mateos, M. , & Muniz, P. (2010). Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. Journal of the Science of Food and Agriculture, 90(7), 1238–1244. 10.1002/jsfa.3959 [DOI] [PubMed] [Google Scholar]
- Chang, K.‐O. , Kim, Y. , Lovell, S. , Rathnayake, A. D. , & Groutas, W. C. (2019). Antiviral drug discovery: Norovirus proteases and development of inhibitors. Viruses, 11(2), 197. 10.3390/v11020197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, L. , Zheng, W. , Li, M. , Huang, J. , Bao, S. , Xu, Q. , & Ma, Z. (2020). Citrus fruits are rich in flavonoids for Immunoregulation and potential targeting ACE2. Preprint, 2020020313. Retrieved from https://www.preprints.org/manuscript/202002.0313/v1 [DOI] [PMC free article] [PubMed]
- Chtourou, Y. , Fetoui, H. , Jemai, R. , Ben Slima, A. , Makni, M. , & Gdoura, R. (2015). Naringenin reduces cholesterol‐induced hepatic inflammation in rats by modulating matrix metalloproteinases‐2, 9 via inhibition of nuclear factor kappaB pathway. European Journal of Pharmacology, 746, 96–105. 10.1016/j.ejphar.2014.10.027 [DOI] [PubMed] [Google Scholar]
- Clark, J. , Zahradka, P. , & Taylor, C. (2015). Efficacy of flavonoids in the management of high blood pressure. Nutrition Reviews, 73(12), 799–822. 10.1093/nutrit/nuv048 [DOI] [PubMed] [Google Scholar]
- Da Pozzo, E. , Costa, B. , Cavallini, C. , Testai, L. , Martelli, A. , Calderone, V. , & Martini, C. (2017). The citrus flavanone Naringenin protects myocardial cells against age‐associated damage. Oxidative Medicine and Cellular Longevity, 2017, 1–12. 10.1155/2017/9536148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabeek, M. W. , & Marra, V. M. (2019). Dietary quercetin and Kaempferol: Bioavailability and potential cardiovascular‐related bioactivity in humans. Nutrients, 11(10), 1–19. 10.3390/nu11102288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas, C. , Alain, G. , Elbez, Y. , Caillard, P. , Zamaria, N. , & Cloarec, M. (2014). Clinical study to assess the efficacy and safety of a citrus polyphenolic extract of red Orange, grapefruit, and Orange (Sinetrol‐XPur) on weight management and metabolic parameters in healthy overweight individuals. Phytotherapy Research, 28(2), 212–218. 10.1002/ptr.4981 [DOI] [PubMed] [Google Scholar]
- Den Hartogh, D. J. , & Tsiani, E. (2019). Antidiabetic properties of Naringenin: A citrus fruit polyphenol. Biomolecules, 9(3), 1–21. 10.3390/biom9030099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, J. H. (2020). Hypothesis: Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID‐19. Journal of Travel Medicine, 27, taaa041. 10.1093/jtm/taaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlund, I. , Meririnne, E. , Alfthan, G. , & Aro, A. (2001). Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. Journal of Nutrition, 131(2), 235–241. 10.1093/jn/131.2.235 [DOI] [PubMed] [Google Scholar]
- Fan, R. , Pan, T. , Zhu, A.‐L. , & Zhang, M.‐H. (2017). Anti‐inflammatory and anti‐arthritic properties of naringenin via attenuation of NF‐κB and activation of the heme oxygenase (HO)‐1/related factor 2 pathway. Pharmacological Reports, 69(5), 1021–1029. 10.1016/j.pharep.2017.03.020 [DOI] [PubMed] [Google Scholar]
- Fouad, A. , Albuali, W. , & Jresat, I. (2016). Protective effect of Naringenin against lipopolysaccharide‐induced acute lung injury in rats. Pharmacology, 97, 224–232. 10.1159/000444262 [DOI] [PubMed] [Google Scholar]
