Abstract
Recommendations were made recently to limit or stop the use of oral and systemic immunotherapies for skin diseases due to potential risks to the patients during the current severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) COVID‐19 pandemic. Herein, we attempt to identify potentially safe immunotherapies that may be used in the treatment of cutaneous diseases during the current COVID‐19 pandemic. We performed a literature review to approximate the risk of SARS‐CoV‐2 infection, including available data on the roles of relevant cytokines, cell subsets, and their mediators in eliciting an optimal immune response against respiratory viruses in murine gene deletion models and humans with congenital deficiencies were reviewed for viral infections risk and if possible coronaviruses specifically. Furthermore, reported risk of infections of biologic and non‐biologic therapeutics for skin diseases from clinical trials and drug data registries were evaluated. Many of the immunotherapies used in dermatology have data to support their safe use during the COVID‐19 pandemic including the biologics that target IgE, IL‐4/13, TNF‐α, IL‐17, IL‐12, and IL‐23. Furthermore, we provide evidence to show that oral immunosuppressive medications such as methotrexate and cyclosporine do not significantly increase the risk to patients. Most biologic and conventional immunotherapies, based on doses and indications in dermatology, do not appear to increase risk of viral susceptibility and are most likely safe for use during the COVID‐19 pandemic. The limitation of this study is availability of data on COVID‐19.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), also named 2019 novel coronavirus disease COVID‐19, is the causative agent of the ongoing pandemic. 1 It is not known if patients on immunotherapies for skin disorders are more susceptible to SARS‐CoV‐2. This uncertainty can result in anxiety for prescribing physicians and treated patients. Several formal and informal recommendations were made to limit or stop immunomodulator therapies in the “COVID‐19 era.” 2 , 3 With our knowledge of the immunopathogenesis of coronaviruses and as our understanding of SARS‐CoV‐2 evolves, it is important to place the emphasis on evidence‐based medicine to objectively evaluate SARS‐CoV‐2 risk in the context of dermatologic indications and doses.
Part 1: Proinflammatory cytokine surge in severe SARS‐CoV‐2 (COVID‐19) infection
The human pathogenic forms of coronaviruses usually cause mild‐to‐moderate upper respiratory tract illnesses (URTI) with few exceptions with life‐threatening implications such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). COVID‐19 is marked by symptoms that can include fever, dry cough, fatigue, and shortness of breath. A subset of COVID‐19 patients succumb to severe disease with manifestations of acute respiratory distress syndrome (ARDS), cardiac injury, and secondary infections with a high mortality rate. 4 It is postulated that a dysregulated immune response to the infection is a consequence of the patients’ comorbidities. 5 Dysregulation of the adaptive T‐cell‐mediated immune response is strongly implicated in pathogenesis of COVID‐19. 5 Elevated levels of proinflammatory cytokines were shown in patients with severe COVID‐19, including plasma levels of tumor necrosis factor α (TNF‐α), interleukin (IL)‐2, IL‐6, G‐CSF, IP10, MCP‐1, and MIP‐1α. 5 , 6 This is consistent with the reported elevation of proinflammatory cytokines in SARS 7 and MERS infections. 8 The massive inflammatory cell infiltration and elevated proinflammatory cytokine/chemokine responses result in acute lung injury and ARDS. 4 , 9 , 10
Part 2: Infectious risks associated with biologics: evaluating cytokine knockout data and reviewing data from randomized controlled trials (RCTs) and biologic treatment registries
TNF‐α
Infecting TNF‐α−/−, TNF receptor 1 (R1)−/−, and TNFR2−/− mice with mouse hepatitis virus‐3 (MHV‐3, belongs to the coronavirus family) revealed that a deficiency of either TNF‐α or TNFR1 decreased morbidity and mortality (Table 1). 11 TNF receptors 1/2 knock‐out mice infected with SARS‐CoV were protected from infection‐related morbidity. 12 Collectively, TNF‐α promotes the deleterious effects of coronavirus infection presumably through excessive inflammation. From clinical trials (Table 2), the relative risk of adalimumab, certolizumab, etanercept, and infliximab for URTI (2.06, 1.54, 2.44, and 0.93) and nasopharyngitis (0.82, 1.5, 1.39, and 0.75), respectively, is elevated compared to placebo, but the absolute risk remains small. Furthermore, in the Psoriasis Longitudinal Assessment and Registry (PSOLAR), biologics that targeted TNF‐α had little‐to‐no increased risk of infections. 13 It is important to note that definitions of URTI and nasopharyngitis in dermatology clinical trials are not adjudicated with nasopharyngeal swabs to confirm the presence of rhinovirus or influenza infection and that upper respiratory symptoms due to allergic phenomena could be a confounder. Given the proposed role of TNF‐α in acute lung injury and ARDS in COVID‐19, TNF‐α is a potential target for treating patients with COVID‐19. 14 Consequently, the efficacy and safety of adalimumab against COVID‐19‐induced cytokine storm are being evaluated in an ongoing clinical trial. 15
Table 1.
Cytokines and their mediators and impact on viral immunity in mice – knockout data
| Target | Respiratory virus susceptibility | Coronavirus susceptibility | Interpretation of effect of knockout | References |
|---|---|---|---|---|
| TNF‐α | TNF‐α−/− mice were less susceptible to MHV‐3 and have improved survival | TNF signaling plays an important role in the pathology of coronavirus mouse hepatitis virus. Interruption of this signaling pathway could be useful for clinical therapy | 11 | |
| TNF receptor |
TNFR1−/−mice were less susceptible to MHV‐3 and had improved survival. TNFRs null mutant mice that were infected with SARS‐CoV were protected from weight loss associated with infection |
Signaling through TNF receptors is implicated in promoting coronaviruses pathogenesis, presumably through excessive inflammation | 11, 12 | |
| IL‐17RA |
IL‐17RA−/− were less susceptible to influenza virus with decreased morbidity and mortality. IL‐17RA knockout protected mice from lung damage |
IL‐17RA is dispensable for the recruitment of CD8+ T cells specific for influenza. IL‐17 signaling in fact plays a key role in promoting a neutrophil response which leads to excessive inflammation in some viral infections | 32 | |
| IL‐12 |
IL‐12 (p35−/−) mice were less susceptible to JHMV. IL‐12 (p35−/−) mice had same susceptibility to MHV as WT |
IL‐12 enhances the magnitude of the inflammatory response in the viral infections after infection, albeit without affecting viral control. MHV‐infected mice lacking IL‐12 produced a polarized Th1‐type cytokine response |
23, 24 | |
| IL‐12/23 | IL‐12 and IL‐23 (p40−/−) mice were less susceptible to JHMV | Reduced morbidity in infected IL‐12‐deficient mice | 24 | |
| IL‐23 | IL‐23 (p19−/−) mice had similar susceptibility to JHMV as WT | IL‐23 appears to be dispensable for the recruitment of specific antiviral immune response | 24 | |
| CD20 | Likely more susceptible. Neutralizing Ab response to adeno‐associated virus was significantly reduced in CD20−/− mice | Reduced humoral immunity to adeno‐associated viral antigens | 16, 17 | |
| IL‐1R | IL‐1R1−/− mice had reduced inflammatory lung pathology but more mortality to influenza virus |
IL‐1R1−/− mice or IL‐1R antagonist (IL‐1Ra) treated mice show reductions in MHV‐3 viral replication, disease progress, and mortality. MyD88−/− mice (defective IL‐1 signaling) were more susceptible to SARS‐CoV virus |
Optimal IL‐1R signaling and inflammatory cell recruitment to the lung appear to be required for protection | 36, 37, 111 |
| IL‐4 | IL‐4−/− or IL‐4 overexpressed mice had same susceptibility to RSV as WT. Overexpression of IL‐4 delayed viral clearance | Absence of IL‐4 signaling does not seem to affect susceptibility to some viruses | 40 |
TNF, tumor necrosis factor; TNFR1, tumor necrosis factor receptor 1; SARS‐CoV, severe acute respiratory syndrome coronavirus 2; IL‐17RA, IL‐17 receptor antagonist; JHMV, JHM strain of mouse hepatitis virus, a neurotropic coronavirus; MHV, mouse hepatitis virus, a coronavirus; RSV, respiratory syncytial virus; WT, wildtype.
Table 2.
