ABSTRACT
BACKGROUND AND PURPOSE
Acute stroke patients may have undiagnosed coronavirus disease 2019 (COVID‐19) infection, transmissible to medical professionals involved in their care. Our aim was to determine the value of incorporating a chest computed tomography (CT) scan during acute stroke imaging, and the factors that influence this decision.
METHODS
We constructed a probabilistic decision tree of the value of acquiring a chest CT scan or not, expressed in quality‐adjusted life months (QALM) of patients and medical professionals. The model was based on the chance of detecting infection by chest CT scan, the case fatality rates of COVID‐19 infection, the risk of COVID‐19 infection after exposure, the expected proportion of medical professionals exposed, and the exposure reduction derived from early disease detection.
RESULTS
The decision to incorporate the chest CT scan was superior to not doing so (12.00 QALM vs 11.99 QALM, respectively), when the probability of patients having undetected COVID‐19 infection is 3.5%, potentially exposing 100% of medical professionals, and if early detection reduces exposure by 50%. The risk of developing symptomatic COVID‐19 infection following exposure casts uncertainty on the results, but this is offset by the potential for reducing exposure.
CONCLUSIONS
We identified a measurable benefit of incorporating a chest CT into the urgent imaging protocol of acute stroke patients in reducing exposure of medical professionals without appropriate precautions. The clinical impact of this benefit, however, may not be materially significant.
Keywords: Computed tomography, COVID‐19, stroke
Introduction
A novel coronavirus is responsible for the coronavirus disease 2019 (COVID‐19) pandemic that is currently affecting multiple continents. 1 , 2 , 3 Recent studies have identified that many persons infected by this virus (the exact number is not known) remain asymptomatic, or display only minor symptoms, which lack specificity for COVID‐19. 4 , 5 , 6 Nevertheless, asymptomatic individuals are capable of transmitting the illness with almost the same infectivity as symptomatic patients. 7 , 8 Exposure to COVID‐19 infection can result in a serious illness characterized by a severe acute respiratory syndrome (SARS) that carries an overall mortality estimated between 3.0% and 30%, although the exact number remains uncertain, and its magnitude seems greater in subpopulations of particularly vulnerable individuals and those with multiorgan involvement. 6 , 9 Therefore, reducing exposure of unaffected individuals to those already infected is a critical strategy to reduce the spread of the COVID‐19 infection and its associated mortality. 10 , 11 , 12 Multiple screening schemes have been put into practice across the globe, each taking into consideration the specific scenario in which it is being instituted. 10 , 11 , 12 Unfortunately, such algorithms may fail to identify half of the individuals who are either asymptomatic or in the prodromal state, and yet capable of infecting others. 12 A uniquely vulnerable population is that of the medical professionals involved in acute stroke evaluation, who may be exposed to stroke patients with undiagnosed COVID‐19 infection at the time of evaluation, although the exact risk of contracting the disease is presently unknown, with indirect evidence suggesting it may be very low. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Recent evidence suggests that COVID‐19 infection may be present in about 5.0% to 6.0% of stroke patients, and that COVID‐19 may in turn increase the risk for stroke. 22 , 23 Still, the recommendations made to date largely emphasize patient screening, widespread use of personal protective equipment (PPE) (ie, masks, gowns, gloves, etc), and limited face‐to‐face interactions. 16 , 17 , 23 Confirmation of COVID‐19 infection requires detection of unique sequences of virus RNA using reverse‐transcription polymerase chain reaction (RT‐PCR) from oropharyngeal and nasopharyngeal swabs and, depending upon the kit being used and the need for an extramural reference laboratory, this can take 8‐72 hours. Therefore, the use of RT‐PCR to detect COVID‐19 infection is not practical in the context of acute stroke management. 16 , 17 , 23 However, increasing evidence suggests that distinct patterns of pulmonary involvement by the COVID‐19 infection are identifiable by chest computed tomography (CT) scan in more than 75% of infected patients, with a sensitivity of 97% for early detection. 24 , 25 , 26 , 27 , 28 , 29 These radiographic changes, including characteristic “ground glass” opacities and consolidation (Fig 1), may be present prior to the onset of symptoms, and even predict future symptom onset in individuals who initially test negative by RT‐PCR. 24 , 25 , 26 , 27 , 28 , 29
Fig 1.