- Frabasile, S. , Koishi, A. , Kuczera, D. , Silveira, G. , Verri, W. , Santos, C. , & Bordignon, J. (2017). The citrus flavanone naringenin impairs dengue virus replication in human cells. Scientific Reports, 7, 1–11. 10.1038/srep41864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galati, G. , Moridani, M. , Chan, T. , & O'Brien, P. (2001). Peroxidative metabolism of apigenin and naringenin versus luteolin and quercetin: Glutathione oxidation and conjugation. Free Radical Biology & Medicine, 30, 370–382. 10.1016/S0891-5849(00)00481-0 [DOI] [PubMed] [Google Scholar]
- Ghaffari, S. , Roshanravan, N. , Tutunchi, H. , Ostadrahimi, A. , & Kafil, B. (2020). Oleoylethanolamide, a bioactive lipid amide, as a promising treatment strategy for coronavirus/COVID‐19. Archives of Medical Research. 10.1016/j.arcmed.2020.04.006. [Epub a head of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwasser, J. , Cohen, P. , Lin, W. , Kitsberg, D. , Balaguer, P. , Polyak, S. , … Nahmias, Y. (2011). Naringenin inhibits the assembly and long‐term production of infectious hepatitis C virus particles through a PPAR‐mediated mechanism. Journal of Hepatology, 55, 963–971. 10.1016/j.jhep.2011.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves, D. , Lima, C. , Ferreira, P. , Costa, P. , Costa, A. , Figueiredo, W. , & Cesar, T. (2017). Orange juice as dietary source of antioxidants for patients with hepatitis C under antiviral therapy. Food & Nutrition Research, 61(1), 1296675. 10.1080/16546628.2017.1296675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal, S. N. , Prajapati, C. P. , Gore, P. R. , Patil, C. R. , Mahajan, U. B. , Sharma, C. , … Ojha, S. K. (2017). Therapeutic potential and pharmaceutical development of Thymoquinone: A multitargeted molecule of natural origin. Frontiers in Pharmacology, 8, 656–656. 10.3389/fphar.2017.00656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y. R. , Cao, Q. D. , Hong, Z. S. , Tan, Y. Y. , Chen, S. D. , Jin, H. J. , … Yan, Y. (2020). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak ‐ an update on the status. Military Medical Research, 7(1), 11. 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez‐Aquino, E. , & Muriel, P. (2018). Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World Journal of Gastroenterology, 24(16), 1679–1707. 10.3748/wjg.v24.i16.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlings, S. J. , & Kalman, D. S. (2017). Curcumin: A review of its' effects on human health. Foods, 6(10), 92. 10.3390/foods6100092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, F. Z. , Ying, J. , Zhang, J. , Wang, X. F. , Hu, Y. H. , Liang, Y. P. , … Xu, G. H. (2016). Naringenin pre‐treatment inhibits neuroapoptosis and ameliorates cognitive impairment in rats exposed to isoflurane anesthesia by regulating the PI3/Akt/PTEN signalling pathway and suppressing NF‐kappaB‐mediated inflammation. International Journal of Molecular Medicine, 38(4), 1271–1280. 10.3892/ijmm.2016.2715 [DOI] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395(10223), 497–506. 10.1016/s0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Sun, F. , Owen, D. M. , Li, W. , Chen, Y. , Gale, M., Jr. , & Ye, J. (2007). Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low‐density lipoproteins. Proceedings of the National Academy of Sciences of the United States of America, 104(14), 5848–5853. 10.1073/pnas.0700760104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman, J. , Jesudoss, V. A. , Menon, V. P. , & Namasivayam, N. (2012). Anti‐inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicology Mechanisms and Methods, 22(7), 568–576. 10.3109/15376516.2012.707255 [DOI] [PubMed] [Google Scholar]
