Summary
Background
The incidence of elevated liver chemistries and the presence of pre‐existing chronic liver disease (CLD) have been variably reported in COVID‐19.
Aims
To assess the prevalence of CLD, the incidence of elevated liver chemistries and the outcomes of patients with and without underlying CLD/elevated liver chemistries in COVID‐19.
Methods
A comprehensive search of electronic databases from 1 December 2019 to 24 April 2020 was done. We included studies reporting underlying CLD or elevated liver chemistries and patient outcomes in COVID‐19.
Results
107 articles (n = 20 874 patients) were included for the systematic review. The pooled prevalence of underlying CLD was 3.6% (95% CI, 2.5‐5.1) among the 15 407 COVID‐19 patients. The pooled incidence of elevated liver chemistries in COVID‐19 was 23.1% (19.3‐27.3) at initial presentation. Additionally, 24.4% (13.5‐40) developed elevated liver chemistries during the illness. The pooled incidence of drug‐induced liver injury was 25.4% (14.2‐41.4). The pooled prevalence of CLD among 1587 severely infected patients was 3.9% (3%‐5.2%). The odds of developing severe COVID‐19 in CLD patients was 0.81 (0.31‐2.09; P = 0.67) compared to non‐CLD patients. COVID‐19 patients with elevated liver chemistries had increased risk of mortality (OR‐3.46 [2.42‐4.95, P < 0.001]) and severe disease (OR‐2.87 [95% CI, 2.29‐3.6, P < 0.001]) compared to patients without elevated liver chemistries.
Conclusions
Elevated liver chemistries are common at presentation and during COVID‐19. The severity of elevated liver chemistries correlates with the outcome of COVID‐19. The presence of CLD does not alter the outcome of COVID‐19. Further studies are needed to analyse the outcomes of compensated and decompensated liver disease.
1. INTRODUCTION
Coronavirus disease‐2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection is a global health problem. Severe COVID‐19 can lead to multi‐organ failure and may be associated with high mortality. Age, severity of the infection and comorbidity are important predictors of poor outcomes in COVID‐19. 1 , 2 However, pandemics may not affect all populations equally, and certain populations are particularly vulnerable. Chronic liver disease (CLD) is one such population with increasing burden worldwide. Moreover patients with cirrhosis, being immunocompromised, are expected to be more susceptible and have worse outcomes in viral illness. 3 The incidence of elevation in aminotransferases and/or bilirubin have been variably reported in COVID‐19. Previous reviews have analysed a limited number of studies. 4 , 5 However, the incidence of elevated liver chemistries at initial presentation or during illness or the presence of pre‐existing CLD on outcomes of COVID‐19 has not been extensively reviewed so far. In this systematic review, we compiled all the data published and analysed the liver involvement in COVID‐19, describing each variable of liver chemistries in COVID‐19 and the effect of COVID‐19 in patients with pre‐existing CLD. We aimed to answer these questions: (a) What is the prevalence of CLD in the global COVID‐19 burden?; (b) What is the incidence of elevated liver chemistries at initial presentation and during the illness in COVID‐19?; and (c) What is the association of elevated liver chemistries and pre‐existing CLD on clinical outcomes of COVID‐19?
2. METHODS
We followed the meta‐analysis of observational studies in epidemiology (MOOSE) guidelines for data extraction and reporting. 6 Two independent reviewers performed a comprehensive search of electronic databases (by AVK and PK) including PUBMED, Excerpta Medica Database (EMBASE) and Scopus since 1 December 2019, to 24 April 2020. Search items included "SARS‐CoV‐2", "Coronavirus," "COVID‐19", "Cirrhosis," "Liver." Articles on drugs causing liver injury was made till 30 April through google search engine. The details of the search strategy for PUBMED are reported in the Appendix 1.
2.1. Study selection and data abstraction
The data were abstracted by two investigators independently (AVK and PK) based on the protocol priori, which was registered on Prospero (CRD42020181962). Case reports (of >2 patients), case series, cross‐sectional studies, case‐control studies, cohort studies (retrospective and prospective), quasi‐randomised and randomised controlled trials that mentioned elevated liver chemistries in patients with COVID‐19 were included irrespective of age and gestational status. Studies that did not report any data on liver chemistries or underlying CLD were excluded. Furthermore, experimental studies, studies describing the mechanism, review articles, virology studies, studies describing only the radiological features or studies describing the usage of artificial intelligence in COVID‐19, epidemiology, transmission, and surveillance‐related articles, editorials, investigations (testing)‐related articles, guidelines, recommendations, unpublished data and management‐related articles were excluded. The following parameters were recorded: first author country of study, number of patients, age, gender, underlying CLD and their outcomes, percentage of patients with elevated liver chemistries (defined as elevation above the laboratory upper limit of normal) at initial presentation, percentage of patients developing elevated liver chemistries during the illness, number of patients with severe disease/non‐severe disease, and percentage of patients developing drug‐induced liver injury (DILI) and the implicated drug and lastly the mortality if reported.
We also noted the percentage of patients who presented with elevated liver chemistries separately for each variable, that is, bilirubin, aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), gamma‐glutamyl transpeptidase (GGT), albumin and prothrombin time (PT). We took AST or ALT levels above 40 U/L (or the upper limit of normal for that laboratory) as elevated. The percentage of patients with elevated AST/ALT (whichever is higher) at initial presentation and during follow‐up was recorded. Percentage of patients with elevated liver chemistries in severely infected and non‐severely infected, if reported, was recorded. We also recorded the percentage of patients with elevated liver chemistries at initial presentation and during illness in alive (survivor) and dead (non‐survivor) patients of COVID‐19. If none of the enzyme elevations was reported, then the percentage of patients presenting with hyperbilirubinaemia was recorded. Two individuals (HT and MP) again separately checked the abstracted data. Any discrepancy in the data was discussed and sorted. Articles not in English were converted using the online google translate tool and included for review. The converted data were confirmed by a Chinese native speaker (XQ).
2.1.1. Definitions
Underlying CLD was defined as any pre‐existing liver disease before the SARS‐CoV‐2 infection. 4 We took CLD/cirrhosis (due to any cause), chronic hepatitis B, chronic hepatitis C, non‐alcoholic fatty liver disease (NAFLD), autoimmune hepatitis as the underlying liver disease. We used the definitions given by the authors of the primary studies to define the rise in enzyme levels for ALP, GGT and prothrombin prolongation. We considered any elevation in liver enzymes or total bilirubin after the initiation of the drugs as DILI in the absence of identified common causes of liver disease (viral, autoimmune, etc) by the primary authors. Severe liver injury was defined as any elevation of enzymes over three times ULN (upper limit of normal) and bilirubin over 2 ULN. 7 In general, we defined severe infection as per Chinese National Health Commission guidelines which defined severe infection as adult patients meeting any of the following criteria (a) respiratory rate ≥30 beats/min in a resting state; or (b) a mean oxygen saturation of ≤93%; or (c) an arterial blood oxygen partial pressure (PaO2)/oxygen concentration (FiO2) ≤300 mm Hg; (d) computed tomographic image showing significant lesion progression >50% within 24‐48 hours or as per the authors of the primary studies. 8 We also included severe pneumonia as those patients satisfying one major criterion or ≥3 minor criteria of American Thoracic Society (ATS) guidelines for community‐acquired pneumonia. 9 In the absence of such definitions, we considered patients with documented pneumonia requiring ICU care/oxygen support as severe disease.
Outcome measures
The study outcome measures were: number of liver disease patients with COVID‐19 and their outcomes (pre‐existing CLD in COVID‐19); the pooled incidence of elevated liver chemistries at initial presentation and during illness in COVID‐19 and the effect of these elevated liver chemistries on the outcome of COVID‐19; the incidence of AST/ALT elevation at initial presentation in COVID‐19; the incidence of ALP/GGT elevation at initial presentation in COVID‐19; the incidence of hyperbilirubinaemia/hypoalbuminaemia at initial presentation in COVID‐19; the incidence of PT prolongation and the incidence of DILI in COVID‐19 patients.