Infection risk associated with biologics reported in randomized clinical trial (RCT)
| Drug | Type of biologic | Trial | Serious infections | URTI | Nasopharyngitis | References |
|---|---|---|---|---|---|---|
| Adalimumab (TNF inhibitor) | Fully human recombinant monoclonal antibody | NCT00237887 | 0.6% of 814 pts vs. 1% of 398 controls | 7.2% of 814 pts vs. 3.5% of 398 controls | 5.3% of 814 pts vs. 6.5% of 398 controls | 112 |
| Certolizumab (TNF inhibitor) | Human IgG1 monoclonal antibody | CIMPASI‐1 (NCT02326298) and CIMPASI‐2 (NCT02326272) | 1.1% of 87 pts vs. 0% of 49 controls | 9.1% of 88 pts vs. 5.9% of 51 controls | 20.5% of 88 pts vs. 13.7% of 51 controls |
Data of higher dose (400 mg) |
| Etanercept (TNF inhibitor) | Dimeric fully human fusion protein receptor (TNF type II receptor linked to IgG1 Fc region) |
ERASURE and FIXTURE Etanercept Psoriasis Study Group |
Not reported 0% of 194 pts vs. 0.52% of 193 controls |
5.6% of 323 pts vs. 0.9% of 327 controls 12.9% of 194 pts vs. 13% of 193 controls |
26.6% of 323 pts vs. 8% of 327 controls Not reported |
Data of higher dose (50 mg) |
| Infliximab (TNF inhibitor) | Chimeric (25% mouse; 75% human) monoclonal antibody (IgG) | EXPRESS | Not reported | 15% of 298 pts v 16% of 76 controls | 6% of 298 pts vs. 8% of 76 controls | 116 |
| Ustekinumab (IL‐12/23 inhibitor) | Fully human monoclonal antibody against p40 subunit | PHOENIX 1 | 0.8% of 255 pts vs. 0.4% of 255 controls | 7.1% of 255 pts vs. 6.3% of 255 controls | 10.2% of 255 pts vs. 8.6% of 255 controls | 117 |
| Brodalumab (IL‐17 inhibitor) | Fully human monoclonal antibody (IgG2) |
AMAGINE‐2 (NCT01708603) AMAGINE‐3 (NCT01708629) |
0.3% of 612 pts vs. 0.3% of 309 controls 0.3% of 622 pts vs. 0.6% of 313 controls |
5.4% of 612 pts vs. 7.4% of 309 controls 5.3% of 622 pts vs. 5.4% of 313 controls |
7.4% of 612 pts vs. 4.5% of 309 controls 5% of 622 pts vs. 7% of 313 controls |
Data of higher dose (210 mg) |
| Ixekizumab (IL‐17 inhibitor) | Humanized monoclonal antibody (IgG4) | UNCOVER‐2 and UNCOVER‐3 | 2% of 734 pts vs. 2% of 360 controls | 4% of 734 pts vs. 3% of 360 controls | 8% of 734 pts vs. 8% of 360 controls | 119 |
| Secukinumab (IL‐17 inhibitor) | Fully human IgG1 monoclonal antibody | ERASURE and FIXTURE | 1% of 349 pts vs. 1.5% of 247 controls | 2.1% of 326 pts vs. 0.9% of 327 controls | 10.7% of 326 pts vs. 8% of 327 controls |
Data of higher dose (300 mg) |
| Guselkumab (IL‐23 inhibitor) | Human immunoglobulin G1 lambda (IgG1λ) monoclonal antibody | VOYAGE 1 (NCT02207231) and VOYAGE 2 (NCT02207244) Phase II and longer‐term safety data |
0.12% of 823 pts vs. 0.24% of 422 controls 0.2% of 494 pts vs. 0.4% of 248 controls |
5% of 823 pts vs. 4.5% of 422 controls 3.2% of 494 pts vs. 4% of 248 controls |
7.9% of 823 pts vs. 7.8% of 422 controls 7.1% of 494 pts vs. 6.5% of 248 controls |
120, 121 |
| Risankizumab (IL‐23 inhibitor) | Fully human IgG monoclonal antibody | UltIMMa‐1 (NCT02684370) and UltIMMa‐2 (NCT02684357) | 0.3% of 304 pts vs. 0% of 102 controls | 5.6% of 304 pts vs. 2% of 102 controls | Not reported | 122 |
| Tildrakizumab (IL‐23 inhibitor) | Humanized, IgG1 κ monoclonal antibody | P05495 (phase II, NCT01225731), reSURFACE 1 (phase III, NCT01722331) and reSURFACE 2 (phase III, NCT01729754) | 0.3% of 708 vs. 0.3% of 355 controls | 3% of 708 vs. 2.8% of 355 controls | 9.3% of 708 vs. 8.2% of 355 controls |
Data of higher dose (200 mg) |
| Rituximab (anti‐CD20) | Chimeric monoclonal antibody against CD20 | REFLEX | 2.3% of 308 pts vs. 1.41% of 209 controls | 7.8% of 308 pts vs. 6.7% of 209 controls | 7.5% of 308 pts vs. 5.7% of 209 controls | 20 |
| Anakinra (IL‐1 inhibitor) | IL‐1 receptor antagonist (recombinant human) | 990145 Study Group | 2.1% of 1,116 pts vs. 0.4% of 283 controls | 21% of 250 pts vs. 16 % of 251 controls | Not reported | 39, 124 |
| Dupilumab (IL‐4/13 inhibitor) | Fully human IgG4 monoclonal antibody directed against IL‐4 receptor α subunit | LIBERTY AD SOLO 1 (NCT02277743) and LIBERTY AD SOLO 2 (NCT02277769) | 0.9% of 465 pts vs. 2.2% of 456 controls | 2.8% of 465 pts vs. 2.2% of 456 controls | 9% of 465 pts vs. 8.6% of 456 controls | 41 |
| Omalizumab | Recombinant IgG antibody against IgE |
ASTERIA I (NCT01287117) ASTERIA II and GLACIAL |
Not reported | 3.4% of 412 pts vs. 2.1% of 242 controls | 6.6% of 412 pts vs. 7% of 242 controls |
Data of higher dose (300 mg) |
| IVIg | Immunoglobulins (mainly IgG) | NCT01545076 | Not reported | 3% of 58 pts vs. 4% of 57 controls | 3% of 58 pts vs. 2% of 57 controls |
Data of higher dose |
URTI, upper respiratory tract infection; TNF, tumor necrosis factor; IgG, immunoglobulin G; Fc, fragment crystallizable; IVIg, intravenous immunoglobin; pts, patients.
CD20
The B‐lymphocyte antigen CD20 is highly expressed on B cells starting at the pre‐B‐cell stage and on mature B cells, and it is downregulated during terminal differentiation into plasma cells. While the precise function of CD20 is not fully elucidated, IgM expression in immature and mature B cells from CD20‐deficient mice was markedly reduced compared to wildtype. 16 Furthermore, reduced humoral immunity to adeno‐associated viral antigens was demonstrated in CD20‐deficient mice. 17 A patient who lacked CD20 expression due to homozygous mutations reported intermittent respiratory infections, associated with persistent hypogammaglobulinemia and strong reductions in circulating memory B cells. 18 No significant differences in URTI, nasopharyngitis, bronchitis, cough, and sinusitis between rituximab (anti‐CD20) 19 and placebo were demonstrated in a double‐blind RCT for rheumatoid arthritis (RA). 20 However, in a prospective, open‐label RCT, it was noted that lung infections/pneumonia were higher in the rituximab treatment arm by more than twofold (11% vs. 5% in control, no confidence intervals were presented). 21 The role of CD20+ cells in presenting antigen to T cells and in generation of antibodies to protect from new infections remains unclear.