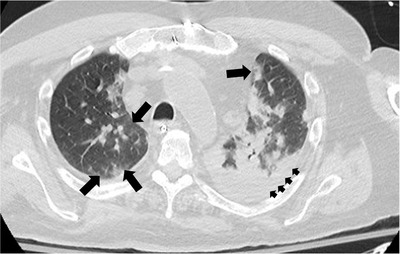
Axial unenhanced chest CT of a patient with COVID‐19 infection, displaying the peripherally located areas of ground‐glass opacity (large arrows) and extensive areas of consolidation (small arrows). Unpublished image courtesy of Saqib A. Chaudhry, MD
Therefore, it seemed reasonable to assess the possible benefit of incorporating a chest CT scan for early detection of COVID‐19‐related pulmonary changes into the urgent imaging protocol routinely used in the evaluation of all stroke patients. The present study explored the utility of a bedside decision‐making model that includes routinely acquiring a chest CT on every stroke patient. Specifically, we examined the implications on the outcomes of both the stroke patients who may be infected with COVID‐19, and on the medical professionals whom they may expose.
Methods
Literature Search and Review
We conducted a search of the English language literature in the National Library of Medicine via PubMed, using permutations of the following search terms: “Stroke,” “myocardial infarction,” “asymptomatic,” “prodrome,” “prodromal,” “risk,” “hospital,” “hospitalization,” “CT,” “computed tomography,” “chest,” “quarantine,” “mortality,” “screening,” and “identification” paired one at a time with one invariable term: “COVID.” The process was then repeated using the invariable term “coronavirus,” paired with each of the others in analogous searches. The resulting citations were entered into a database using dedicated software (EndNote™. Clarivate Analytics, Inc. London, United Kingdom), and the resulting publications examined for relevance. Specifically, we selected those articles that provided quantified chances and risks pertaining to the different variables necessary to construct our probabilistic clinical decision model. In addition, we also reviewed the references listed by each of these citations, in order to identify any additional publications with pertinent risk and outcomes information. Finally, we reviewed the Morbidity and Mortality Weekly Report (MMWR) of the Centers for Disease Control and Prevention (CDC) (ie, https://www.cdc.gov/mmwr/) and further identified applicable publications. Once all relevant publications were selected, we reviewed them with particular attention to the following parameters: (a) risk of stroke patients harboring an asymptomatic COVID‐19 infection, (B) risk of developing symptomatic COVID‐19 infection following exposure, (c) chance of the chest CT being diagnostic (ie, consistent with COVID‐19 infection) in asymptomatic COVID‐19‐infected patients, (d) case fatality rates in asymptomatic COVID‐19‐infected patients with diagnostic findings on chest CT, (e) case fatality rates in symptomatic COVID‐19‐infected patients, and (f) proportion of COVID‐19 patients who require hospitalization. This information was used to construct the decision analysis model. In instances when the specific probabilities could not be directly found in the literature, we used the published information to derive their values by approximation. We concluded our literature data collection, and began our analysis on April 10, 2020.
Clinical Decision Model
We constructed a probabilistic decision tree to analyze the soundness of routinely incorporating a chest CT scan in the urgent imaging studies for the evaluation of acute stroke patients, in order to identify asymptomatic COVID‐19 infections. We did not address those patients who present with signs and symptoms suggestive of a respiratory infection, because completing chest CT under these circumstances is wholly justified based on the existing literature and, therefore, does not require further assessment. 24 , 25 We used, for all input variables, the proportional risk that had been either published or that we approximated from the literature, as described above. Table 1 summarizes the baseline values of all probability and outcome estimations used as inputs for the decisions tree, as well as the relevant literature sources for each. Each one represents the calculated mean (rounded to the nearest multiple of five) of all probabilities found in the literature and these, in turn, encompass the plausible range of values for each variable. The logical arguments we used to choose the baseline values for each input a variable are specified in the following paragraphs.
Table 1.