Assessment of study quality
Two independent reviewers (AVK, PK) did the quality appraisal using the Appraisal tool for Cross‐Sectional Studies (AXIS) checklist for cross‐sectional studies and the Institute of Health Economics (IHE) checklist tool for case series. 10 , 11 AXIS checklist has a total of 20 points of which seven questions are related to the quality of reporting, seven related to study design and six questions are aimed to assess the bias in the study. IHE is a comprehensive checklist of quality of study which covers both the risk of bias and quality of reporting. Randomised studies were assessed using the Cochrane collaboration tool. 12 New‐castle Ottawa scale tool was used to assess the bias in case–control and cohort studies. 13 Any discrepancy in the study quality assessment was discussed, and the best score was decided after confirming with a third independent individual (PNR) who acted as the ombudsman.
2.2. Statistical methods
A database was generated in Microsoft Excel, and meta‐analysis was performed using the Comprehensive Meta‐analysis package (Ver. 3.3.070; 2014). We expressed the percentages as the event rate and transformed the pooled data by Logit transformation method to assess the pooled incidence. The sample size (n) was entered, and the analysis was run. Studies with sample size <10 were excluded from the pooled meta‐analysis. We included all patients irrespective of age, gender, comorbidity, type of infection (mild or severe) across different countries. And we also included pregnant patients. Hence, we expected high heterogeneity and used the random model effect. We used the odds ratio to express the measured effect. For studies reporting the proportion of patients with elevated liver chemistries for two comparative groups, odds ratios were used to describe the difference between severe vs non‐severe and survivor vs non‐survivor groups. We assessed the statistical heterogeneity by visual inspection of forest plots, and using the Chi‐squared and the I2 statistics. A P value <0.10 was considered to indicate statistically significant heterogeneity. Q measure was used to evaluate the significance of heterogeneity and was considered statistically significant when P was <0.1. Publication bias was assessed by funnel plot asymmetry and quantified using Egger's intercept.
3. RESULTS
A total of 4213 articles were screened for eligibility. (Figure 1) Of the 169 full‐text articles deemed eligible, 33 publications had neither mentioned the percentage of patients with elevated liver chemistries or underlying CLD, 21 articles were case reports of ≤2 patients, and eight were liver transplant related (but had not mentioned any elevated liver chemistries). The details of the excluded articles are provided as Appendix 2 in the Supplementary Material. A total of 107 articles were included for the systematic review (Figure 1). Ninety‐two articles (of which four included pregnant patients) reported adult patients infected with SARS‐CoV‐2. Eleven articles included paediatric population, and four articles reported both adults and children infected with SARS‐CoV‐2. 2 , 7 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 (Tables S1, S2 and S3) A total of 20 874 patients were included, of which 38 were pregnant patients, 395 were paediatric patients and the rest were adult patients. Fifty‐eight percent (11 882/20 479) were adult males. Fifty‐one percent (202/395) were males in the paediatric group. Twelve articles were not from China. Three articles from the United States of America, 14 , 75 , 78 one each from United Kingdom (UK), 81 Italy, 100 Thailand, 57 France, 64 South Korea, 51 Singapore, 115 Hong Kong 76 and Iran. 66 One was a multicentre trial on remdesivir conducted simultaneously at USA, Japan, Italy, Austria, France, Germany, Netherlands, Spain and Canada. 93 A total of 7627 patients were reported from countries other than China. (Figure 2).
FIGURE 1.
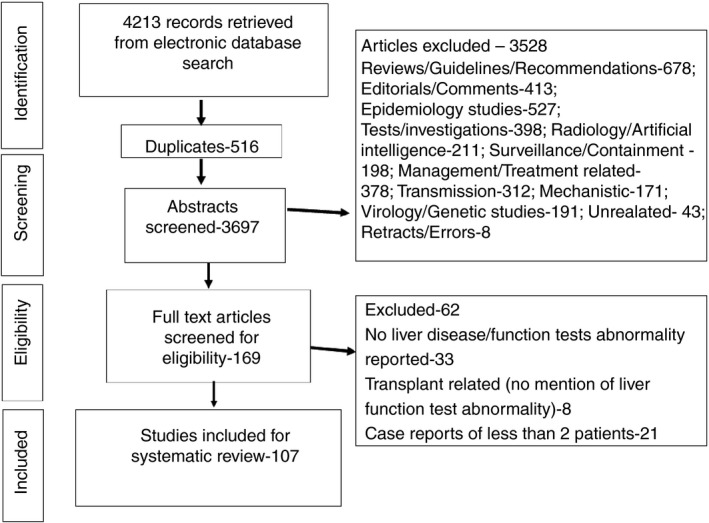
Flowchart depicting the articles screened, excluded and included for the systematic review
FIGURE 2.
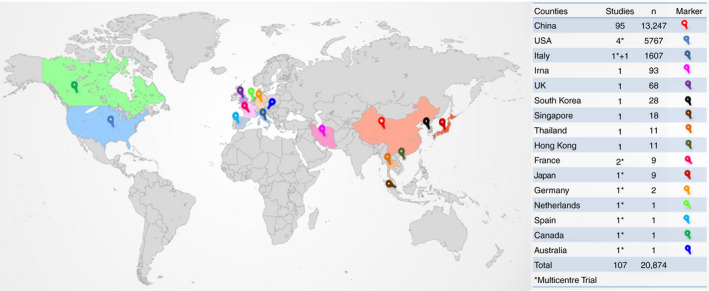
Countries from which studies were analysed. The number of studies and number of patients from each country in the table
3.1. Pre‐existing liver disease in COVID‐19
A total of 409 patients had underlying liver disease among the 50 articles which reported it. 7 , 14 , 16 , 17 , 19 , 21 , 23 , 27 , 31 , 32 , 33 , 34 , 36 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 48 , 50 , 51 , 52 , 57 , 63 , 65 , 67 , 68 , 70 , 71 , 75 , 77 , 80 , 83 , 85 , 86 , 87 , 88 , 91 , 92 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 113 The pooled prevalence of underlying CLD was 3.6% (95% CI, 2.5‐5.1) among the 15 407 COVID‐19 patients (Figure 3). The representation of data was not uniform in most of the studies regarding the underlying disease. CLD was reported in 61.12% (95% CI, 56.21‐65.87), followed by NAFLD and chronic hepatitis B in 19.56% (95% CI, 15.82‐23.74) and 17.85% (95% CI, 14.25‐21.91) of patients respectively. Of the 250 CLD patients 11.6% (8%‐16.2%) had cirrhosis (Table 1) There was no mention about the decompensations of liver disease in any of the articles, although one of the studies did mention the cause of death as variceal bleed in a SARS‐CoV‐2–infected patient. 48
FIGURE 3.
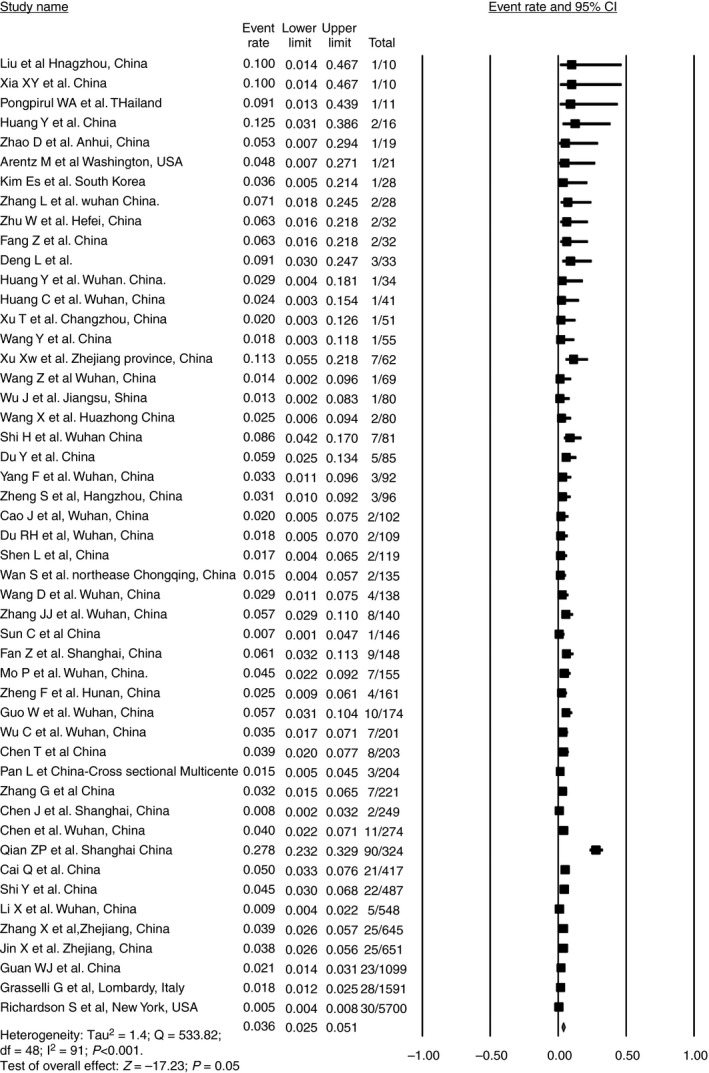
Pooled prevalence of liver disease in COVID‐19 patients
TABLE 1.