IL‐12/23
The IL‐12/IL‐23 common pathway plays a key role in the induction of inflammation in adaptive immune responses, where IL‐12 induces a Th1 immune response with a downstream induction of cytokines such as TNF, interferon (IFN)‐γ, and IL‐23 promotes a Th17 immune response through the induction of inflammatory cytokines such as IL‐17 and IL‐22. 22 Mice defective in both IL‐12/23 (p40−/−) and IL‐12 alone (p35−/−) were infected with a murine coronavirus (MHV). 23 IL‐12 and IL‐12/23 knockout mice had similar survival to wild‐type animals. 23 Therefore, IL‐12 does not seem to contribute to antiviral function or survival. Mice deficient in IL‐23 alone (p19−/−) were infected with murine coronavirus, and viral control was similar to wild‐type mice, demonstrating that IL‐23 does not significantly confer protection from infection. 24 This was also demonstrated thorough neutralization of mice using anti‐IL‐23p19‐specific and anti‐IL‐12/23p40 antibodies, followed by infection of mice with MHV. 25 In the absence of IL‐12/23 signaling, specific antiviral T‐cell response was intact. 25 Clinical trials using IL‐12/23 or IL‐23 inhibitors demonstrated no significant increase in respiratory adverse events (Table 2). Furthermore, the PSOLAR study reported that ustekinumab had no increased risk of serious infections. 13 Of note, a recent case study reported COVID‐19 in a patient during IL‐23 inhibitor (guselkumab) treatment for psoriasis, and the patient had a good outcome. 26
IL‐17
IL‐17 is a proinflammatory cytokine with important roles in T‐cell activation and neutrophil mobilization and activation. 27 IL‐17 expression is induced during influenza infection as part of the Th1 immune response that contributes to viral clearance. 28 However, a growing body of evidence suggests that IL‐17 is also associated with promotion of viral infections and tissue pathology. This is thought to occur through direct suppression of IFN‐γ and the pivotal regulators of Th1‐cell development T‐bet and eomesodermin. 29 , 30 IL‐17 in some settings was shown to induce tissue pathology in response to viral infections through neutrophil infiltration. Mouse models developed increased IL‐17A‐dependent lung pathology upon respiratory syncytial virus (RSV) infection. 31 IL‐17RA‐/‐ mice challenged with influenza had decreased morbidity and mortality, and this correlated with decreased levels of proinflammatory cytokines including TNF‐α, IL‐1β, and IL‐6. 32 In humans, chronic mucocutaneous candidiasis has been attributed to the disruption of Th1 and Th17 pathways. This was illustrated in patients with identified mutations in IL‐17RA and STAT1 genes. 33 These patients have no increased risk of viral infections. 34 Clinical trials using IL‐17 inhibitors demonstrated no significant increase in respiratory adverse events (Table 2). A recent case report reported a patient receiving therapy with an IL‐17 inhibitor (ixekizumab) who was completely asymptomatic but tested positive for COVID‐19. 35
IL‐1
IL‐1 is a key player in the regulation of inflammation. IL‐1 signaling may enhance or attenuate viral replication depending on the setting. Mice deficient in MyD88, an adapter protein that mediates Toll‐like receptor (TLR), IL‐1R, and IL‐18R signaling, are more susceptible to SARS‐CoV infection. 36 On the other hand, mice that were infected with MHV‐3 had high levels of IL‐1β in the serum and liver. 37 IL‐1β receptor‐I deficient (IL‐1R1 ‐/‐ ) or IL‐1R antagonist (IL‐1Ra)‐treated mice infected with MHV‐3 showed attenuation in viral replication and mortality, demonstrating that IL‐1 may contribute to the pathogenesis of coronavirus in mice. 37 Patients with unopposed activation of IL‐1 due to recessive mutations in IL1RN, the gene encoding IL‐1–receptor antagonist, had elevated levels of proinflammatory cytokines TNF‐α, IL‐6, and IL‐17, and some of these patients presented with respiratory distress. 38 Treatment of these patients with IL‐1 receptor antagonist decreased mortality. 38 The use of anakinra in clinical trials was associated with a slightly higher frequency of serious infectious episodes, primarily pneumonia (2.1% vs. 0.4%, comparative risk 5.25), than the placebo group. 39 It appears that normal IL‐1 expression/function is required to mount an optimal antiviral immune response.
IL‐4
IL‐4 is a key regulator in humoral and Th2 adaptive immunity. Mouse models demonstrated that the constitutive overexpression of IL‐4 prior to RSV infection delayed viral clearance, increased the density of the lymphocytic infiltrate in the lungs, and diminished induction of primary cytotoxic T lymphocyte responses. 40 Conversely, IL‐4−/− mice cleared RSV readily after primary infection, with minimal pathology. 40 A pooled analysis of two phase III RCTs demonstrated safety of dupilumab, where URTIs, nasopharyngitis, and severe infection rates were comparable to the placebo group. 41 Recently, several case reports demonstrated no evidence of increased risk for COVID‐19 infection in patients treated with dupilumab. 42 , 43 , 44 , 45
Anti‐immunoglobulin E
Anti‐IgE biologics (e.g., omalizumab) block IgE molecule binding to receptors on mast cells and basophils and are approved for urticaria. Omalizumab was shown in multiple trials to be a safe biological therapy with no significant increase in adverse respiratory events. 46 , 47 , 48
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIg) is used for several dermatological diseases. IVIg has been shown to have a good safety profile with no significant increase in the rates of nasopharyngitis and URTI. 49 , 50 Of note, a clinical trial on IVIg and pemphigus demonstrated that the incidence of adverse drug reactions was 6/21(28.6%) in the 400 mg/kg/day group and 7/20 (35.0%) in the 200 mg group including one URTI vs. 5/20(25.0%) in the placebo group. 51
Parts 3: Non‐biologic systemic agents and risk of infection
Cyclosporine
Cyclosporine is a calcineurin inhibitor that blocks IL‐2 signaling and T‐cell proliferation. 52 , 53 The most common infectious side effects from cyclosporine were flu‐like symptoms seen in 15% of patients enrolled in an RCT for chronic idiopathic urticaria. 54 Psoriasis registries examining cyclosporine reported infection rates of 8.1–17.7 infections per 100 patient‐years 55 , 56 , 57 with severe or serious infection rates of 1.4 and 2.0 per 100 patient‐years, slightly higher than comparators. 56 , 57 Of note, cyclosporine has been shown to inhibit the replication of diverse coronaviruses including SARS as demonstrated by in vitro experiments. 58 , 59
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is an antimetabolite that blocks B‐cell and T‐cell maturation. 60 , 61 Most reported trials examined MMF with an oral corticosteroid or other steroid‐sparing agent. Trials that combined MMF with corticosteroids had significantly higher rates of infections, up to 59%. 62 MMF is reported to increase patients’ susceptibility to viral infections, 63 and an increase in nasopharyngitis and URTIs was noted comparing prednisone plus MMF to prednisone monotherapy in pemphigus vulgaris. 62 Of note, MMF was used to treat eight patients with MERS with a 100% survival rate; however, when analyzing the severity of illness and treatment, MMF was given to less severely ill patients. 64
Azathioprine
Azathioprine inhibits purine synthesis and downregulates B‐cell and T‐cell function. 65 , 66 Documented types of infection with use of azathioprine include lower respiratory tract infections (LRTI) and URTI, which had rates of 5% and 5–20%, respectively. 67 , 68 Thirty‐six percent of patients in one study had infections of moderate intensity. 69 There were no registries evaluating the prevalence of infections during azathioprine therapy for dermatologic uses. One systematic review evaluating the off‐label use of azathioprine found mild infections reported in 0.36% of patients and severe infections in only 0.30% of patients 70 (Table 3).
Table 3.