Input Variables used in the Construction of the Decision Tree
| Variable | Baseline Value | References |
|---|---|---|
| Estimated probabilities at the chance nodes | ||
| Risk of stroke patients harboring asymptomatic COVID‐19 infection | .035 | 4, 30‐40 |
| Risk of developing symptomatic COVID‐19 infection once exposed | .10 | 1, 18‐21, 31‐40 |
| Chance of chest CT (+) in asymptomatic COVID‐19 infection | .75 | 3, 24‐30, 41 |
| Case fatality rate in asymptomatic COVID‐19 with chest CT scan (+) | .25 | 10, 15, 22, 27, 42 |
| Case fatality rate in symptomatic COVID‐19 infections | .03 | 3, 6, 9, 34, 40, 43 |
| Proportion of COVID19 infected patients requiring hospitalization | .50 | 1, 4, 25, 30, 34, 44 |
| Proportion of medical professionals exposed to asymptomatic COVID‐19 infected patient | 1.0 | (*) |
| Predicted rate of medical professionals exposure reduction by COVID‐19 identification | .50 | (*) |
| Estimated outcome values at the endpoints (QALM) | ||
| Person with no evidence of COVID‐19 infection | 12.00 | (*) |
| Person Quarantined due to COVID‐19 infection | 11.00 | 1, 34, 39, 50 |
| Person hospitalized due to COVID‐19 infection | 9.00 | 1, 34, 39, 50 |
| Person dying from COVID‐19 infection | .00 | (*) |
QALM = Quality‐adjusted life month; (*) = Derived from the literature.
The first variable, the probability that any given stroke patient harbors an asymptomatic COVID‐19 infection, depends on the proportion of all COVID19‐infected individuals who remain asymptomatic, augmented by a factor representative of the relationship between respiratory infection and stroke, as noted above. 22 , 23 The magnitude of COVID‐19 asymptomatic carriers is not known with certainty, and it is believed to be underestimated. 3 The published data suggest that the proportion is between 1.5% and 17.9%, averaging 3.5%, which corresponds to the value we assigned it in our decision tree (ie, baseline value = .035 and plausible range = .015‐.05). 4 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 Next, we extrapolated the risk of developing symptomatic COVID‐19 infection following exposure, considering the various levels of exposure risk and the estimated transmissibility of COVID‐19, expressed in part by its basic reproductive number (R 0). 1 , 18 , 19 , 20 , 21 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 We reasoned that, considering the ongoing systematic precautions being instituted across medical facilities, the average risk could be estimated at 10% across all levels of exposure, although it would clearly be higher in very specific high‐risk scenarios, and perhaps even lower if some of the recent reports are accurate. 18 , 19 , 20 Thus, we expressed this risk as a baseline value = .10 plus a plausible range = .05‐.60.
The chance of a chest CT scan being diagnostic (ie, consistent with COVID‐19 infection) in an asymptomatic COVID‐19‐infected patient varies between 10% and 100%. 24 , 25 , 26 , 27 , 28 , 29 Based on this information, and considering larger clinical series, we assigned this variable a Baseline Value = .75 and a Plausible Range = .05‐1.00. 3 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 41 The case fatality rate in those same patients does not seem to be materially different than that reported in series of hospitalized patients, most likely due to the fact that the CT abnormalities frequently precede the development of active signs of infection. 26 , 27 Still, because it is reasonable to think that early identification of COVID‐19 infection has a beneficial impact on outcome, 10 , 15 , 42 we conservatively assigned this variable a baseline value = .25 and a plausible range = .10‐.40. 10 , 15 , 22 , 27 , 42 However, we chose an overall case fatality rate for symptomatic COVID‐19 patients of 3.0% (ie, baseline value = .03 and plausible range = .015‐.20), based on the published studies, which have reported the rate to be as low as 1.4% and as high as 17%. 3 , 6 , 9 , 34 , 40 , 43 The proportion of COVID‐19‐infected patients who require hospitalization is difficult to establish because the published series are based on populations of individuals already hospitalized at the time of the data collection. 1 , 30 , 34 , 44 However, if we consider that those who either remain asymptomatic or develop very mild symptoms (ie, approximately 5‐30%) 4 , 25 , 30 are less likely to require hospitalization, and could be managed by quarantine, then the reciprocal proportion would constitute the remainder subset of hospitalized individuals. 4 , 25 , 30 Thus, we assigned a baseline value = .50 and a plausible range = .25‐.75 to this variable. 1 , 4 , 25 , 30 , 34 , 44 It also seemed reasonable to expect that any given asymptomatic CODIV‐19‐infected patient would likely expose all staff members (with varying magnitude) with whom he came in contact, unless appropriate preventive measures were instituted. 10 , 15 , 16 , 17 , 23 , 45 That is, the proportion of medical professionals exposed to an infected, yet asymptomatic, patient would likely have a baseline value = 1.0 and a plausible range = .10‐1.00, the latter accounting for the widespread precautions instituted in most hospitals, including the use of PPE. 10 , 15 , 16 , 17 , 23 , 45 We then hypothesized that the identification of the COVID‐19 infection by the chest CT scan would reduce the chances of exposure by half (ie, proportion of staff exposed = .50).