Type of liver disease reported in the included studies
| Type of liver disease | N | Incidence % (95% CI, lower limit‐upper limit) | References |
|---|---|---|---|
| Chronic liver disease/Cirrhosis | 250 | 61.12% (56.21‐65.87) | 7, 14, 16, 17, 19, 21, 31, 32, 33, 36, 38, 39, 42, 43, 48, 50, 51, 57, 63, 65, 67, 68, 70, 80, 83, 85, 87, 88, 92, 94, 95, 96, 97, 98, 99, 100, 101, 113 |
| NAFLD | 80 | 19.56% (15.82‐23.74) | 41, 45, 71, 80 |
| Chronic hepatitis B | 73 | 17.85% (14.25‐21.91) | 23, 27, 40, 44, 52, 71, 75, 91, 98 |
| HBV ‐HCC | 2 | 0.49% (0.05‐1.75) | 77 |
| Chronic Hepatitis C | 3 | 0.73% (0.15‐2.12) | 75 |
| Chronic Hepatitis (unspecified) | 1 | 0.24% (0.006‐1.35) | 34 |
Abbreviations: CI, confidence interval; HBV, Hepatitis B virus; HCC, Hepatocellular carcinoma; NAFLD, non‐alcoholic fatty liver disease.
The pooled prevalence of CLD among 1587 severely infected patients was 3.9% (3%‐5.2%) among 18 articles which reported it. 14 , 16 , 27 , 34 , 36 , 39 , 41 , 43 , 50 , 57 , 67 , 77 , 85 , 87 , 91 , 96 , 99 , 101 The pooled prevalence of CLD among 2699 non‐severely infected patients was 3.1% (2.5‐3.9) among 19 articles which reported it. 16 , 27 , 34 , 36 , 38 , 41 , 43 , 44 , 50 , 67 , 77 , 80 , 85 , 87 , 91 , 95 , 96 , 99 , 101 (Figure S1A,B) The prevalence of CLD was 4.7% (95% CI, 2.9‐7.7) among 326 non‐survivors reported in five articles. 48 , 52 , 63 , 88 , 92 The prevalence of CLD was 3.6% (95% CI, 2‐6.6) amongst 292 survivors reported in four articles. 38 , 52 , 63 , 88 (Figure S1C,D) Of these 23 patients were hepatitis B, and the rest were CLD patients. The odds of developing severe COVID‐19 in CLD patients was 0.81 (0.31‐2.09; P = 0.67) compared to non‐CLD patients among three articles which reported 70 CLD and 2161 non‐CLD patients. 27 , 43 , 87 (Figure S2) Most of the articles had n <10 patients (CLD) hence we could not meta‐analyse the mortality difference among CLD and non‐CLD patients. The incidence of mortality among 11 hepatitis B patients was 45.5% which reported it. 57
3.2. COVID‐19 and elevated liver chemistries
3.2.1. Incidence of elevated liver chemistries in COVID‐19 at initial presentation
Seventy‐six articles, including 13 141 patients, described elevated liver chemistries at initial presentation. 7 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 46 , 47 , 49 , 50 , 51 , 52 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 86 , 88 , 89 , 90 , 91 , 92 , 93 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 The incidence of elevated liver chemistries varied from 1.1% to 68% in COVID‐19 patients. 84 , 89 Furthermore, five studies reported normal liver functions tests at initial presentation. 56 , 60 , 61 , 73 , 111 Two of which reported pregnant patients with COVID‐19 having normal liver chemistries at initial presentation. 61 , 73 One study reported paediatric patients, and the other two reported adult patients with normal liver chemistries. 56 , 60 , 111
For the pooled meta‐analysis, we excluded studies reporting the incidence of elevated liver chemistries among <10 patients. 24 , 47 , 61 , 64 , 73 , 104 , 105 , 108 The pooled incidence of elevated liver chemistries was 23.1% (95% CI, 19.3‐27.3) among 13 056 COVID‐19 patients and 68 studies. (Figure 4) The incidence of elevated liver chemistries in adults was 24.1% (95% CI, 20‐28.8) among 12 756 patients. The incidence of elevated liver chemistries in children was 17.8% (95% CI, 9.9‐29.8) among 283 patients. The incidence of elevated liver chemistries in pregnant patients was 2.8% (95% CI, 2‐32.2) among 17 patients. From the reviewed literature, COVID‐19 did not have any adverse effects on foetal or maternal outcomes. Of the 58 patients who had gastrointestinal (GI) manifestations in COVID‐19, elevated liver chemistries were the most common GI manifestation, followed by diarrhoea. 84
FIGURE 4.
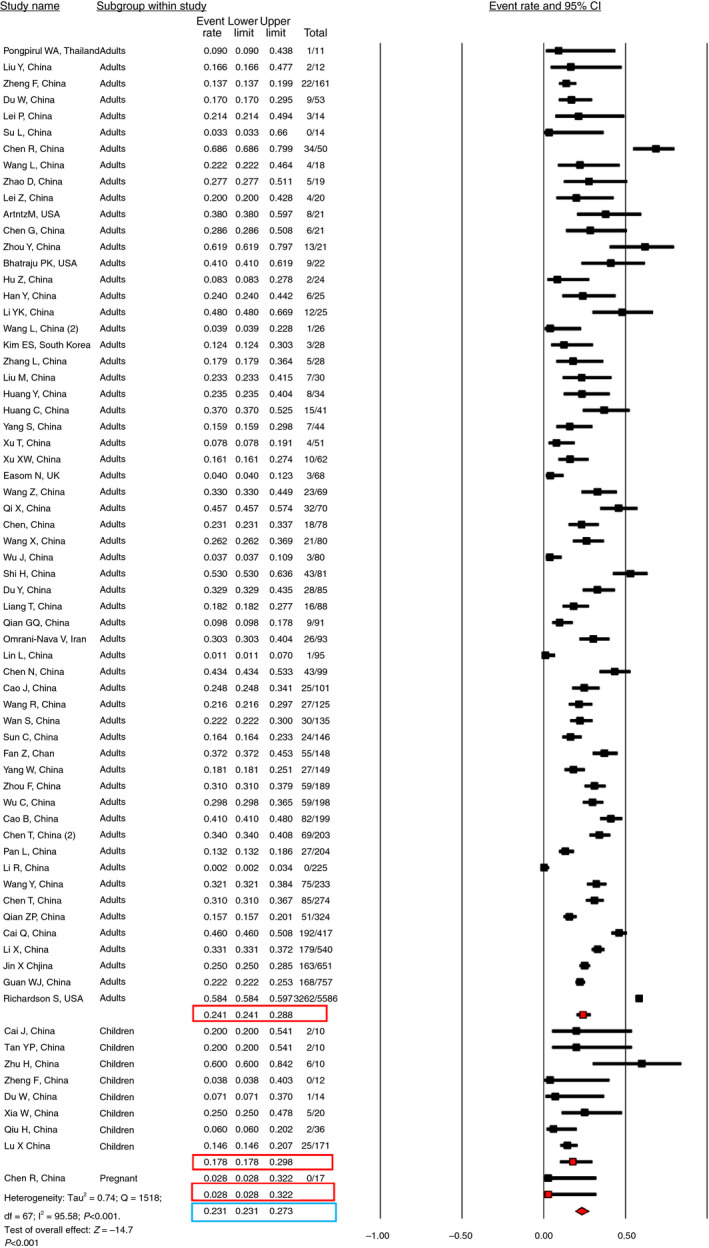
Pooled incidence of elevated liver chemistries at initial presentation in COVID‐19 patients
3.2.2. Elevated liver chemistries and the mortality/severity in COVID‐19
Forty‐six studies reported mortality. 14 , 15 , 16 , 17 , 20 , 21 , 27 , 29 , 34 , 35 , 36 , 39 , 40 , 48 , 52 , 53 , 54 , 55 , 60 , 63 , 64 , 65 , 66 , 67 , 68 , 70 , 74 , 75 , 77 , 78 , 80 , 82 , 83 , 85 , 88 , 89 , 90 , 91 , 93 , 96 , 98 , 100 , 102 , 103 , 118 Of which two of the studies had n <10. 55 , 64 The pooled incidence of mortality was 12.7% (95% CI, 9.9‐16.2) among 12 778 patients. (Figure S3) The pooled incidence of mortality reported from China was 10.6% (95% CI, 7.5‐14.8) among 38 articles. One article each from Italy and Iran reported 26% (95% CI, 24‐28.2) and 18.3% (95% CI, 11.7‐27.5) mortality respectively. 66 , 100 Three studies from the USA reported 38.5% (95% CI, 17.1‐65.4) and one multicentre trial reported 13% (95% CI, 6.3‐25) mortality. 14 , 75 , 78 , 118 Only two articles reported mortality in the paediatric population. 102 , 103 The pooled incidence of mortality was 2.3% (95% CI, 0.1‐33.2) in children.