Trial data on systemic medications and the risk of infection
| Trial | Trial Type | Type of infectious risk assessed | Number | |
|---|---|---|---|---|
| Cyclosporine | Grattan et al. 54 | Randomized, double‐blind, placebo controlled | URTI | 10% of 20 vs. not reported/10 placebo |
| Flu‐like symptoms | 15% of 20 vs. not reported/10 placebo | |||
| Vena et al. 125 | Randomized, double‐blind, placebo controlled | Infections | 3.2% of 62 vs. 8.6% of 35 | |
| Karanikolas et al. 126 | Non‐randomized, unblinded, ADA vs. CsA | Any infection | 3.5% CsA of 57 vs. 10.3% of 58 ADA | |
| Any serious infection | 0% of 57 CsA vs. 1.7% of 58 ADA | |||
| URTI | 1.8% of 57 CAsA vs. 8.6% of 58 ADA | |||
| Lai et al. 127 | Randomized, double‐blind, placebo controlled | Infections (UTI a ) | 5.6% of 18 vs. 0% of 18 placebo | |
| Mycophenolate mofetil | Beissert et al. 128 | Randomized, non‐blinded, methylpred + MMF vs. methylpred + AZA | Grade 3 Infections (severe) b | 11% of 35 Methylpred + MMF vs. 0% of 38 Methylpred + AZA |
| Grade 4 Infections (life threatening) | 0% of 35 Methylpred + MMF vs. 3% of 38 Methylpred + AZA | |||
| Beissert et al. 62 | Randomized non‐blinded, Prednisone (Pred) + MMF vs. Pred monotherapy c | Nasopharyngitis | 12% of 58 Pred + MMF vs. 0% of 36 Pred | |
| URTI | 10% of 58 Pred + MMF vs. 3% of 36 Pred | |||
| Influenza viral | 0% of 58 Pred + MMF vs. 3% of 36 Pred | |||
| LRTI | 3% of 58 Pred + MMF vs. 0% of 36 Pred | |||
| Overall Infections | 59% of 58 Pred + MMF vs. 36% of 36 Pred P = 0.04 | |||
| Akhyani et al. 129 | Randomized, open‐label MMF vs. MTX | Infections d | 0% of 20 vs. 0% of 18 MTX | |
| Ioannides et al. 130 | Randomized, non‐blinded, methylpred vs. methylpred + MMF | Internal Infection | 8% of 24 Methylpred + MMF vs. 4% of 23 Methylpred (P = 1.0000) | |
| Zhou et al. 131 | Open‐label | Infection | 0% of 23 | |
| Azathioprine | Meggitt et al. 68 | Randomized, double‐blind, placebo controlled | LRTI | 5% of 41 vs. 0% of 20 |
| URTI | 5% of 41 vs. 5% of 20 | |||
| Berth‐Jones et al. 67 | Double blind, randomized, placebo crossover | URTI | 20% of 25 vs. 8% of 25 | |
| Schram et al. 69 | Randomized, single blind compared to methotrexate | Infection | 70% of 22 vs. 64% of 20 MTX | |
| Moderate intensity infection | 36% of 22 vs. 25% of 20 MTX | |||
| Methotrexate | METOP 73 | Randomized, double‐blind, placebo‐controlled | Any infection | 44% of 91 weeks 0–16 and 41% of 76 weeks 16–52 vs. 45% of 29 weeks 0–16 placebo |
| Serious infection | 0% of 91 vs. 3% of 29 placebo | |||
| Pasnoor et al. 74 | Randomized, double‐blind, placebo‐controlled | Infection | 16% of 175 vs. 11% of 161 placebo | |
| Kingsley et al. 75 | Randomized, double‐blind, placebo‐controlled | Respiratory tract infection | 28% of 109 vs. 22% of 112 placebo | |
| Apremilast | UNVEIL 84 | Double‐blind, placebo‐controlled, 52 weeks | Nasopharyngitis | 10% of 211 vs. N/A placebo |
| URTI | 7% of 211 vs. N/A placebo | |||
| LIBERATE 85 | Randomized, double‐blind, Aprem vs. Enbrel vs. placebo with Aprem extension a | URTI | 7% of 74 vs. 7% of 73 placebo/Aprem | |
| Nasopharyngitis | 3% of 74 vs. 6% of 73 placebo/Aprem | |||
| Bronchitis | 5% of 74 vs. 1% of 73 placebo/Aprem | |||
| Bissonette et al. 86 | Randomized, double‐blind, placebo‐controlled | URTI | 26% of 53 vs. 14% of 50 placebog | |
| Bronchitis | 6% of 50 vs. 0% of 50 placebo | |||
| ESTEEM 1 87 | Randomized, double‐blind, placebo‐controlled | URTI |
10% of 560 EAIR/100 py = 37.6 vs. 7% of 282 EAIR/100 py = 27.3 placebo |
|
| Nasopharyngitis |
7% of 560 EAIR/100 py = 26.6 vs. 8% of 282 EAIR/100 py = 30.1 |
|||
| ESTEEM 2 88 | Randomized, double‐blind, placebo‐controlled | URTI | 5% of 272 EAIR/100 py = 17.3 vs. 4% of 136 EAIR/100 py = 16.7 | |
| Nasopharyngitis |
7% of 272 EAIR/100 py = 27.3 vs. 4% of 136 EAIR/100 py = 16.9 placebo |
|||
| Any type of infection | 25% vs. 21% placebo | |||
| Vossen et al. 88 | Randomized, double‐blind, placebo‐controlled | Common cold | 26% of 15 vs. 20% of 5 placebo | |
| Thalidomide | Droitcourt et al. 132 | Randomized, double‐blind, placebo‐controlled | Cough and fever | 5% of 20 vs. 0% of 19 placebo |
| Kaur et al. 133 | Randomized, double‐blind, thalidomide vs. prednisolone | Infection d , e | 0% of 30 vs. 0% of 30 prednisolone | |
| Lazzerini et al. 134 | Randomized, double‐blind, placebo‐controlled | Infection d | 0% of 12 vs. 0% of 31 placebo | |
| Hamuryudan et al. 135 | Randomized, double‐blind, placebo‐controlled | Infection d | 0% of 63 vs. 0% of 32 placebo |
URTI, upper respiratory infection; ADA, adalimumab; CsA, Cyclosporine; UTI, urinary tract infection; MEP, methylprednisolone; MMF, mycophenolate mofetil; AZA, azathioprine; Pred, prednisone; LRTI, lower respiratory infection; MTX, methotrexate; Aprem, apremilast; EAIR, exposure‐adjusted incidence rate; py, patient years.
Urinary tract infection.
Three infections were URTIs; one infection was recurrent HSV.
No patients withdrew due to infection.
No infections reported in paper.
One patient had amoebic dysentery within 2 weeks of initiation of study and stopped therapy.
Methotrexate
The use of methotrexate (MTX), 71 a folic acid antagonist that inhibits nucleotide synthesis, 72 had slightly increased risk of infections ranging from 16 to 44% vs. 3 to 45% compared to placebo in three RCTs. 73 , 74 , 75 A large cardiovascular trial using 15–20 mg doses of methotrexate showed rates of serious infection were similar to the placebo group. 76 A review of infectious risks in rheumatoid arthritis (RA) patients indicated that although MTX has previously been implicated not only with increased risk of infection but also increased severity, the evidence was not clear. 77 The review concluded that MTX appears to be associated with minimal, if any, increased infection risk in the RA population. 77
Hydroxychloroquine
Hydroxychloroquine is an antimalarial medication that inhibits lysosomal functions and interferes with a myriad of immune pathways. 78 Its exact mechanism in many dermatologic processes has never been fully elucidated. Hydroxychloroquine has been shown to have a favorable side effect profile in terms of infection risk in many clinical trials. 79 , 80 It is currently under investigation in numerous phase 2 clinical trials as treatment for COVID‐19 as it may inhibit viral fusion to the host cell and inhibit viral assembly and release. 81
Apremilast
Apremilast is a phosphodiesterase 4 (PDE4) inhibitor, 82 with side effects including nasopharyngitis and URTI. 83 The incidence of URTI in the apremilast‐treated groups is comparable to placebo ranging from 4.8 to 26.0% and 4.4 to 14.0%, with higher rates being accounted for from one study examining apremilast in palmoplantar psoriasis (Table 3). Overall, rates of infection were not increased in patients treated with apremilast. 84 , 85 , 86 , 87 , 88 , 89 A recent case was reported of a patient with erythrodermic psoriasis, with contraindication to most treatments due to a recurrent brain oligodendroglioma who had psoriasis partially controlled on apremilast. The patient contracted COVID‐19 while on apremilast treatment and has fully recovered despite being at high risk of complications from COVID‐19 (obesity, recent chemotherapy, and active malignancy); his apremilast treatment was not interrupted. 90
Thalidomide
Thalidomide, 91 an immunomodulatory drug with a range of activity that is not fully characterized, 92 is effective for various refractory dermatoses, but its side effect profile is unfavorable, and risks of teratogenicity and neuropathy often preclude its use. 91 Table 3 highlights four RCTs where there was no increased risk of infection in thalidomide compared to placebo.
Oral corticosteroids
Prolonged use of oral corticosteroids is generally avoided due to side effects. 93 None of the following studies reported infection as an adverse reaction. 94 , 95 , 96 , 97 A meta‐analysis including 2,382 patients from 28 studies showed a rate of infectious adverse events of 9% in all patients (AE/100 py = 12, 95% CI: 8–16). 98 Pooled data from 71 RCTs for steroids vs. no steroids found the relative risk of infections was increased by 60% (95% CI 30–90) for those receiving steroids (Table 4). 99 In a large cohort of patients with inflammatory bowel disease that was collected through an international registry, the outcomes of the use of high‐dose corticosteroids, among other immunosuppressives, in COVID‐19‐positive patients was evaluated. 100 The study demonstrated a strong positive association between systemic corticosteroid use and increased mortality/ICU admission of COVID‐19 patients. The study also indicated that TNF antagonist, methotrexate, and IL‐12/23 inhibitors do not appear to be associated with severe COVID‐19. 100 We note that the effects of low‐dose dexamethasone against COVID‐19 are currently being evaluated in the RECOVERY trial. 101
Table 4.