The decision tree outcomes (ie, the utility values of the terminal nodes) were quantified in quality‐adjusted life months (QALM) of the survivors, rather than quality‐adjusted life years due to the paucity of data on the long‐term outcomes of COVID‐19 infection. 6 , 9 , 46 , 47 , 48 , 49 The utility values were assigned using the following arguments: (a) the QALM of individuals not infected by COVID‐19 is at least 12.0, allowing disease‐free survival up to the subsequent season (if, in fact COVID‐19 turns out to be a seasonal virus); (b) the QALM of patients who test positive for COVID‐19, and are either asymptomatic or do not require hospitalization, is probably around 11.00, having lost 1 month due to either quarantine or the known time course of the illness; 1 , 34 , 38 , 39 , 50 (c) the QALM of patients infected with COVID‐19, and who require hospitalization, probably averages 9.0, having lost 3 months (conservatively) due to the impact of the illness, 1 , 34 , 38 , 39 , 50 and (d) the QALM of patients who die from COVID‐19 infection is .0.
Once the results of “folding back” the decision tree became available, in order to minimize the risk of structural and programming errors negatively affecting them, we conducted individual univariate sensitivity analyses of all variables over their entire range, evaluating them graphically and specifically comparing the slopes of the different strategies and the rank order of the extreme values. All of the decision tree calculations (ie, “folding back”) and sensitivity analyses were carried out using dedicated computer software (TreePlan™ v.2.03 and SensIt™ v.1.53. TreePlan Software, Inc. San Francisco, CA), following generally accepted rules. 51 , 52
Results
The results of the decision tree analysis are presented in Figure 2. The utility of incorporating a chest CT scan into the urgent stroke imaging is superior to not doing so (12.00 QALM vs 11.99 QALM, respectively), when the probability of detecting asymptomatic COVID‐19 infection by chest CT scan in a stroke patient is 3.5%, the proportion of medical professionals exposed is 100% if the infection remains unidentified, and if such an exposure is reduced by 50% due to early detection of COVID‐19 infection by chest CT scan. These results appear fairly robust, as evidenced by the sensitivity analyses displayed in Table 2. In fact, only one of the eight variables (ie, the risk of developing symptomatic COVID‐19 infection following exposure) was found to have a threshold value within its plausible range, indicating that variations in this particular parameter directly affect the results of the decision tree (Table 2 and Fig 3A). The finding suggests that, at values greater than the threshold (ie, 16% chance of becoming symptomatic following exposure), the incorporation of a chest CT scan loses the beneficial effect. This is further supported by direct comparison of the relative importance of all the input variables. The tornado chart (Fig 3B) demonstrates how this same variable has the greatest swing and, therefore, introduces the greatest degree of uncertainty when interpreting the value of decision tree. Interestingly, the input variable of the proportion of medical professionals exposed to COVID‐19‐infected patients has a degree of swing of comparable magnitude but in the opposite direction (Fig 3B). These findings support incorporating a chest CT scan during acute stroke evaluation to reduce exposure to COVID‐19 infection. This is particularly noticeable when comparing the effect of marginal changes of the input variables on the decision tree results (Fig 3C). The proportion of medical professionals exposed to an asymptomatic COVID‐19‐infected patient continued to have the largest effect when expressed as a function of percentage changes from the baseline value (ie, the steepest slope in Fig 3C).