The incidence of elevated liver chemistries was significantly higher in non‐survivors (43.3% [95% CI, 30‐57.6]) than survivors (19.2% [95% CI, 16.4‐22.3]). (Figure S4) Six articles mentioned elevated liver chemistries in non‐survivors (n = 468 patients) and four articles (n = 995 patients) mentioned elevated liver chemistries at initial presentation among survivors. 20 , 27 , 48 , 52 , 88 , 89 Non‐survivors had a higher risk of presenting with elevated liver chemistries at initial presentation than survivors (OR‐3.46 [2.42‐4.95, P < 0.001]) among the four articles which compared the elevated liver chemistries between survivors (n = 995) and non‐survivors (n = 326) (Figure S5). 20 , 27 , 52 , 88
The incidence of elevated liver chemistries in non‐severe COVID‐19 patients was 19.9% (95% CI‐14.8‐26.3) among nine articles, which reported 1290 non‐severely infected patients. 16 , 17 , 27 , 29 , 34 , 67 , 74 , 91 , 102 The definitions of severely infected and non‐severely infected in the included studies are explained in Table S4. While the incidence was 41.1% (95% CI, 33.1‐49.5) in severely infected patients among the nine articles, which reported 780 sick patients. 16 , 17 , 29 , 34 , 50 , 67 , 91 , 102 (Figure S6) Severely infected patients had higher odds of presenting with elevated liver chemistries, that is, OR‐2.87 (95% CI, 2.29‐3.6, P < 0.001) among nine articles which compared severe (n = 690) and non‐severe patients (n = 1290) (Figure S7). 16 , 17 , 27 , 29 , 34 , 67 , 74 , 91 , 102
3.2.3. Elevated liver chemistries during illness in COVID‐19
The incidence of elevated liver chemistries during illness was 24.4% (95% CI, 13.5‐40) among the eighteen articles which reported 5762 infected patients. 7 , 14 , 35 , 38 , 48 , 52 , 53 , 54 , 65 , 68 , 75 , 76 , 84 , 85 , 87 , 88 , 91 , 92 (Figure S8) Five articles reported severe liver injury during illness. 7 , 14 , 52 , 75 , 88 Only three of them defined liver injury. 7 , 52 , 75 Liver injury was defined as an elevation in total bilirubin level by ≥3 mg/dL and an acute increase in ALT ≥5 ULN and/or an increase ALP ≥2 ULN. 52 Another study defined liver injury as any elevation of enzymes over three times ULN and bilirubin over 2 ULN. 7 Third study defined acute liver injury as >15 times ULN elevation in aminotransferases. 75 The incidence of severe liver injury was 10.7% (95% CI, 3%‐32.1%) during the illness among 3440 patients· The incidence was 24.9% (95% CI, 10.3%‐49%) in 358 non‐severe patients vs 41.5% (95% CI, 15.1%‐73.8%) in 317 severely infected patients during the illness. 53 , 54 , 77 Severely infected patients had higher odds of developing elevated liver chemistries during the illness, that is, OR‐2.46 (95% CI, 1.12‐5.39, P = 0.02) among three articles which compared severe (n = 312) and non‐severe COVID‐19 patients (352) (Figure S9A). 53 , 54 , 91 The incidence of elevated liver chemistries was 26.2% (7.8‐59.8) in non‐survivors (n = 151) vs 18.5% (2.4‐67.9) in survivors (n = 270). Non‐survivors had higher odds of developing elevated liver chemistries during the illness (OR‐2.07 [95% CI, 0.4‐10.72; P = 0.38]) among the two studies which reported it. 35 , 52 (Figure S9B).
3.3. COVID‐19 and liver function tests
3.3.1. Incidence of AST elevation at initial presentation in COVID‐19
The pooled incidence of AST elevation was 22.5% (95% CI, 18.1‐27.6) among 47 studies and 11 914 adult patients. AST elevation was reported in six studies, including 251 children. The incidence was 18.4% (95% CI, 9.4%‐33.1%) among children. The pooled incidence of AST elevation was 22.5% (95% CI, 18.1‐27.6). (Figure S10).
3.3.2. Incidence of ALT elevation at initial presentation in COVID‐19
The pooled incidence of ALT elevation was 20.1% (95% CI, 16.8‐23.8) among the 48 articles and 11 431 adult patients. The incidence of ALT elevation in children was 12.6% (95% CI, 8.9‐17.3) among the six studies, including 261 patients which reported ALT elevation. The pooled incidence of ALT elevation was 17.9% (95% CI, 15.3%‐21%). (Figure S11).
3.3.3. Incidence of hyperbilirubinaemia at initial presentation in COVID‐19
The incidence of hyperbilirubinaemia was 13.4% (95% CI, 9‐19.4) among the 19 articles (n = 3248) in adults. 7 , 27 , 28 , 29 , 31 , 49 , 52 , 58 , 62 , 65 , 66 , 71 , 77 , 82 , 86 , 89 , 90 , 91 , 92 (Figure S12A).
3.3.4. Incidence of prothrombin time prolongation at initial presentation in COVID‐19
Eleven articles reported prolongation in PT in adults ranging from 2.1% to 58% at initial presentation. The pooled incidence of PT prolongation was 9.7% (95% CI, 4.6%‐19.2%) among the 11 articles which reported it (n = 1739). 2 , 21 , 32 , 36 , 49 , 70 , 71 , 79 , 82 , 86 , 92 The incidence of PT prolongation was 7.1% (95% CI, 1%‐37%) in only 1 article, which reported PT prolongation amongst children. 79 The pooled incidence of PT prolongation was 9.5% (95% CI, 4.7%‐18.6%). (Figure S12B).
3.3.5. ALP, GGT and albumin in COVID‐19
Alkaline phosphatase elevation was reported among 5 articles (n = 918 patients). The incidence of ALP elevation was 6.1% (95% CI, 2.4%‐14.2%). 7 , 65 , 66 , 71 , 72 Incidence of GGT elevation was 21.1% (95% CI, 12.8‐32.9) among six articles (n = 972) which reported it. 7 , 23 , 59 , 65 , 71 , 82 (Figure S13A,B) And the incidence of hypoalbuminaemia was 55.5% (95% CI, 42.8‐67.6) among 1990 patients reported in 14 articles. 18 , 28 , 29 , 32 , 49 , 52 , 58 , 63 , 71 , 72 , 77 , 82 , 91 , 92 (Figure S13C) Non‐severely infected patients had hypoalbuminaemia ranging from 1.1%‐45.8%. 15 , 91 While the severely infected patients had significant hypoalbuminaemia reaching up to 72.9%. 22 , 29 , 39 , 55 , 67 , 82 Hypoalbuminaemia was reported among 78%‐100% of deceased patients. 48 , 92
3.4. Incidence of drug‐induced liver injury (DILI) in COVID‐19
In total, 12 articles reported DILI. 7 , 15 , 64 , 65 , 84 , 93 , 113 , 114 , 115 , 116 , 117 , 118 Two article sample size <10 and were excluded from the pooled analysis. 64 , 114 The pooled incidence of DILI was 25.4% (95% CI, 14.2‐41.4). The incidence of DILI in 208 patients treated with remdesivir was 15.2% (95% CI, 6.4‐32). While the incidence was higher with lopinavir/ritonavir (LPV/r), that is, 37.2% (95% CI, 22.7‐54.6) among 775 COVID‐19 patients who were treated with LPV/r (Table 2) Hyperbilirubinaemia was the most frequent adverse effect of LPV/r, followed by elevated aminotransferases. 7 , 15 , 114 While remdesivir frequently led to elevated aminotransferases. 64 , 118 In some studies DILI was attributed to more than one drug. 65 , 115 (Figure S14) A recent trial included 158 patients in remdesivir arm and 79 in the placebo arm. 93 The incidence of adverse effects were reported in 66% of remdesivir recipients versus 64% in placebo recipients. Hyperbilirubinaemia developed in 10% (15/155) and AST elevation in 5% (7/155) in remdesivir group. 93 Remdesivir was stopped early because of adverse events in 18 (12%) patients versus four (5%) patients who stopped placebo early. 93 Interestingly, 28% in remdesivir group and 38% in the placebo group had received concomitant LPV/r. Three patients had to discontinue due to deranged liver function tests, that is, elevated ALT (2 patients) and bilirubin (1 patient). In another multicentre study of 53 patients, a total of 23% (12/53) developed elevated aminotransferases, and two patients discontinued the drug due to elevated aminotransferase. 118
TABLE 2.