- Jin, L. , Zeng, W. , Zhang, F. , Zhang, C. , & Liang, W. (2017). Naringenin ameliorates acute inflammation by regulating intracellular cytokine degradation. Journal of Immunology, 199(10), 3466–3477. 10.4049/jimmunol.1602016 [DOI] [PubMed] [Google Scholar]
- Jo, S. , Kim, S. , Shin, D. H. , & Kim, M. S. (2020). Inhibition of SARS‐CoV 3CL protease by flavonoids. Journal of Enzyme Inhibition and Medicinal Chemistry, 35(1), 145–151. 10.1080/14756366.2019.1690480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaze, F. I. , Bounartzi, M. I. , Georgarakis, M. , & Niopas, I. (2007). Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. European Journal of Clinical Nutrition, 61(4), 472–477. 10.1038/sj.ejcn.1602543 [DOI] [PubMed] [Google Scholar]
- Khaerunnisa, S. , Kurniawan, H. , Awaluddin, R. , Suhartati, S. , & Soetjipto, S. (2020). Potential inhibitor of COVID‐19 Main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints 2020, 2020030226. Retrieved from 10.20944/preprints202003.0226.v1. [DOI]
- Letko, M. , Marzi, A. , & Munster, V. (2020). Functional assessment of cell entry and receptor usage for SARS‐CoV‐2 and other lineage B betacoronaviruses. Nature Microbiology, 5, 562–569. 10.1038/s41564-020-0688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Wang, N. , Fan, S. , Zheng, X. , Yang, Y. , Zhu, Y. , … Zheng, J. (2016). The citrus flavonoid naringenin confers protection in a murine endotoxaemia model through AMPK‐ATF3‐dependent negative regulation of the TLR4 signalling pathway. Scientific Reports, 6, 1–14. 10.1038/srep39735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , & Wang, X.‐J. (2020). Potential inhibitors against 2019‐nCoV coronavirus M protease from clinically approved medicines. Journal of Genetics and Genomics, 47, 119–121. 10.1016/j.jgg.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. , Wu, H. , … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet, 395(10224), 565–574. 10.1016/s0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madjid, M. , Safavi‐Naeini, P. , Solomon, S. D. , & Vardeny, O. (2020). Potential effects of coronaviruses on the cardiovascular system: A Review. JAMA Cardiology. 10.1001/jamacardio.2020.1286. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Malik, Y. , Sircar, S. , Bhat, S. , Khan, S. , Dhama, K. , Dadar, M. , … Chaicumpa, W. (2020). Emerging novel coronavirus (2019‐nCoV) ‐ current scenario, evolutionary perspective based on genome analysis and recent developments. The Veterinary Quarterly, 40(1), 1–10. 10.1080/01652176.2020.1727993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchope, M. F. , Casagrande, R. , & Verri, W. A., Jr. (2017). Naringenin: An analgesic and anti‐inflammatory citrus flavanone. Oncotarget, 8(3), 3766–3767. 10.18632/oncotarget.14084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini, F. M. , Alche, L. E. , & Bueno, C. A. (2018). Virucidal, antiviral and immunomodulatory activities of beta‐escin and Aesculus hippocastanum extract. The Journal of Pharmacy and Pharmacology, 70(11), 1561–1571. 10.1111/jphp.13002 [DOI] [PubMed] [Google Scholar]
- Mileva, M. , & Galabov, A. (2018). Vitamin E and influenza virus infection. In Vitamin E in health and disease, England: IntechOpen. 10.5772/intechopen.80954 [DOI] [Google Scholar]