Registry, databases, systematic reviews, and meta‐analyses on systemic medications and the risk of infection
| Level of evidence | Type of infectious risk assessed | Outcome | |
|---|---|---|---|
| Cyclosporine | Biobadaderm Registry 55 2019 | Infections and infestations | Incidence per 1,000 py = 177 (136–231) |
| Biobadaderm Registry 57 2017 | Infection | Rate/1,000 py = 171.6 (127.3–231.4) | |
| Serious and deadly infections | Rate/1,000 py = 20 (8.3–47.9) | ||
| PsoBest Registry 56 | Infections (non‐severe a ) | Rate/100 py = 8.1 [95% CI 5–13] | |
| Infections (severe b ) | Rate/100 py = 1.4 [95% CI 0.25–4] c | ||
| Schmitt et al. 136 Meta‐analysis | Infections | 0–12% per month of treatment | |
| Mycophenolate mofetil | Sparse data | ||
| Azathioprine | Sood et al. 137 Prospective database | Flu‐like illness | 13/255 (5%) |
| Schram et al. 70 Systematic review | Mild infection | 36/1,128 (0.36%) | |
| Severe infection | 3/1,128 (0.3%) | ||
| Methotrexate | Biobadaderm Registry 55 2019 | Infections and infestations | Incidence per 1,000 patient years = 112 (98–129) |
| Biobadaderm Registry 128 2017 | Infection | Rate/1,000 py = 113.1 (95.2–134.3) | |
| Serious and fatal infection d | Rate/1,000 py = 9.6 (5.3–17.3) | ||
| SDNTT Registry 138 | Infections | 0/66 (0%) | |
| PsoBest Registry 56 | Infections (non‐severe a ) | Rate/100 py = 6 (95% CI 5–8) c | |
| Infections (severe b ) | Rate/100 py = 0.75 (95% CI 0.25–1.50) c | ||
| Apremilast | Biobadaderm Registry 55 | Infections and infestations | Incidence per 1,000 patient years = 105 (95% CI 64–175) |
| Papadavid et al. 139 Prospective observational | Infection | 3/50 (6.0) | |
| Thalidomide | Sparse data | ||
| Systemic Corticosteroids | Hoes et al. 98 Meta‐analysis (low‐ to medium‐dose oral glucocorticoids) | Infections | 9% AE/100 py = 12 (95% CI 8–16) |
| Non‐biologic Systemics | Rate/1,000 py (95% CI) | ||
| Biobadaderm Registry 140 | All infections | 88.35 (75.19–103.15) | |
| Serious infections | 9.80 (5.90–15.31) | ||
| Clalit Database 140 | All infections | 48.14 (42.50–54.32) | |
| Serious infections | 32.6 (28.00–37.67) | ||
| Psocare Registry 140 | All infections | 21.77 (17.00–37.46) | |
| Serious infections | 12.21 (8.73–16.63) |
py, patient years; CI, confidence interval; AE, adverse event.
Non‐severe infections: all other.
Severe infections: requiring antibiotics, inpatient stay or life‐threatening.
Estimated from a bar graph.
Serious infections: resulted in death, life‐threatening, required prolonged hospitalization, caused persistent disability.
Part 4: Non‐biologic agents in transplant recipients with coronavirus
It is known that transplant patients are at higher risk of severe infections, including more severe and complicated influenza. 102 However, coronaviruses have not been shown to cause more severe disease in transplant recipients compared to other common viruses such as adenovirus and rhinovirus. 103
COVID‐19 in transplant recipients
Immunosuppression is not a comorbidity that is commonly reported in COVID‐19 patients despite it commonly being referred to as a risk factor. 104 The limited data do not suggest increased risk of severe complications compared to the general population. Lei et al. 105 reported two heart transplant patients in China who survived COVID‐19 infections. Two reported renal transplant patients who contracted COVID‐19 and succumbed to the illness had similar clinical courses compared to non‐transplant patients. 106 Transplant recipients may practice more stringent physical distancing practices compared to the general population, resulting in falsely low numbers.
SARS in transplant recipients
The literature surrounding SARS and transplant recipients is sparse. Risk factors for severe SARS included hypertension, diabetes, coronary heart disease, hepatitis, and pregnancy with a mortality rate with ≥1 risk factor compared to none of 54.5% vs. 7.5%; P < 0.01. 107 There is no evidence that suggests transplant recipients had poorer outcome in the SARS epidemic.
MERS in transplant recipients
A retrospective cohort study of a MERS outbreak in Korea revealed that the number of affected immunosuppressed patients was low and did not identify any transplant patients. 108 Immunosuppression was not identified as a poor prognostic factor in MERS infection. 109
Closing remarks
Immunomodulatory regimens have revolutionized the treatment of dermatological diseases. With the current COVID‐19 pandemic, it is imperative to examine the evidence and conduct a risk–benefit analysis for each patient. There may be patients who require more or less treatment, for instance some patients with existing comorbidities may require a more conservative approach. 110 The greatest risk of infections in biologics appear to occur with CD20 inhibition (Fig. 1). For non‐biologic immunotherapies, the greatest risk of infection appears to occur with the use of high doses of oral corticosteroids. A slight increased infection risk is seen with cyclosporine, although cyclosporine has been shown to inhibit coronavirus replication and did not increase susceptibility in transplant patients.
Figure 1.
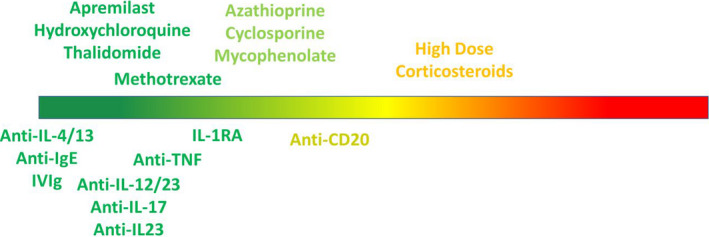
A pictorial representation of COVID‐19 risk assessment of dermatologic treatments where green represents "safe" and red represents "higher risk"
Acknowledgments
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. JPD is a Senior Scientist at BC Children's Hospital Research Institute.
Conflict of interest: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding source: None.
References
- 1. Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease‐2019 (COVID‐19): the epidemic and the challenges. Int J Antimicrob Agents 2020; 55: 105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conforti C, Giuffrida R, Dianzani C, et al. Zalaudek. COVID‐19 and psoriasis: is it time to limit treatment with immunosuppressants? A call for action. Dermatol Ther 2020: e13298. 10.1111/dth.13298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lebwohl M, Rivera‐Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID‐19? J Am Acad Dermatol 2020; 82(5): 1217–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis 2020: ciaa248. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004; 136: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahallawi WH, Khabour OF, Zhang Q, et al. MERS‐CoV infection in humans is associated with a pro‐inflammatory Th1 and Th17 cytokine profile. Cytokine 2018; 104: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J 2020; 133: 1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi Y, Wang Y, Shao C, et al. COVID‐19 infection: the perspectives on immune responses. Cell Death Differ 2020; 27: 1451–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu H, Li H, Cao D, et al. Tumor necrosis factor alpha (TNF‐alpha) receptor‐I is required for TNF‐alpha‐mediated fulminant virus hepatitis caused by murine hepatitis virus strain‐3 infection. Immunol Lett 2014; 158: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McDermott JE, Mitchell HD, Gralinski LE, et al. The effect of inhibition of PP1 and TNFalpha signaling on pathogenesis of SARS coronavirus. BMC Syst Biol 2016; 10: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the psoriasis longitudinal assessment and registry (PSOLAR). JAMA Dermatol 2015; 151: 961–969. [DOI] [PubMed] [Google Scholar]
- 14. Hussell T, Pennycook A, Openshaw PJ. Inhibition of tumor necrosis factor reduces the severity of virus‐specific lung immunopathology. Eur J Immunol 2001; 31: 2566–2573. [DOI] [PubMed] [Google Scholar]
- 15. A randomized, open‐label, controlled trial for the efficacy and safety of Adalimumab Injection in the treatment of patients with severe novel coronavirus pneumonia (COVID‐19). Shanghai, China: Chinese Clinical Trial Registry: ChiCTR2000030089. 2020. Available at: http://www.chictr.org.cn/showprojen.aspx?proj=49889.