Incidence of liver injury for different drugs
| Drugs | N | Incidence % (95% CI, lower limit‐upper limit) | References |
|---|---|---|---|
| Remdesivir | 208 | 15.2% (6.4‐32) | 93, 118 |
| LPV/r | 775 | 37.2% (22.7‐54.6) | 7, 15, 65, 113, 115, 116, 117 |
| Arbidol | 64 | 18.7% (12.5‐63.6) | 65, 117 |
| Antibiotic | 130 | 38% (10.3‐77.1) | 65, 84 |
| Darunavir | 13 | 45.4% (21.8‐71.2) | 65 |
| Antivirals | 170 | 36.4% (14.1‐66.7) | 65, 84 |
| Placebo (38% had received LPV/r) | 78 | 9% (4.4‐17.7) | 93 |
| Multiple drugs | 217 | 13.8% (9.8‐19.1) | 115 |
| Chloroquine | 37 | 4.2% (0.09‐17.9) | 115 |
| Umifenovir | 119 | 18.1% (12.2‐26.1) | 115 |
Antivirals: umifenovir, oseltamivir, acyclovir in Ref. [7]. The study by Lin et al 84 has not mentioned the drugs.
Antibiotics: levofloxacin, azithromycin, cephalosporin in Ref. [7]. The study by Lin et al 84 has not mentioned the drugs.
Abbreviations: CI, confidence interval; LPV/r, lopinavir/ritonavir.
3.5. Publication bias and study quality
We included a vast number of trials ranging from case series to large studies which described 5700 patients with different age groups. There was no publication bias when the studies reporting underlying liver disease were assessed by funnel plot symmetry and Eggers's test (Egger's intercept: −1.28 [−2.97 to 0.39], P = 0.13). (Figure 5A) There was publication bias when the studies reporting elevated liver chemistries at initial presentation were evaluated. Egger's intercept was −4.25 ([−5.37 to −3.14], P < 0.001) for 68 articles reporting elevated liver chemistries, and there was funnel plot asymmetry. (Figure 5B) However, there was no publication bias when 18 studies describing elevated liver chemistries during the illness were assessed by Eggers test (Egger's intercept: 0.68 [95% CI, −7.72 to 9.1; P = 0.86]) and funnel plot symmetry. (Figure 5C) And, for the 10 studies describing the DILI, there was publication bias based on Egger's test (Eggers's intercept: −5.77 [−12.72 to 1.18], P = 0.09) and funnel plot asymmetry. (Figure 5D) The quality assessment of 30 case series, 20 cross‐sectional studies, 55 cohort studies and two randomised controlled trials is reported in Table S5.
FIGURE 5.
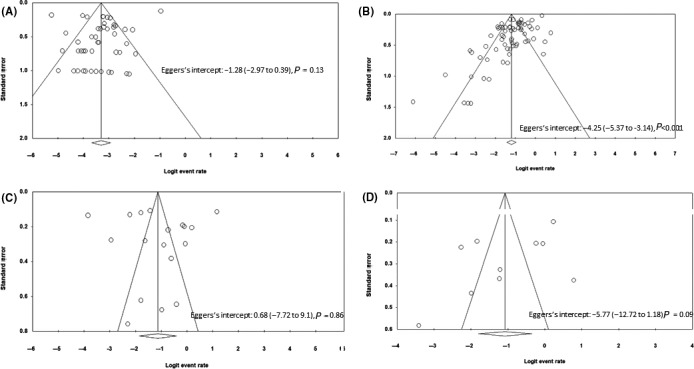
Funnel plot of the studies included for (A) prevalence of chronic liver disease (B) incidence of elevated liver chemistries at initial presentation (C) incidence of elevated liver chemistries during the illness (D) incidence of drug‐induced liver injury
4. DISCUSSION
In this systematic review, we evaluated the incidence of elevated liver chemistries in patients with COVID‐19. The salient features noted in our exhaustive review of 107 studies from various countries are: (a) The pooled prevalence of underlying CLD is 3.6% (95% CI, 2.5‐5.1) in COVID‐19; (b) The pooled prevalence of CLD among severely infected patients is 3.9% (3%‐5.2%); (c) The odds of developing severe COVID‐19 in CLD patients is 0.81(0.31‐2.09; P = 0.67) compared to non‐CLD patients; (d) Elevated liver chemistries occurs in 23.1% (95% CI, 19.3‐27.3) of patients at initial presentation in COVID‐19; (e) The incidence of elevated liver chemistries and severe liver injury during the illness was 24.4% (95% CI, 13.5‐40) and 10.7% (95% CI, 3%‐32.1%) respectively; (f) Patients with elevated liver chemistries have higher risk of severe COVID‐19 (g) Hypoalbuminaemia indicates severe infection; (h) The incidence of DILI was 25.4% (95% CI, 14.2‐41.4); (i) DILI due to of remdesivir, lopinavir/ritonavir and arbidol is common but not life‐threatening.
We have also compared our results with previous meta‐analysis (Table 3). 4 , 5 , 119 , 120 , 121 , 122 , 123 , 124 , 125 This is the first study to report the liver involvement of all adults, pregnant patients and paediatric patients with COVID‐19. We have analysed in‐depth about the liver involvement in COVID‐19, including elevated liver chemistries at initial presentation, during illness and the impact of this on the outcome. We have also reported the elevation in each variable of liver function tests, that is, AST, ALT, bilirubin, albumin, ALP, GGT and prolongation in PT. We also reported the incidence of DILI and the implicated drugs from the available data. Elevated liver chemistries at initial presentation or during illness is an important marker of disease severity. Serum albumin, a negative acute phase reactant, also indicates severe disease. Liver injury is more common in COVID‐19 than non‐COVID‐19 infections. 23 , 126
TABLE 3.