- Moballegh Nasery, M. , Abadi, B. , Poormoghadam, D. , Zarrabi, A. , Keyhanvar, P. , Khanbabaei, H. , … Sethi, G. (2020). Curcumin delivery mediated by bio‐based nanoparticles: A review. Molecules, 25(3), 689. 10.3390/molecules25030689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone, G. , & Ardizzone, S. (2020). Are patients with inflammatory bowel disease at increased risk for Covid‐19 infection? Journal of Crohn's and Colitis, jjaa061. 10.1093/ecco-jcc/jjaa061. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj, V. H. , Shridhar, B. S. , Nirguna Babu, P. , & Gowrishankar, B. S. (2012). Antimicrobial activity of zerumbone from zingiber zurumbet against staphylococcus epidermidis and aspergillus SPP. IJABPT, 3(4), 40–43. [Google Scholar]
- Nahmias, Y. , Goldwasser, J. , Casali, M. , van Poll, D. , Wakita, T. , Chung, R. T. , & Yarmush, M. L. (2008). Apolipoprotein B‐dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology, 47(5), 1437–1445. 10.1002/hep.22197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz‐Andrade, R. , Sánchez‐Salgado, J. , Navarrete‐Vázquez, G. , Webster, S. , Binnie, M. , Sara, G.‐j. , … Estrada‐Soto, S. (2008). Antidiabetic and toxicological evaluations of naringenin in normoglycaemic and NIDDM rat models and its implications on extra‐pancreatic glucose regulation. Diabetes, Obesity & Metabolism, 10, 1097–1104. 10.1111/j.1463-1326.2008.00869.x [DOI] [PubMed] [Google Scholar]
- Panche, A. , Diwan, A. , & Chandra, S. (2016). Flavonoids: An overview. Journal of Nutritional Science, 5, 1–15. 10.1017/jns.2016.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes, A. , Alzuru, M. , Mendez, J. , & Rodriguez‐Ortega, M. (2003). Anti‐Sindbis activity of flavanones Hesperetin and Naringenin. Biological & Pharmaceutical Bulletin, 26, 108–109. 10.1248/bpb.26.108 [DOI] [PubMed] [Google Scholar]
- Peng, X. , Xu, X. , Li, Y. , Cheng, L. , Zhou, X. , & Ren, B. (2020). Transmission routes of 2019‐nCoV and controls in dental practice. International Journal of Oral Science, 12(1), 1–6. 10.1038/s41368-020-0075-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietta, P. G. (2000). Flavonoids as antioxidants. Journal of Natural Products, 63, 1035–1042. 10.1021/np9904509 [DOI] [PubMed] [Google Scholar]
- Prasannan, R. , Kalesh, K. A. , Shanmugam, M. K. , Nachiyappan, A. , Ramachandran, L. , Nguyen, A. H. , … Sethi, G. (2012). Key cell signaling pathways modulated by zerumbone: Role in the prevention and treatment of cancer. Biochemical Pharmacology, 84(10), 1268–1276. 10.1016/j.bcp.2012.07.015 [DOI] [PubMed] [Google Scholar]
- Rajendran, P. , Li, F. , Shanmugam, M. K. , Vali, S. , Abbasi, T. , Kapoor, S. , … Sethi, G. (2012). Honokiol inhibits signal transducer and activator of transcription‐3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP‐1. Journal of Cellular Physiology, 227(5), 2184–2195. 10.1002/jcp.22954 [DOI] [PubMed] [Google Scholar]
- Rashmi, R. , Bojan Magesh, S. , Mohanram Ramkumar, K. , Suryanarayanan, S. , & Venkata SubbaRao, M. (2018). Antioxidant potential of Naringenin helps to protect liver tissue from Streptozotocin‐induced damage. Reports of Biochemistry & Molecular Biology, 7(1), 76–84. [PMC free article] [PubMed] [Google Scholar]
- Russell, B. , Moss, C. , Rigg, A. , & Van Hemelrijck, M. (2020). COVID‐19 and treatment with NSAIDs and corticosteroids: Should we be limiting their use in the clinical setting? Ecancermedicalscience, 14, 1023–1023. 10.3332/ecancer.2020.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu, S. C. , & Gray, G. C. (1997). Lipid peroxidation and dna damage induced by morin and naringenin in isolated rat liver nuclei. Food and Chemical Toxicology, 35(5), 443–447. 10.1016/S0278-6915(97)00011-2 [DOI] [PubMed] [Google Scholar]