- 16. Uchida J, Lee Y, Hasegawa M, et al. Mouse CD20 expression and function. Int Immunol 2004; 16: 119–129. [DOI] [PubMed] [Google Scholar]
- 17. Morsy DE, Sanyal R, Zaiss AK, et al. Reduced T‐dependent humoral immunity in CD20‐deficient mice. J Immunol 2013; 191: 3112–3118. [DOI] [PubMed] [Google Scholar]
- 18. Kuijpers TW, Bende RJ, Baars PA, et al. CD20 deficiency in humans results in impaired T cell‐independent antibody responses. J Clin Invest 2010; 120: 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gleghorn K, Wilson J, Wilkerson M. Rituximab: uses in dermatology. Skin Therapy Lett 2016; 21: 5–7. [PubMed] [Google Scholar]
- 20. Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti‐tumor necrosis factor therapy: results of a multicenter, randomized, double‐blind, placebo‐controlled, phase III trial evaluating primary efficacy and safety at twenty‐four weeks. Arthritis Rheum 2006; 54: 2793–2806. [DOI] [PubMed] [Google Scholar]
- 21. Joly P, Maho‐Vaillant M, Prost‐Squarcioni C, et al. First‐line rituximab combined with short‐term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel‐group, open‐label randomised trial. Lancet 2017; 389: 2031–2040. [DOI] [PubMed] [Google Scholar]
- 22. Teng MW, Bowman EP, McElwee JJ, et al. IL‐12 and IL‐23 cytokines: from discovery to targeted therapies for immune‐mediated inflammatory diseases. Nat Med 2015; 21: 719–729. [DOI] [PubMed] [Google Scholar]
- 23. Schijns VE, Haagmans BL, Wierda CM, et al. Mice lacking IL‐12 develop polarized Th1 cells during viral infection. J Immunol 1998; 160: 3958–3964. [PubMed] [Google Scholar]
- 24. Kapil P, Atkinson R, Ramakrishna C, et al. Interleukin‐12 (IL‐12), but not IL‐23, deficiency ameliorates viral encephalitis without affecting viral control. J Virol 2009; 83: 5978–5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Held KS, Glass WG, Orlovsky YI, et al. Generation of a protective T‐cell response following coronavirus infection of the central nervous system is not dependent on IL‐12/23 signaling. Viral Immunol 2008; 21: 173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Messina F, Piaserico S. SARS‐CoV‐2 infection in a psoriatic patient treated with IL‐23 inhibitor. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cypowyj S, Picard C, Marodi L, et al. Immunity to infection in IL‐17‐deficient mice and humans. Eur J Immunol 2012; 42: 2246–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma WT, Yao XT, Peng Q, et al. The protective and pathogenic roles of IL‐17 in viral infections: friend or foe? Open Biol 2019; 9: 190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mukherjee S, Lindell DM, Berlin AA, et al. IL‐17‐induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol 2011; 179: 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuan J, Yu M, Lin QW, et al. Th17 cells contribute to viral replication in coxsackievirus B3‐induced acute viral myocarditis. J Immunol 2010; 185: 4004–4010. [DOI] [PubMed] [Google Scholar]
- 31. Reed M, Morris SH, Owczarczyk AB, et al. Deficiency of autophagy protein Map1‐LC3b mediates IL‐17‐dependent lung pathology during respiratory viral infection via ER stress‐associated IL‐1. Mucosal Immunol 2015; 8: 1118–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crowe CR, Chen K, Pociask DA, et al. Critical role of IL‐17RA in immunopathology of influenza infection. J Immunol 2009; 183: 5301–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin‐17 immunity. Science 2011; 332: 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van de Veerdonk FL, Plantinga TS, Hoischen A, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med 2011; 365: 54–61. [DOI] [PubMed] [Google Scholar]
- 35. Balestri R, Rech G, Girardelli CR. SARS‐CoV‐2 infection in a psoriatic patient treated with IL‐17 inhibitor. J Eur Acad Dermatol Venereol 2020. in press. 10.1111/jdv.16571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sheahan T, Morrison TE, Funkhouser W, et al. MyD88 is required for protection from lethal infection with a mouse‐adapted SARS‐CoV. PLoS Pathog 2008; 4: e1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guo S, Yang C, Diao B, et al. The NLRP3 inflammasome and IL‐1beta accelerate immunologically mediated pathology in experimental viral fulminant hepatitis. PLoS Pathog 2015; 11: e1005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aksentijevich I, Masters SL, Ferguson PJ, et al. An autoinflammatory disease with deficiency of the interleukin‐1‐receptor antagonist. N Engl J Med 2009; 360: 2426–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fleischmann RM, Schechtman J, Bennett R, et al. Anakinra, a recombinant human interleukin‐1 receptor antagonist (r‐metHuIL‐1ra), in patients with rheumatoid arthritis: a large, international, multicenter, placebo‐controlled trial. Arthritis Rheum 2003; 48: 927–934. [DOI] [PubMed] [Google Scholar]
- 40. Fischer JE, Johnson JE, Kuli‐Zade RK, et al. Overexpression of interleukin‐4 delays virus clearance in mice infected with respiratory syncytial virus. J Virol 1997; 71: 8672–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thaçi D, L. Simpson E, Deleuran M, et al. Efficacy and safety of dupilumab monotherapy in adults with moderate‐to‐severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J Dermatol Sci 2019; 94(2): 266–275. [DOI] [PubMed] [Google Scholar]
- 42. Ferrucci S, Romagnuolo M, Angileri L, et al. Safety of dupilumab in severe atopic dermatitis and infection of Covid‐19: two case reports. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carugno A, Raponi F, Locatelli AG, et al. No evidence of increased risk for COVID‐19 infection in patients treated with Dupilumab for atopic dermatitis in a high‐epidemic area ‐ Bergamo, Lombardy, Italy. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Caroppo F, Biolo G, Belloni Fortina A. SARS‐CoV‐2 asymptomatic infection in a patient under treatment with dupilumab. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Forster‐Ruhrmann U, Szczepek AJ, Claus Bachert H, et al. COVID‐19 infection in a patient with severe chronic rhinosinusitis with nasal polyps during therapy with dupilumab. J Allergy Clin Immunol 2020. 10.1016/j.jaci.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gimenez‐Arnau AM. Omalizumab for treating chronic spontaneous urticaria: an expert review on efficacy and safety. Expert Opin Biol Ther 2017; 17: 375–385. [DOI] [PubMed] [Google Scholar]
- 47. Maurer M, Rosen K, Hsieh HJ, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med 2013; 368: 924–935. [DOI] [PubMed] [Google Scholar]
- 48. Casale TB, Bernstein JA, Maurer M, et al. Similar efficacy with omalizumab in chronic idiopathic/spontaneous urticaria despite different background therapy. J Allergy Clin Immunol Pract 2015; 3: 743–750.e1. [DOI] [PubMed] [Google Scholar]
- 49. van Schaik IN, Bril V, van Geloven N, et al. Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Neurol 2018; 17: 35–46. [DOI] [PubMed] [Google Scholar]
- 50. Amagai M, Ikeda S, Hashimoto T, et al. A randomized double‐blind trial of intravenous immunoglobulin for bullous pemphigoid. J Dermatol Sci 2017; 85: 77–84. [DOI] [PubMed] [Google Scholar]
- 51. Amagai M, Ikeda S, Shimizu H, et al. A randomized double‐blind trial of intravenous immunoglobulin for pemphigus. J Am Acad Dermatol 2009; 60: 595–603. [DOI] [PubMed] [Google Scholar]
- 52. Spisani S, Fabbri E, Muccinelli M, et al. Inhibition of neutrophil responses by cyclosporin A. An insight into molecular mechanisms. Rheumatology 2001; 40: 794–800. [DOI] [PubMed] [Google Scholar]
- 53. Cyclosporine. Product monograph. Available at: https://pdf.hres.ca/dpd_pm/00014004.PDF. Accessed 13 April, 2020.
- 54. Grattan CE, O'Donnell BF, Francis DM, et al. Randomized double‐blind study of cyclosporin in chronic 'idiopathic' urticaria. Br J Dermatol 2000; 143: 365–372. [DOI] [PubMed] [Google Scholar]
- 55. Dauden E, Carretero G, Rivera R, et al. Long term safety of nine systemic medications for psoriasis: a cohort study using the Biobadaderm Registry. J Am Acad Dermatol 2020. 10.1016/j.jaad.2020.03.033 [DOI] [PubMed] [Google Scholar]
- 56. Reich K, Mrowietz U, Radtke MA, et al. Drug safety of systemic treatments for psoriasis: results from the German Psoriasis Registry PsoBest. Arch Dermatol Res 2015; 307: 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davila‐Seijo P, Dauden E, Descalzo MA, et al. Infections in moderate to severe psoriasis patients treated with biological drugs compared to classic systemic drugs: findings from the BIOBADADERM registry. J Invest Dermatol 2017; 137: 313–321. [DOI] [PubMed] [Google Scholar]
- 58. Tanaka Y, Sato Y, Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses 2013; 5: 1250–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de Wilde AH, Zevenhoven‐Dobbe JC, van der Meer Y, et al. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol 2011; 92: 2542–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Allison AC, Eugui EM. Preferential suppression of lymphocyte proliferation by mycophenolic acid and predicted long‐term effects of mycophenolate mofetil in transplantation. Transplant Proc 1994; 26: 3205–3210. [PubMed] [Google Scholar]
- 61. Mycophenolate mofetil. Product monograph. Available at: https://www.sandoz.ca/sites/www.sandoz.ca/files/Mycophenolate%20Mofetil%20Product%20Monograph.pdf. Accessed 13 April, 2020.