Reported meta‐analysis of liver function/disease in COVID‐19
| Author (date of publication)Reference number | Included studies | Total number of patients | Liver disease (Yes/No) | Deranged LFT (yes/no) and % | Paediatric/pregnant patients included a (yes/no) | Impact of altered LFT or underlying CLD on outcome | Comments |
|---|---|---|---|---|---|---|---|
| Mantovani A et al (April 4th) 4 |
11 studies Chronic liver disease or deranged liver function tests. |
2034 |
3% (95% CI, 2‐4) had CLD. Main cause was hepatitis B/C. |
No | No | No |
No meta‐analysis of deranged liver function tests. Only Chinese patients. |
| Zheng Z et al (April 23rd) 5 | 13 studies | 3027 | No | No | No | Increased AST predicted severe disease. | Analysed risk factors for severe disease. |
| Parohan M et al (May 9th) 119 | 20 studies | 3428 | No | Yes. (mean differences between mild and severe cases) | No | Severely infected patients had higher AST, ALT and total bilirubin and hypoalbuminemia. | Only Chinese patients. |
| Sultan S et al (May 11th) 120 | 32 studies with liver abnormalities | 2711 | No | Yes. abnormal AST and ALT in 15% and bilirubin in 16.7% | No | No | Included both published and preprint studies. |
| Kukla M et al (11th May) 121 | SARS CoV‐2‐11 studies; SARS‐CoV‐1‐ 23. MERS‐ 9 studies | 2541 | No | No | No | No | Systematic review comparing liver function/histology in SARS‐CoV‐2 vs SARS‐CoV‐1 and MERS CoV. |
| Mao R et al (May 12th) 122 | 12 studies ith liver abnormalities. (4 paediatric studies) | 1267 | Pooled prevalence of digestive system comorbidities was 4% (95% CI 2‐5) | Abnormal LFT in 19% of adults. Increased ALT, AST, and total bilirubin in 18%, 21% and 6% |
Yes. GI symptoms similar to adults. Liver involvement in paediatric population not analysed. |
No | Mainly Chinese patients. Combined liver disease and other digestive comorbidities. |
| Wang H et al (May 12th) 123 | 16 studies reported liver function abnormalities | 3678 | 0.8%‐11% had chronic liver comorbidities | Abnormal LFT in 2.6%‐53%. | No | No | Mainly Chinese patients. Meta‐analysis of GI symptoms and Systematic review of liver function tests. |
| Oyelade T et al (May 15th) 124 | 22 studies (kidney and liver disease) | 5595 | 3% (95% CI; 2%‐3%) (CLD, Hepatitis B/C) | No | No | No | Systematic review −57.33% of liver disease patients had severe COVID‐19. |
| Youssef M et al (May 23rd) 125 |
20 studies Mild vs Severe |
3428 | No | No | No | Liver dysfunction associated with poor outcome. | Only Chinese patients. Lack of liver dysfunction definition. |
| Current meta‐analysis | 107 studies | 20 874 |
Yes 3.6% (95% CI, 2.5‐5.1) |
Yes, described each variable. | Yes | Yes. | Included worldwide data spanning 3 continents. Also described DILI. |
Abbreviations: DILI, drug induced liver injury; LFT, liver function test; MERS CoV, Middle East respiratory syndrome coronavirus; SARS‐CoV, severe acute respiratory syndrome coronavirus.
None of the previous studies included pregnant patients.
Liver biopsy in COVID‐19 is documented to show moderate microvesicular steatosis with mild lobular and portal activity. 90 , 127 SARS‐CoV‐2 enters cells through the angiotensin‐converting enzyme 2 (ACE2) receptor. 128 ACE2 receptors are located on alveolar cells, bile duct epithelial cells, and hepatocytes. Interestingly in our review, we noted hyperbilirubinaemia, elevated aminotransferases, a significant elevation in ALP and GGT indicating direct or indirect liver injury in COVID‐19. But liver biopsy in three COVID‐19 patients did not detect the virus on PCR testing and liver immunohistochemistry, implying other plausible mechanisms for liver injury. 129
The second mechanism for liver injury is the cytokine storm, which leads to a surge in inflammatory cytokines and affects the liver. 130 SARS‐CoV‐2 induced acute respiratory distress syndrome (ARDS) and systemic inflammatory response syndrome lead to hypoxia and shock, which can cause liver ischaemia and hypoxia‐reperfusion injury. 131 SARS‐CoV‐2 can infect the endothelial cells directly and result in widespread endotheliitis. 132 , 133 Post‐mortem histological evaluation of three patients infected with SARS‐CoV‐2 has demonstrated lymphocytic endotheliitis in lung, heart, kidney and liver along with hepatocyte necrosis. 132 Further electron microscopy of one of the patients transplanted kidneys revealed viral inclusion structures in endothelial cells. 132 The authors hypothesised that recruitment of immune cells, either by direct viral infection of the endothelium or immune‐mediated, can result in widespread endothelial dysfunction associated with apoptosis. 132 Similarly, another study of 48 severe COVID‐19 demonstrated significant vascular thrombosis along with steatosis, lobular inflammation and portal fibrosis on post mortem liver biopsy. 133 Lastly, drugs itself may cause liver injury. Most patients tend to receive multiple drugs due to the absence of any proven therapy (polypharmacy). Drugs such as remdesivir, lopinavir, ritonavir, oseltamivir, umifenovir and hydroxychloroquine in addition to paracetamol, have hepatotoxic potential which may exacerbate liver injury in patients of COVID‐19. In search of newer drugs for the treatment of SARS‐CoV‐2, many of which have now been shown to be hepatotoxic, careful evaluation of newer drugs, especially in patients pre‐existing elevated liver chemistries are needed.
The prevalence of CLD among COVID‐19 patients is low. However, patients who got infected had lower odds of developing severe COVID‐19, although the data is limited and hence skewed. The relationship between ACE2 receptor, SARS‐CoV‐2 and angiotensin II (Ang II) is intriguing. 134 The ACE2 receptor facilitates the entry of the SARS‐CoV‐2 into type 2 pneumocytes. After endocytosis of the viral complex, ACE2 is downregulated, which results in unopposed angiotensin II accumulation and worsening systemic complications. 134 In a small recent study, patients with COVID‐19 appeared to have elevated levels of plasma Ang II, which were in turn, correlated with total viral load and degree of lung injury. 30 Recombinant ACE2 infusion is proven to reduce disease severity by decreasing Ang II along with increasing ACE2 levels in respiratory viral infection. 135 Increased renin‐angiotensin‐aldosterone system (RAAS) activity is involved in the pathogenesis of various complications in patients with liver cirrhosis. As the liver disease progresses and worsens, the alternate pathway of the renin‐angiotensin system is activated, resulting in a decrease in Ang II and a rise in ACE2. 136 , 137 ACE2 activity is upregulated more than twofold in cirrhotic subjects. 137 ACE2 upregulation contributes to splanchnic vasodilatation by degrading Ang II. 137 Increased ACE2 and low Ang II is responsible for increased splanchnic vasodilatation and worsening systemic haemodynamics in cirrhosis. 136 , 137 Although the prevalence of CLD in COVID‐19 is low, we wonder whether high ACE2 and low Ang II reduces the severity of SARS‐CoV‐2 in advanced cirrhotic patients. Though this is highly speculative, more data is required to assess the outcomes of advanced liver disease patients with COVID‐19. We concur with the recent review, which suggested that patients with CLD are not at greater risk for acquiring the infection based on our meta‐analysis. 138 However, we recommend for further focused research on the effect of existing liver‐related comorbidities on treatment and outcome of COVID‐19.
The main limitation of this meta‐analysis is the high heterogeneity in the published articles. The lack of uniformity in classifying the liver disease to compensated or decompensated liver disease or chronic hepatitis in the included studies may limit the generalisability of our findings in pre‐existing CLD patients. We also did not analyse the data on liver transplant recipients as it is still evolving. There are some reports of increased mortality in liver transplant recipients due to post‐transplant metabolic complications which might outweigh immunosuppression as a risk factor for the development of severe COVID‐19 disease. 139 , 140 Conversely, some authors have suggested the absence of the role of comorbidities in predicting outcomes of COVID‐19 in transplant recipients. 141 Elevated liver chemistries should be uniformly defined in COVID‐19, and the details of outcomes of liver disease patients should be appraised separately.
To date, this is the largest systematic review done on pre‐existing CLD as well as elevated liver chemistries in COVID‐19. We have extensively analysed all groups of the population, including paediatric and pregnant patients detailing the effect of elevated liver chemistries on their outcomes. This is also the first comprehensive review to analyse the effects of drugs, namely remdesvir, lopinavir/ritonavir on hepatic injury in patients with SARS‐CoV‐2 infection.
AUTHORSHIP
Guarantor of the article: Anand V Kulkarni & Pramod Kumar.
Author contributions: AVK and PK conceptualised and designed the study, and retrieved data. HT and MP rechecked the data. XQ cross‐checked the retrieved Chinese data. AVK, RC and RT conducted study analyses. AVK, PK and HT did the initial drafting. RT, JPA, MS, RC, DNR and PNR critically assessed the data and provided intellectual inputs. All members approved the final draft.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Declaration of personal interest: None.
Kulkarni AV, Kumar P, Tevethia HV, et al. Systematic review with meta‐analysis: liver manifestations and outcomes in COVID‐19. Aliment Pharmacol Ther. 2020;52:584–599. 10.1111/apt.15916
As part of AP&T's peer‐review process, a technical check of this meta‐analysis was performed by Dr Y Yuan. The Handling Editor for this article was Professor Grace Wong, and it was accepted for publication after full peer‐review.