Fig 2.
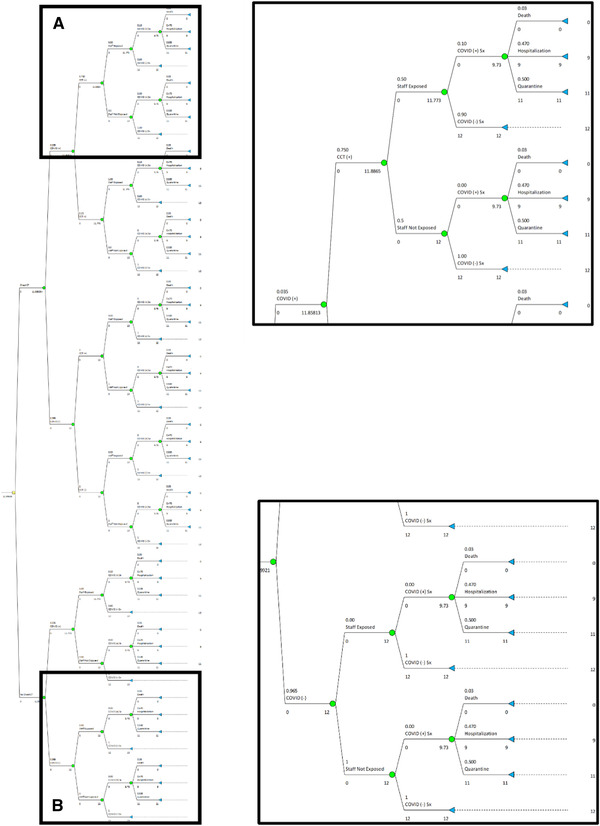
Decision tree in its completed form, with inserts showing details of its two extremes. Insert (A): Reduced probability of medical professionals (ie, “Staff”) being exposed when the chest CT scan is diagnostic, and subsequent probability of developing symptomatic COVID‐19 infection, including the consequent impact on outcomes, measured in quality adjusted life months (QALM). Insert (B): Reduced impact of exposing medical professionals (ie, “Staff”) to individuals not infected by COVID‐19, in whom acquiring a chest CT scan is likely to provide no marginal benefit. Maximally subsequent probability of developing symptomatic COVID‐19 infection, including the consequent impact on outcomes, measured in QALM.
Table 2.
Univariate Sensitivity Analyses of the Decision Tree Results
| Variable | Baseline Value | Plausible Range | Threshold Value | Sensitive? |
|---|---|---|---|---|
| Risk of stroke patients having asymptomatic COVID‐19 infection | .035 | .015.05 | NT | N |
| Risk of developing symptomatic COVID‐19 infection once exposed | .10 | .05‐.20 | 0.16 | Y |
| Chance of chest CT scan (+) in asymptomatic COVID‐19 infected patients | .75 | .05‐1.00 | NT | N |
| Case fatality rate in asymptomatic COVID‐19 infection with chest CT scan (+) | .25 | .10‐.40 | NT | N |
| Case fatality rate in symptomatic COVID‐19 infections | .03 | .015‐.10 | NT | N |
| Proportion of COVID‐19 infected patients requiring hospitalization | .50 | .25‐.75 | NT | N |
| Proportion of medical professionals exposed to asymptomatic COVID‐19 infected patient | 1.00 | .10‐1.00 | NT | N |
| Predicted rate of medical professionals exposure reduction by COVID‐19 infection identification | .50 | .10‐.80 | NT | N |
NT = No threshold value found; N = Not sensitive; Y = Sensitive.
Fig 3.