- 62. Beissert S, Mimouni D, Kanwar AJ, et al. Treating pemphigus vulgaris with prednisone and mycophenolate mofetil: a multicenter, randomized, placebo‐controlled trial. J Invest Dermatol 2010; 130: 2041–2048. [DOI] [PubMed] [Google Scholar]
- 63. Saha M, Black MM, Groves RW. Risk of herpes zoster infection in patients with pemphigus on mycophenolate mofetil. Br J Dermatol 2008; 159: 1212–1213. [DOI] [PubMed] [Google Scholar]
- 64. Al Ghamdi M, Alghamdi KM, Ghandoora Y, et al. Treatment outcomes for patients with Middle Eastern Respiratory Syndrome Coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect Dis 2016; 16: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tiede I, Fritz G, Strand S, et al. CD28‐dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 2003; 111: 1133–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Imuran® (azathioprine). Product monograph. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/016324s034s035lbl.pdf. Accessed 13 April, 2020.
- 67. Berth‐Jones J, Takwale A, Tan E, et al. Azathioprine in severe adult atopic dermatitis: a double‐blind, placebo‐controlled, crossover trial. Br J Dermatol 2002; 147: 324–330. [DOI] [PubMed] [Google Scholar]
- 68. Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate‐to‐severe atopic eczema: a double‐blind, randomised controlled trial. Lancet 2006; 367: 839–846. [DOI] [PubMed] [Google Scholar]
- 69. Schram ME, Roekevisch E, Leeflang MM, et al. A randomized trial of methotrexate versus azathioprine for severe atopic eczema. J Allergy Clin Immunol 2011; 128: 353–359. [DOI] [PubMed] [Google Scholar]
- 70. Schram ME, Borgonjen RJ, Bik CM, et al. Off‐label use of azathioprine in dermatology: a systematic review. Arch Dermatol 2011; 147: 474–488. [DOI] [PubMed] [Google Scholar]
- 71. Methotrexate. Product monograph. Available at: https://www.pfizer.ca/sites/default/files/201908/Methotrexate_Injection_PM_E_224776_08July2019.pdf. Accessed 13 April, 2020.
- 72. Inoue K, Yuasa H. Molecular basis for pharmacokinetics and pharmacodynamics of methotrexate in rheumatoid arthritis therapy. Drug Metab Pharmacokinet 2014; 29: 12–19. [DOI] [PubMed] [Google Scholar]
- 73. Warren RB, Mrowietz U, von Kiedrowski R, et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque‐type psoriasis (METOP): a 52 week, multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2017; 389: 528–537. [DOI] [PubMed] [Google Scholar]
- 74. Pasnoor M, He J, Herbelin L, et al. A randomized controlled trial of methotrexate for patients with generalized myasthenia gravis. Neurology 2016; 87: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kingsley GH, Kowalczyk A, Taylor H, et al. A randomized placebo‐controlled trial of methotrexate in psoriatic arthritis. Rheumatology 2012; 51: 1368–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ridker PM, Everett BM, Pradhan A, et al. Low‐dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2019; 380: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McLean‐Tooke A, Aldridge C, Waugh S, et al. Methotrexate, rheumatoid arthritis and infection risk: what is the evidence? Rheumatology 2009; 48: 867–871. [DOI] [PubMed] [Google Scholar]
- 78. Ponticelli C, Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin Drug Saf 2017; 16: 411–419. [DOI] [PubMed] [Google Scholar]
- 79. Yokogawa N, Eto H, Tanikawa A, et al. Effects of hydroxychloroquine in patients with cutaneous lupus erythematosus: a multicenter, double‐blind, randomized, Parallel‐Group Trial. Arthritis Rheumatol 2017; 69: 791–799. [DOI] [PubMed] [Google Scholar]
- 80. Costedoat‐Chalumeau N, Galicier L, Aumaitre O, et al. Hydroxychloroquine in systemic lupus erythematosus: results of a French multicentre controlled trial (PLUS Study). Ann Rheum Dis 2013; 72: 1786–1792. [DOI] [PubMed] [Google Scholar]
- 81. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis 2020: ciaa237. 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Afra TP, Razmi TM, Dogra S. Apremilast in psoriasis and beyond: big hopes on a small molecule. Indian Dermatol Online J 2019; 10: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Otezla® (apremilast). Product monograph. Available at: https://media.celgene.com/content/uploads/sites/23/Otezla‐Product‐Monograph‐English.pdf. Accessed 13 April, 2020.
- 84. Stein Gold L, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in systemic‐ and biologic‐naive patients with moderate plaque psoriasis: 52‐week results of UNVEIL. J Drugs Dermatol 2018; 17: 221–228. [PubMed] [Google Scholar]
- 85. Reich K, Gooderham M, Bewley A, et al. Safety and efficacy of apremilast through 104 weeks in patients with moderate to severe psoriasis who continued on apremilast or switched from etanercept treatment: findings from the LIBERATE study. J Eur Acad Dermatol Venereol 2018; 32: 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bissonnette R, Haydey R, Rosoph LA, et al. Apremilast for the treatment of moderate‐to‐severe palmoplantar psoriasis: results from a double‐blind, placebo‐controlled, randomized study. J Eur Acad Dermatol Venereol 2018; 32: 403–410. [DOI] [PubMed] [Google Scholar]
- 87. Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol 2015; 73: 37–49. [DOI] [PubMed] [Google Scholar]
- 88. Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate‐to‐severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol 2015; 173: 1387–1399. [DOI] [PubMed] [Google Scholar]
- 89. Vossen A, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol 2019; 80: 80–88. [DOI] [PubMed] [Google Scholar]
- 90. Mugheddu C, Pizzatti L, Sanna S, et al. COVID‐19 pulmonary infection in erythrodermic psoriatic patient with oligodendroglioma: safety and compatibility of apremilast with critical intensive care management. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thalidomid® (Thalidomide). Product monograph. Available at: http://media.celgene.com/content/uploads/sites/23/Thalomid‐Product_Monograph_‐_English_Version.pdf. Accessed 13 April, 2020.
- 92. Turk BE, Jiang H, Liu JO. Binding of thalidomide to alpha1‐acid glycoprotein may be involved in its inhibition of tumor necrosis factor alpha production. Proc Natl Acad Sci USA 1996; 93: 7552–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Prednisone. Product monograph. Available at: https://pdf.hres.ca/dpd_pm/00030893.PDF. Accessed 13 April, 2020.