REFERENCES
- 1. Yang J, Zheng YA, Gou XI, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in‐hospital death of patients with COVID‐19. Kidney Int. 2020;97:829‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. European Association for the Study of the Liver . EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406‐460. [DOI] [PubMed] [Google Scholar]
- 4. Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: a meta‐analysis. Liver Int. 2020;40:1316‐1320. [DOI] [PubMed] [Google Scholar]
- 5. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis [published online ahead of print, 2020. Apr 23]. J Infect. 10.1016/j.jinf.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008‐2012. [DOI] [PubMed] [Google Scholar]
- 7. Cai Q, Huang D, Yu H, et al. COVID‐19: abnormal liver function tests [published online ahead of print, 2020. Apr 13]. J Hepatol. 10.1016/j.jhep.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(Released by National Health Commission & National Administration of Traditional Chinese Medicine on March 3, 2020). Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chinese Medical Journal. 2020;133:1087–1095. 10.1097/cm9.0000000000000819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community‐acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45‐e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross‐sectional studies (AXIS). BMJ Open. 2016;6:e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo B, Moga C, Harstall C, Schopflocher D. A principal component analysis is conducted for a case series quality appraisal checklist. J Clin Epidemiol. 2016;69:199‐207.e2. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wells GA, Shea B, O'connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2013. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed May 23, 2020.
- 14. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington state. JAMA. 2020;323:1612‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao B, Wang Y, Wen D, et al. A trial of lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qian G‐Q, Yang N‐B, Ding F, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID‐19 in Zhejiang, China: a retrospective, multi‐centre case series. QJM. 2020. 10.1093/qjmed/hcaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu T, Chen C, Zhu Z, et al. Clinical features and dynamics of viral load in imported and non‐imported patients with COVID‐19. Int J Infect Dis. 2020;94:68‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu M, He P, Liu HG, et al. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E016. [DOI] [PubMed] [Google Scholar]
- 23. Zhao D, Yao F, Wang L, et al. A comparative study on the clinical features of COVID‐19 pneumonia to other pneumonias. Clin Infect Dis. 2020. 10.1093/cid/ciaa247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hu Z, Song CI, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang L, Gao YH, Lou LL, Zhang GJ. The clinical dynamics of 18 cases of COVID‐19 outside of Wuhan, China. Eur Respir J. 2020;55:2000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang W, Cao Q, Qin LE, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID‐19): a multi‐center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang Y, Tu M, Wang S, et al. Clinical characteristics of laboratory confirmed positive cases of SARS‐CoV‐2 infection in Wuhan, China: a retrospective single center analysis. Travel Med Infect Dis. 2020:101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID‐19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. 2020. 10.1093/cid/ciaa199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu X‐W, Wu X‐X, Jiang X‐G, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China [published online ahead of print, 2020. Mar 16]. Clin Infect Dis. 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang D, Hu BO, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan J‐W, Yuan S, Kok K‐H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu F, Xu A, Zhang Y, et al. Patients of COVID‐19 may benefit from sustained lopinavir‐combined regimen and the increase of eosinophil may predict the outcome of COVID‐19 progression. Int J Infect Dis. 2020;95:183‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mo P, Xing Y, Xiao YU, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen J, Qi T, Liu LI, et al. Clinical progression of patients with COVID‐19 in Shanghai, China. J Infect. 2020;80:e1‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang J‐J, Dong X, Cao Y‐Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 42. Zhu W, Xie K, Lu H, Xu L, Zhou S, Fang S. Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei, China. J Med Virol. 2020. 10.1002/jmv.25763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID‐19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Y, Liu Y, Liu L, Wang X, Luo N, Li L. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J Infect Dis. 2020;221:1770‐1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64‐68. [DOI] [PubMed] [Google Scholar]
- 46. Chen X, Yang YI, Huang M, et al. Differences between COVID‐19 and suspected then confirmed SARS‐CoV‐2‐negative pneumonia: a retrospective study from a single center. J Med Virol. 2020. 10.1002/jmv.25810 [DOI] [PubMed] [Google Scholar]
- 47. Yu N, Li W, Kang Q, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID‐19 in Wuhan, China: a retrospective, single‐centre, descriptive study. Lancet Infect Dis. 2020;20:559‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Analysis of 92 deceased patients with COVID‐19. J Med Virol. 2020. 10.1002/jmv.25891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang L, Duan Y, Zhang W, et al. Epidemiologic and clinical characteristics of 26 cases of COVID‐19 arising from patient‐to‐patient transmission in Liaocheng, China. Clin Epidemiol. 2020;12:387‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID‐19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24:3404‐3410. [DOI] [PubMed] [Google Scholar]
- 51. Kim ES, Chin BS, Kang CK, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID‐19. J Korean Med Sci. 2020;35:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen T, Wu DI, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen G, Wu DI, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620‐2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cai Y, Hao Z, Gao YI, et al. Coronavirus disease 2019 in the perioperative period of lung resection: a brief report from a single thoracic surgery department in Wuhan, People's Republic of China. J Thorac Oncol. 2020;15:1065‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Su L, Ma X, Yu H, et al. The different clinical characteristics of corona virus disease cases between children and their families in China ‐ the character of children with COVID‐19. Emerg Microbes Infect. 2020;9:707‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pongpirul WA, Mott JA, Woodring JV, et al. Clinical characteristics of patients hospitalized with coronavirus disease, Thailand. Emerg Infect Dis. 2020;26. 10.3201/eid2607.200598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Han Y‐N, Feng Z‐W, Sun L‐N, et al. A comparative‐descriptive analysis of clinical characteristics in 2019‐coronavirus‐infected children and adults. J Med Virol. 2020. 10.1002/jmv.25835 [DOI] [PubMed] [Google Scholar]
- 59. Yang S, Shi Y, Lu H, et al. Clinical and CT features of early stage patients with COVID‐ 19: a retrospective analysis of imported cases in Shanghai, China. Eur Respir J. 2020;55:2000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li R, Tian J, Yang F, et al. Clinical characteristics of 225 patients with COVID‐19 in a tertiary Hospital near Wuhan, China. J Clin Virol. 2020;127:104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen R, Zhang Y, Huang L, Cheng BH, Xia ZY, Meng QT. Safety and efficacy of different anesthetic regimens for parturients with COVID‐19 undergoing Cesarean delivery: a case series of 17 patients. Can J Anaesth. 2020;67:655‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lei Z, Cao H, Jie Y, et al. A cross‐sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID‐19) in Wuhan and outside Wuhan, China [published online ahead of print, 2020. Apr 9]. Travel Med Infect Dis;101664. 10.1016/j.tmaid.2020.101664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID‐19) in Wuhan, China (2019): a single‐centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020. 10.1093/gerona/glaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lescure F‐X, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID‐19 in Europe: a case series. Lancet Infect Dis. 2020;20:697‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fan Z, Chen L, Li J, et al. Clinical features of COVID‐19‐related liver damage. Clin Gastroenterol Hepatol. 2020;18:1561‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Omrani‐Nava V, Maleki I, Ahmadi A, et al. Evaluation of hepatic enzymes changes and association with prognosis in COVID‐19 patients. Hepat Mon. 2020;20:e103179. [Google Scholar]
- 67. Wan S, Xiang YI, Fang W, et al. Clinical features and treatment of COVID‐19 patients in northeast Chongqing. J Med Virol. 2020;92:797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jin XI, Lian J‐S, Hu J‐H, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms. Gut. 2020;69:1002‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liang T, Liu Z, Wu CC, et al. Evolution of CT findings in patients with mild COVID‐19 pneumonia. Eur Radiol. 2020. 10.1007/s00330-020-06823-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang X, Liu W, Zhao J, et al. Clinical characteristics of 80 hospitalized frontline medical workers infected with COVID‐19 in Wuhan, China. J Hosp Infect. 2020. 10.1016/j.jhin.2020.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Qian ZP, Mei X, Zhang YY, et al. Analysis of baseline liver biochemical parameters in 324 cases with novel coronavirus pneumonia in Shanghai area. Zhonghua Gan Zang Bing Za Zhi. 2020;28:229‐233. [DOI] [PubMed] [Google Scholar]