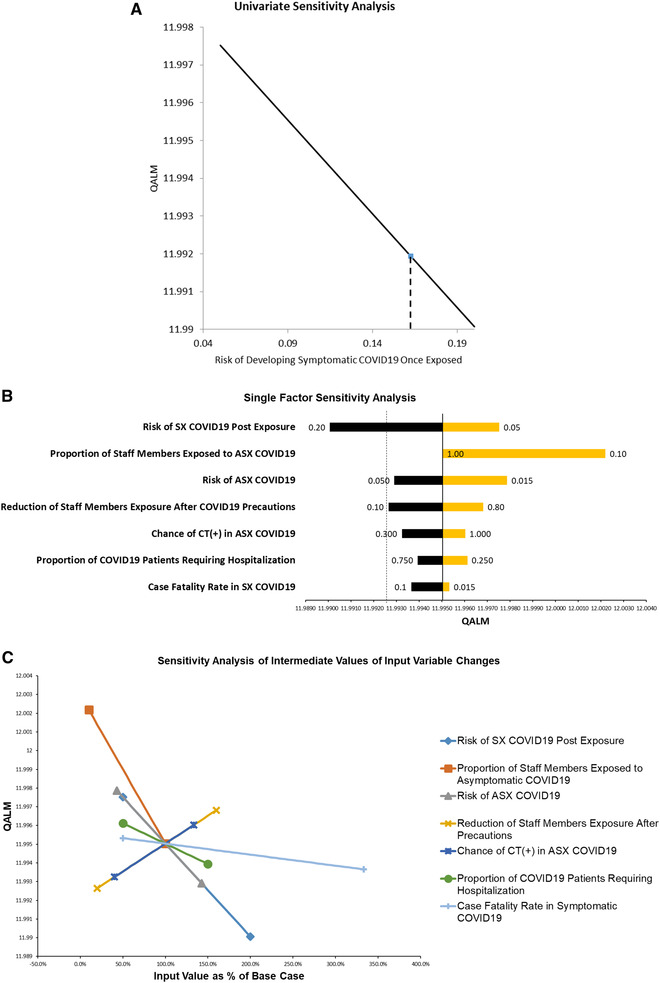
Graphical depictions of the univariate sensitivity analyses of the effect of changes in the different variables on the results of the decision tree: (A) The risk of a medical professional (ie, “Staff Members”) developing symptomatic COVID‐19 infection once exposed marginally decreases the utility of routinely completing chest CT studies if equal to or greater than 16% (ie, threshold value). (B) Single factor tornado chart sorted by degree of swing, demonstrating how dependent the decision tree result is on the plausible ranges specified for the different input variables (see text for description). (C) Multiple input, one output spider chart demonstrates how intermediate ranges (expressed as percentage change from baseline) affect the decision tree result (see text for description).
Discussion
The value of incorporating a chest CT scan into acute stroke management protocols has to be interpreted with the understanding of the number of stroke patients who have concurrent COVID‐19 infection, and the exposure risk to medical professionals. A recent estimation suggests that approximately 35,867 stroke patients who also have a COVID‐19 infection may be seen worldwide. 23 Moreover, recent data from the CDC show that approximately 20% of COVID‐19‐infected persons in the United States have been medical professionals, highlighting the high exposure risk within hospitals. 53 Among medical professionals with data available on age and outcomes, 8‐10% were hospitalized, 2‐5% were admitted to an intensive care unit, and .3‐.6% died. However, the mortality reached 37% in the subset of medical professionals older than 65 years. Therefore, exposure to COVID‐19 infection within the hospital remains an important issue. Nevertheless, the question that remains unanswered is “what is the actual risk of contracting COVID‐19 infection after being exposed in the hospital, particularly to an asymptomatic or mildly symptomatic patient?” Counterbalancing the CDC data mentioned above, recent publications suggest that this risk may be quite small. 18 , 19 , 20 , 21 On the other hand, these represent small retrospective series, with multiple limitations, including a failure to take into account that the sensitivity of RT‐PCR testing for COVID‐19 has a considerably high rate of false‐negative results (ie, about 30%). 24 The uncertainty surrounding this issue is illustrated by the sensitivity analysis of our decision model, although the threshold value of the input variable being higher than the baseline value suggests that lower than average risks would have little effect on the results.