- Salehi, B. , Fokou, P. V. T. , Sharifi‐Rad, M. , Zucca, P. , Pezzani, R. , Martins, N. , & Sharifi‐Rad, J. (2019). The therapeutic potential of Naringenin: A review of clinical trials. Pharmaceuticals, 12(1), 1–18. 10.3390/ph12010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawikowska, A. (2020). Meta‐analysis of flavonoids with antiviral potential against coronavirus. Biometrical Letters, 0. 10.2478/bile-2020-0002. [Epub ahead of print]. [DOI] [Google Scholar]
- Schoeman, D. , & Fielding, B. C. (2019). Coronavirus envelope protein: Current knowledge. Virology Journal, 16(1), 1–22. 10.1186/s12985-019-1182-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehn, L. H. (2020). Balancing risk and benefit during Corona virus. Blood, 135, 1817. 10.1182/blood.2020006279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi, G. , Ahn, K. S. , Sung, B. , & Aggarwal, B. B. (2008). Pinitol targets nuclear factor‐kappaB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Molecular Cancer Therapeutics, 7(6), 1604–1614. 10.1158/1535-7163.mct-07-2424 [DOI] [PubMed] [Google Scholar]
- Shan, S. , Zhang, Y. , Wu, M. , Yi, B. , Wang, J. , & Li, Q. (2017). Naringenin attenuates fibroblast activation and inflammatory response in a mechanical stretch‐induced hypertrophic scar mouse model. Molecular Medicine Reports, 16(4), 4643–4649. 10.3892/mmr.2017.7209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen, M. A. , Khan, S. , Kazmi, A. , Bashir, N. , & Siddique, R. (2020). COVID‐19 infection: Origin, transmission, and characteristics of human coronaviruses. Journal of Advanced Research, 24, 91–98. 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, J. , Lima, C. , Pereira, C. , Bittar, C. , Batista, M. , Nazaré, A. C. , … Jardim, A. (2017). Flavonoids from Pterogyne nitens inhibit hepatitis C virus entry. Scientific Reports, 7, 1–9. 10.1038/s41598-017-16336-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siveen, K. S. , Ahn, K. S. , Ong, T. H. , Shanmugam, M. K. , Li, F. , Yap, W. N. , … Sethi, G. (2014). Y‐tocotrienol inhibits angiogenesis‐dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget, 5(7), 1897–1911. 10.18632/oncotarget.1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siveen, K. S. , Mustafa, N. , Li, F. , Kannaiyan, R. , Ahn, K. S. , Kumar, A. P. , … Sethi, G. (2014). Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF‐κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget, 5(3), 634–648. 10.18632/oncotarget.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing, J. , Phelan, A. , Griffin, I. , Tucker, C. , Oechsle, O. , Smith, D. , & Richardson, P. (2020). COVID‐19: Combining antiviral and anti‐inflammatory treatments. The Lancet Infectious Diseases, 20(4), 400–402. 10.1016/S1473-3099(20)30132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir Ul Qamar, M. , Alqahtani, S. , Alamri, M. , & Chen, L.‐L. (2020). Structural basis of SARS‐CoV‐2 3CLpro and anti‐COVID‐19 drug discovery from medicinal plants. Journal of Pharmaceutical Analysis. 10.1016/j.jpha.2020.03.009. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, S. M. , Li, F. , Rajendran, P. , Kumar, A. P. , Hui, K. M. , & Sethi, G. (2010). Identification of beta‐escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus‐activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. The Journal of Pharmacology and Experimental Therapeutics, 334(1), 285–293. 10.1124/jpet.110.165498 [DOI] [PubMed] [Google Scholar]