- 72. Lei P, Huang Z, Liu G, et al. Clinical and computed tomographic (CT) images characteristics in the patients with COVID‐19 infection: What should radiologists need to know? J Xray Sci Technol. 2020;28:369‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen S, Liao E, Cao D, Gao Y, Sun G, Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020. 10.1002/jmv.25789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li Y‐K, Peng S, Li L‐Q, et al. Clinical and transmission characteristics of covid‐19 ‐ a retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci. 2020;40:295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lui G, Ling L, Lai CKC, et al. Viral dynamics of SARS‐CoV‐2 across a spectrum of disease severity in COVID‐19. J Infect. 2020. 10.1016/j.jinf.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID‐19‐infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020. 10.1016/j.annonc.2020.03.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid‐19 in critically ill patients in the Seattle region ‐ case series. N Engl J Med. 2020;382:2012‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Du W, Yu J, Wang H, et al. Clinical characteristics of COVID‐19 in children compared with adults in Shandong Province, China. Infection. 2020;48:445‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pan L, Mu MI, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional multicenter study. Am J Gastroenterol. 2020;115:766‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Easom N, Moss P, Barlow G, et al. Sixty‐eight consecutive patients assessed for COVID‐19 infection: Experience from a UK Regional infectious diseases Unit. Influenza Other Respir Viruses. 2020. 10.1111/irv.12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID‐19. Clin Transl Sci. 2020. 10.1111/cts.12805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sun C, Zhang XB, Dai Y, Xu XZ, Zhao J. Clinical analysis of 150 cases of 2019 novel coronavirus infection in Nanyang City, Henan Province. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E042. [DOI] [PubMed] [Google Scholar]
- 84. Lin LU, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS‐CoV‐2 infection. Gut. 2020;69:997‐1001. [DOI] [PubMed] [Google Scholar]
- 85. Du R‐H, Liu L‐M, Yin W, et al. Hospitalization and critical care of 109 decedents with COVID‐19 pneumonia in Wuhan, China. Ann Am Thorac Soc. 2020. 10.1513/AnnalsATS.202003-225OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang R, Pan M, Zhang X, et al. Epidemiological and clinical features of 125 hospitalized patients with COVID‐19 in Fuyang, Anhui, China. Int J Infect Dis. 2020;95:421‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang X, Cai H, Hu J, et al. Epidemiological, clinical characteristics of cases of SARS‐CoV‐2 infection with abnormal imaging findings. Int J Infect Dis. 2020;94:81‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cao J, Tu WJ, Cheng W, et al. Clinical features and short‐term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020. 10.1093/cid/ciaa243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020. 10.1016/j.chest.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Qi X, Liu C, Jiang Z, et al. Multicenter analysis of clinical characteristics and outcome of COVID‐19 patients with liver injury. J Hepatol. 2020. 10.1016/j.jhep.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID‐19 From Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395:1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fang Z, Zhang Y, Hang C, Ai J, Li S, Zhang W. Comparisons of viral shedding time of SARS‐CoV‐2 of different samples in ICU and non‐ICU patients. J Infect. 2020;81:147‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Xia XY, Wu J, Liu HL, Xia H, Jia B, Huang WX. Epidemiological and initial clinical characteristics of patients with family aggregation of COVID‐19. J Clin Virol. 2020;127:104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhang G, Hu C, Luo L, et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. J Clin Virol. 2020;127:104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Huang Y, Chen S, Yang Z, et al. SARS‐CoV‐2 viral load in clinical samples of critically ill patients. Am J Respir Crit Care Med. 2020;201:1435‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19 [published online ahead of print, 2020. Mar 31]. Diabetes Metab Res Rev;e3319. 10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS‐CoV‐2 in Zhejiang province, China, January‐March 2020: retrospective cohort study. BMJ. 2020;369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Shen L, Li S, Zhu Y, et al. Clinical and laboratory‐derived parameters of 119 hospitalized patients with coronavirus disease 2019 in Xiangyang, Hubei Province, China. J Infect. 2020;81:147‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382:1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019‐nCoV pneumonia. Transl Pediatr. 2020;9:51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Liu W, Zhang QI, Chen J, et al. Detection of Covid‐19 in children in early january 2020 in Wuhan, China. N Engl J Med. 2020;382:1370‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sun D, Li H, Lu XX, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020. 10.1093/cid/ciaa198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatr Pulmonol. 2020;55:1169‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shen Q, Guo W, Guo T, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol. 2020;55:1424‐1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Xu YI, Li X, Zhu B, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tan YP, Tan BY, Pan J, Wu J, Zeng SZ, Wei HY. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol. 2020;127:104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zheng F, Liao C, Fan Q‐H, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40:275‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Deng L, Li C, Zeng QI, et al. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020;81:e1‐e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA. 2020;323:1488‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Sun JI, Deng X, Chen X, et al. Incidence of adverse drug reactions in covid‐19 patients in China: an active monitoring study by Hospital Pharmacovigilance System. Clin Pharmacol Ther. 2020. 10.1002/cpt.1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ye XT, Luo YL, Xia SC, et al. Clinical efficacy of lopinavir/ritonavir in the treatment of Coronavirus disease 2019. Eur Rev Med Pharmacol Sci. 2020;24:3390‐3396. [DOI] [PubMed] [Google Scholar]
- 117. Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID‐19. J Infect. 2020;81:e21‐e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid‐19. N Engl J Med. 2020. 382:2327‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Parohan M, Yaghoubi S, Seraj A. Liver injury is associated with severe Coronavirus disease 2019 (COVID‐19) infection: a systematic review and meta‐analysis of retrospective studies. Hepatol Res. 2020. 10.1111/hepr.13510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sultan S, Altayar O, Siddique SM, et al. AGA Institute rapid review of the GI and liver manifestations of COVID‐19, meta‐analysis of international data, and recommendations for the consultative management of patients with COVID‐19. Gastroenterology. 2020. 10.1053/j.gastro.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kukla M, Skonieczna‐Żydecka K, Kotfis K, et al. COVID‐19, MERS and SARS with concomitant liver injury‐systematic review of the existing literature. J Clin Med. 2020;9:1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mao R, Qiu Y, He J‐S, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID‐ 19: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2020. 10.1016/S2468-1253(20)30126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang H, Qiu P, Liu J, Wang F, Zhao Q. The liver injury and gastrointestinal symptoms in patients with Coronavirus Disease 19: a systematic review and meta‐analysis. Clin Res Hepatol Gastroenterol. 2020. 10.1016/j.clinre.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID‐19 in patients with liver and kidney diseases: an early systematic review and meta‐analysis. Trop Med Infect Dis. 2020;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Youssef M, Hussein M, Attia AS, et al. COVID‐19 and Liver dysfunction: a systematic review and meta‐analysis of retrospective studies. J Med Virol. 2020. 10.1002/jmv.26055ka [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:411‐417. [DOI] [PubMed] [Google Scholar]
- 130. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hu LL, Wang WJ, Zhu QJ, Yang L. Novel coronavirus pneumonia‐related liver injury: etiological analysis and treatment strategy. Zhonghua Gan Zang Bing Za Zhi. 2020;28:97‐99. [DOI] [PubMed] [Google Scholar]
- 132. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395:1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sonzogni A, Previtali G, Seghezzi M, et al. Liver and COVID 19 infection: a very preliminary lesson learnt from histological post‐mortem findings in 48 patients. Preprints. 2020. 10.20944/preprints202004.0438.v1 [DOI]
- 134. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020;382:1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Monteil V, Kwon H, Prado P, et al. Inhibition of SARS‐CoV‐2 infections in engineered human tissues using clinical‐grade soluble human ACE2. Cell. 2020;181:905‐913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Casey S, Schierwagen R, Mak K, et al. Activation of the alternate renin‐angiotensin system correlates with the clinical status in human cirrhosis and corrects post liver transplantation. J Clin Med. 2019;8:419. 10.3390/jcm8040419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Paizis G, Tikellis C, Cooper ME, et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Garrido I, Liberal R, Macedo G. Review article: COVID‐19 and liver disease ‐ what we know on 1st May 2020. Aliment Pharmacol Ther. 2020. 10.1111/apt.15813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID‐19 in long‐term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020. 10.1111/ajt.15941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Webb GJ, Moon AM, Barnes E, Barritt AS, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID‐19. Lancet. Gastroenterol Hepatol. 2020. 10.1016/S2468-1253(20)30125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