The urgent management of acute stroke patients, some of whom may have unrecognized COVID‐19 infection, has multiple challenges. 16 , 17 , 45 Acute stroke care is very time‐sensitive, requiring rapid clinical and imaging assessments, which may not allow time‐consuming COVID‐19 infection screening procedures. 16 , 17 , 45 Also, the history of exposure and symptoms suggestive of COVID‐19 infection may not be reliably ascertained due to aphasia, altered consciousness, and other neurologic deficits inherent to the stroke syndrome. Moreover, the exposure risk is not limited to the emergency department but extends to other sites such as angiographic suites if endovascular intervention is necessary and/or intensive care units when patients require multimodal monitoring. Therefore, early identification of stroke patients who have COVID‐19 infection may considerably reduce the exposure of medical professionals working at multiple hospital locations. The acquisition of a chest CT scan concurrently with a head CT scan has been recommended for the evaluation of acute stroke patients during the COVID‐19 infection pandemic, but such suggestion has been based on expert opinion, and the need for a data‐driven assessment of this issue has been acknowledged. 23 Presently, however, prospective clinical studies addressing this question are not available, thereby limiting the information from which to derive practical guidance. Thus, in the face of uncertainty, using a probabilistic clinical decision model based on the only existing data constitutes the best alternative method of addressing such an important clinical question. 51 , 52
The results of our decision tree demonstrate that incorporating a chest CT scan within the urgent imaging protocol used to evaluate acute stroke patients constitutes a better strategy than not performing such a scan. However, the value of this approach is small and appears sensitive to (a) attributes that would influence the risk of transmission of the COVID‐19 infection following exposure and (b) the proportion of medical professionals inadvertently exposed to patients with unidentified COVID‐19 infection (Table 2 and Fig 3). Thus, as the risk of developing symptomatic COVID‐19 infection increases, the benefit of screening stroke patients with a chest CT scan is reduced, probably due to the effect of increased infectivity of those with nondiagnostic chest CT studies, with a greater tendency for the infection to spread within the hospitalized population (Figs 3A and 3B).
Our clinical decision model has inherent limitations that must be considered in the interpretation of the results. First, we used somewhat conservative baseline estimations of the variables, thereby increasing the burden of proof that incorporating a chest CT scan would be of any benefit. For example, using a 50% reduction of medical professionals’ exposure following early identification of infected patients by a diagnostic chest CT scan does not reflect the use of comprehensive isolation protocols, which may reduce exposure to 80% or more (ie, at rates comparable to those specified earlier) and may result in a higher magnitude of benefit. Second, our decision tree modeling is an oversimplification of the true pattern of spread of such an infective virus, because it selectively focuses on patient‐to‐medical professionals’ exposure, and does not consider the subsequent exposure from one medical professional to another, as predicted by the exponential growth rate and the R 0 of the infection, originally estimated at .1‐.14/day and 2.2‐2.7, respectively. 32 , 34 , 36 , 38 , 39 , 40 Therefore, any beneficial effect of an intervention destined to reduce exposure would be underestimated, by not accounting for second‐degree exposures. This underestimation becomes even more pronounced when considering more recent data that suggest that the true R 0 of COVID‐19 may be within the 5.0‐7.0 interval. 38 , 54 A third limitation is that the model does not take into account the effect on the actual stroke patient whose urgent imaging protocol will incorporating a chest CT scan, particularly with regard to the time‐sensitive therapeutic decisions required in this clinical scenario. 16 , 17 , 45 However, chest CT scans can be presently completed in just a few minutes following any other neurologic CT studies, without removing the patient from the table. Such an addition is unlikely to interfere with treatment algorithms for intravenous thrombolysis and/or endovascular techniques. Finally, we are compelled to address the magnitude of the gain of incorporating a chest CT scan in the evaluation of every single patient. Although undoubtedly “numerically” significant, the results of our decision model may be viewed as not “clinically” significant, because the difference in value (ie, .01 QALM) equates to a fraction of 1 day. Although decision‐analytic purists have argued that it does not matter, 55 such a small margin could also be interpreted as either of the two courses of action being “not‐inferior” to the other.
In conclusion, incorporating a chest CT into the urgent imaging protocol of acute stroke patients seems to reduce exposure of medical professionals, with a beneficial effect measurable in QALMs. The clinical impact of this benefit, however, is questionable, although it is likely to be magnified when comprehensive isolation techniques applied to patients identified by chest CT scan lead to greater than 80% reduction of the chance of exposure of medical professionals.