- Tapas, D. A. , Sakarkar, D. M. , & Kakde, R. (2008). Flavonoids as nutraceuticals: A review. Tropical Journal of Pharmaceutical Research, 7(3), 1090–1099 10.4314/tjpr.v7i3.14693 [DOI] [Google Scholar]
- The, L. (2020). Redefining vulnerability in the era of COVID‐19. The Lancet, 395(10230), 1089. 10.1016/S0140-6736(20)30757-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, A. , Zhao, W. , Xu, Z. , & Gu, J. (2020). Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID‐19) is urgently needed. Diabetes Researchand Clinical Practic, 162, 1–2. 10.1016/j.diabres.2020.108118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, P. C. Y. , & Lau, S. K. P. (2019). Viruses and Bats. Viruses, 11(10), 1–4. 10.3390/v11100884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2020). Coronavirus disease 2019 (COVID‐19): Retrieved from https://covid19.who.int
- Xiao, K. , Zhai, J. , Feng, Y. , Zhou, N. , Zhang, X. , Zou, J.‐J. , … Shen, Y. (2020). Isolation and characterization of 2019‐nCoV‐like coronavirus from Malayan pangolins. bioRxiv. Retrieved from 10.1101/2020.02.17.951335 [DOI]
- Xu, H. , Kulkarni, K. , Singh, R. , Yang, Z. , Wang, S. , Tam, V. , & Hu, M. (2009). Disposition of naringenin via glucuronidation pathway is affected by compensating efflux transporters of hydrophilic glucuronides. Molecular Pharmaceutics, 6, 1703–1715. 10.1021/mp900013d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, N. , Yang, R. , Yoshinaka, Y. , Amari, S. , Nakano, T. , Cinatl, J. , … Yamamoto, N. (2004). HIV protease inhibitor nelfinavir inhibits replication of SARS‐associated coronavirus. Biochemical and Biophysical Research Communications, 318(3), 719–725. 10.1016/j.bbrc.2004.04.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, H. , Takamura, N. , Shuto, T. , Ogata, K. , Tokunaga, J. , Kawai, K. , & Kai, H. (2010). The citrus flavonoids hesperetin and naringenin block the lipolytic actions of TNF‐alpha in mouse adipocytes. Biochemical and Biophysical Research Communications, 394(3), 728–732. 10.1016/j.bbrc.2010.03.060 [DOI] [PubMed] [Google Scholar]
- Zaidun, N. H. , Thent, Z. C. , & Latiff, A. A. (2018). Combating oxidative stress disorders with citrus flavonoid: Naringenin. Life Science, 208, 111–122. 10.1016/j.lfs.2018.07.017 [DOI] [PubMed] [Google Scholar]
- Zandi, K. , Teoh, B.‐T. , Sam, S.‐S. , Wong, P.‐F. , Mustafa, M. , & Abu Bakar, S. (2011). In vitro antiviral activity of Fisetin, Rutin and Naringenin against dengue virus type‐2. Journal of Medicinal Plants Research, 5, 5534–5539. 10.5897/JMPR11.1046 [DOI] [Google Scholar]
- Zhang, Y.‐Z. , & Holmes, E. C. (2020). A genomic perspective on the origin and emergence of SARS‐CoV‐2. Cell, 181, 223–227. 10.1016/j.cell.2020.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, N. S. , Zheng, B. J. , Li, Y. M. , Poon L. L. M., Xie, Z. H. , Chan, K. H. , … Guan, Y. (2003). Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet, 362(9393), 1353–1358. 10.1016/S0140-6736(03)14630-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, N. , Zhang, D. , Wang, W. , Li, X. , Yang, B. , Song, J. , … Tan, W. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 382(8), 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobeiri, M. , Belwal, T. , Parvizi, F. , Naseri, R. , Farzaei, M. H. , Nabavi, S. , … Nabavi, S. (2018). Naringenin and its nano‐formulations for fatty liver: Cellular modes of action and clinical perspective. Current Pharmaceutical Biotechnology, 19(3), 196–205. 10.2174/1389201019666180514170122 [DOI] [PubMed] [Google Scholar]