- 94. El Mofty M, Essmat S, Youssef R, et al. The role of systemic steroids and phototherapy in the treatment of stable vitiligo: a randomized controlled trial. Dermatol Ther 2016; 29: 406–412. [DOI] [PubMed] [Google Scholar]
- 95. Kar BR, Handa S, Dogra S, et al. Placebo‐controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol 2005; 52: 287–290. [DOI] [PubMed] [Google Scholar]
- 96. Kurosawa M, Nakagawa S, Mizuashi M, et al. A comparison of the efficacy, relapse rate and side effects among three modalities of systemic corticosteroid therapy for alopecia areata. Dermatology 2006; 212: 361–365. [DOI] [PubMed] [Google Scholar]
- 97. Singh H, Kumaran MS, Bains A, et al. A randomized comparative study of oral corticosteroid minipulse and low‐dose oral methotrexate in the treatment of unstable vitiligo. Dermatology 2015; 231: 286–290. [DOI] [PubMed] [Google Scholar]
- 98. Hoes JN, Jacobs JW, Verstappen SM, et al. Adverse events of low‐ to medium‐dose oral glucocorticoids in inflammatory diseases: a meta‐analysis. Ann Rheum Dis 2009; 68: 1833–1838. [DOI] [PubMed] [Google Scholar]
- 99. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis 1989; 11: 954–963. [DOI] [PubMed] [Google Scholar]
- 100. Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID‐19 outcomes in patients with inflammatory bowel diseases: results from an International Registry. Gastroenterology 2020. 10.1053/j.gastro.2020.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wilkinson E. RECOVERY trial: the UK covid‐19 study resetting expectations for clinical trials. BMJ 2020; 369: m1626. [DOI] [PubMed] [Google Scholar]
- 102. Memoli MJ, Athota R, Reed S, et al. The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts. Clin Infect Dis 2014; 58: 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. D'Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl 2020; 26(6): 832–834. [DOI] [PubMed] [Google Scholar]
- 104. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID‐19) infection: a systematic review and meta‐analysis. Int J Infect Dis 2020; 94: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Li F, Cai J, Dong N. First cases of COVID‐19 from China. J Heart Lung Transplant 2020; 39: 496–497. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gandolfini I, Delsante M, Fiaccadori E, et al. COVID‐19 in kidney transplant recipients. Am J Transplant 2020. 10.1111/ajt.15967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zou Z, Yang Y, Chen J, et al. Prognostic factors for severe acute respiratory syndrome: a clinical analysis of 165 cases. Clin Infect Dis 2004; 38: 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ko JH, Seok H, Park GE, et al. Host susceptibility to MERS‐CoV infection, a retrospective cohort study of the 2015 Korean MERS outbreak. J Infect Chemother 2018; 24: 150–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hui DS, Azhar EI, Kim YJ, et al. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis 2018; 18: e217–e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Centers for Disease Control and Prevention Coronavirus (COVID‐19) . Available at: https://www.cdc.gov/coronavirus/2019‐ncov/index.html (accessed 24 May 2020).
- 111. Schmitz N, Kurrer M, Bachmann MF, et al. Interleukin‐1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol 2005; 79: 6441–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2008; 58: 106–115. [DOI] [PubMed] [Google Scholar]
- 113. Gottlieb AB, Blauvelt A, Thaci D, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double‐blinded, placebo‐controlled studies (CIMPASI‐1 and CIMPASI‐2). J Am Acad Dermatol 2018; 79: 302–314 e306. [DOI] [PubMed] [Google Scholar]
- 114. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014; 371: 326–338. [DOI] [PubMed] [Google Scholar]
- 115. Papp KA, Tyring S, Lahfa M, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol 2005; 152: 1304–1312. [DOI] [PubMed] [Google Scholar]
- 116. Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet 2005; 366: 1367–1374. [DOI] [PubMed] [Google Scholar]
- 117. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 76‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 1). Lancet 2008; 371: 1665–1674. [DOI] [PubMed] [Google Scholar]
- 118. Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med 2015; 373: 1318–1328. [DOI] [PubMed] [Google Scholar]
- 119. Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015; 386: 541–551. [DOI] [PubMed] [Google Scholar]
- 120. Reich K, Papp KA, Armstrong AW, et al. Safety of guselkumab in patients with moderate‐to‐severe psoriasis treated through 100 weeks: a pooled analysis from the randomized VOYAGE 1 and VOYAGE 2 studies. Br J Dermatol 2019; 180: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 121. Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76: 418–431. [DOI] [PubMed] [Google Scholar]
- 122. Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate‐to‐severe plaque psoriasis (UltIMMa‐1 and UltIMMa‐2): results from two double‐blind, randomised, placebo‐controlled and ustekinumab‐controlled phase 3 trials. Lancet 2018; 392: 650–661. [DOI] [PubMed] [Google Scholar]
- 123. Blauvelt A, Reich K, Papp KA, et al. Safety of tildrakizumab for moderate‐to‐severe plaque psoriasis: pooled analysis of three randomized controlled trials. Br J Dermatol 2018; 179: 615–622. [DOI] [PubMed] [Google Scholar]
- 124. Cohen SB, Moreland LW, Cush JJ, et al. A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis 2004; 63: 1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Vena GA, Cassano N, Colombo D, et al. Cyclosporine in chronic idiopathic urticaria: a double‐blind, randomized, placebo‐controlled trial. J Am Acad Dermatol 2006; 55: 705–709. [DOI] [PubMed] [Google Scholar]
- 126. Karanikolas GN, Koukli EM, Katsalira A, et al. Adalimumab or cyclosporine as monotherapy and in combination in severe psoriatic arthritis: results from a prospective 12‐month nonrandomized unblinded clinical trial. J Rheumatol 2011; 38: 2466–2474. [DOI] [PubMed] [Google Scholar]
- 127. Lai VWY, Chen G, Gin D, et al. Cyclosporine for moderate‐to‐severe alopecia areata: a double‐blind, randomized, placebo‐controlled clinical trial of efficacy and safety. J Am Acad Dermatol 2019; 81: 694–701. [DOI] [PubMed] [Google Scholar]
- 128. Beissert S, Werfel T, Frieling U, et al. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of bullous pemphigoid. Arch Dermatol 2007; 143: 1536–1542. [DOI] [PubMed] [Google Scholar]
- 129. Akhyani M, Chams‐Davatchi C, Hemami MR, et al. Efficacy and safety of mycophenolate mofetil vs. methotrexate for the treatment of chronic plaque psoriasis. J Eur Acad Dermatol Venereol 2010; 24: 1447–1451. [DOI] [PubMed] [Google Scholar]
- 130. Ioannides D, Apalla Z, Lazaridou E, et al. Evaluation of mycophenolate mofetil as a steroid‐sparing agent in pemphigus: a randomized, prospective study. J Eur Acad Dermatol Venereol 2012; 26: 855–860. [DOI] [PubMed] [Google Scholar]
- 131. Zhou Y, Rosenthal D, Dutz J, et al. Mycophenolate mofetil (CellCept) for psoriasis: a two‐center, prospective, open‐label clinical trial. J Cutan Med Surg 2003; 7: 193–197. [DOI] [PubMed] [Google Scholar]
- 132. Droitcourt C, Rybojad M, Porcher R, et al. A randomized, investigator‐masked, double‐blind, placebo‐controlled trial on thalidomide in severe cutaneous sarcoidosis. Chest 2014; 146: 1046–1054. [DOI] [PubMed] [Google Scholar]
- 133. Kaur I, Dogra S, Narang T, et al. Comparative efficacy of thalidomide and prednisolone in the treatment of moderate to severe erythema nodosum leprosum: a randomized study. Australas J Dermatol 2009; 50: 181–185. [DOI] [PubMed] [Google Scholar]
- 134. Lazzerini M, Martelossi S, Magazzu G, et al. Effect of thalidomide on clinical remission in children and adolescents with refractory Crohn disease: a randomized clinical trial. JAMA 2013; 310: 2164–2173. [DOI] [PubMed] [Google Scholar]
- 135. Hamuryudan V, Mat C, Saip S, et al. Thalidomide in the treatment of the mucocutaneous lesions of the Behcet syndrome. A randomized, double‐blind, placebo‐controlled trial. Ann Intern Med 1998; 128: 443–450. [DOI] [PubMed] [Google Scholar]
- 136. Schmitt J, Schmitt N, Meurer M. Cyclosporin in the treatment of patients with atopic eczema ‐ a systematic review and meta‐analysis. J Eur Acad Dermatol Venereol 2007; 21: 606–619. [DOI] [PubMed] [Google Scholar]
- 137. Sood R, Ansari S, Clark T, et al. Long‐term efficacy and safety of azathioprine in ulcerative colitis. J Crohns Colitis 2015; 9: 191–197. [DOI] [PubMed] [Google Scholar]
- 138. Drach M, Papageorgiou K, Maul JT, et al. Effectiveness of methotrexate in moderate to severe psoriasis patients: real‐world registry data from the Swiss Dermatology Network for Targeted Therapies (SDNTT). Arch Dermatol Res 2019; 311: 753–760. [DOI] [PubMed] [Google Scholar]
- 139. Papadavid E, Rompoti N, Theodoropoulos K, et al. Real‐world data on the efficacy and safety of apremilast in patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol 2018; 32: 1173–1179. [DOI] [PubMed] [Google Scholar]
- 140. Garcia‐Doval I, Cohen AD, Cazzaniga S, et al. Risk of serious infections, cutaneous bacterial infections, and granulomatous infections in patients with psoriasis treated with anti‐tumor necrosis factor agents versus classic therapies: prospective meta‐analysis of Psonet registries. J Am Acad Dermatol 2017; 76: 299–308.e16. [DOI] [PubMed] [Google Scholar]