Acknowledgment and Disclosure: The work leading to this manuscript has not received any external funding. The authors have not conflicts of interests to disclose.
References
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lai CC, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (sars‐cov‐2): facts and myths. J Microbiol Immunol Infect 2020;53:404‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID‐19) cases on board the diamond princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 2020;25:2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nishiura H, Kobayashi T, Suzuki A, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID‐19). Int J Infect Dis 2020;94:154‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gaye B, Fanidi A, Jouven X. Denominator matters in estimating COVID‐19 mortality rates. Eur Heart J 2020. 10.1093/eurheartj/ehaa282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu X, Yang R. COVID‐19 transmission through asymptomatic carriers is a challenge to containment. Influenza Other Respir Viruses 2020. 10.1111/irv.12743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA 2020;323:1406‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baud D, Qi X, Nielsen‐Saines K, et al. Real estimates of mortality following COVID‐19 infection. Lancet Infect Dis 2020. 10.1016/S1473-3099(20)30195-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng C, Wang J, Guo H, et al. Risk‐adapted treatment strategy for COVID‐19 patients. Int J Infect Dis 2020;94:74‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agrawal S, Goel AD, Gupta N. Emerging prophylaxis strategies against COVID‐19. Monaldi Arch Chest Dis 2020;90. 10.4081/monaldi.2020.1289 [DOI] [PubMed] [Google Scholar]
- 12. Gostic K, Gomez AC, Mummah RO, et al. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID‐19. Elife 2020;9:e55570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams JG, Walls RM. Supporting the health care workforce during the COVID‐19 global epidemic. JAMA 2020;323(15):1439‐1440. [DOI] [PubMed] [Google Scholar]
- 14. Chughtai AA, Seale H, Islam MS, et al. Policies on the use of respiratory protection for hospital health workers to protect from coronavirus disease (COVID‐19). Int J Nurs Stud 2020;105:103567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang D, Xu H, Rebaza A, et al. Protecting health‐care workers from subclinical coronavirus infection. Lancet Respir Med 2020;8:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. AHA/ASA Stroke Council Leadership . Temporary emergency guidance to us stroke centers during the COVID‐19 pandemic: on behalf of the American Heart Association/American Stroke Association Stroke Council Leadership. Stroke 2020;51:1910‐2. [DOI] [PubMed] [Google Scholar]
- 17. Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019 (COVID‐19) pandemic. Stroke 2020;51:1891‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Canova V, Lederer Schläpfer H, Piso RJ, et al. Transmission risk of sars‐cov‐2 to healthcare workers ‐ observational results of a primary care hospital contact tracing. Swiss Med Wkly 2020;150:w20257. [DOI] [PubMed] [Google Scholar]
- 19. Heinzerling A, Stuckey MJ, Scheuer T, et al. Transmission of COVID‐19 to health care personnel during exposures to a hospitalized patient ‐ Solano county, California, February 2020. MMWR Morb Mortal Wkly Rep 2020;69:472‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ng K, Poon BH, Kiat Puar TH, et al. COVID‐19 and the risk to health care workers: a case report. Ann Intern Med 2020;172:766‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 22. Li Y, Wang M, Zhou Y, Chang J. Acute cerebrovascular disease following COVID‐19: a single center, retrospective, observational study. Available at: https://ssrn.com/abstract=3550025. Accessed March 3, 2020. [DOI] [PMC free article] [PubMed]
- 23. Qureshi AI, Adbd ‐Allah F, Alsenani F, etal. Management of acute ischemic stroke in patients with COVID‐19 infection: recommendations from an international panel. Int J Stroke 2020. 10.1177/1747493020923234 [DOI] [PubMed] [Google Scholar]
- 24. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology 2020. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li K, Fang Y, Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID‐19). Eur Radiol 2020. 10.1007/s00330-020-06817-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019‐ncov). Radiology 2020;295:202‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with covid‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoon SH, Lee KH, Kim JY, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID‐19): analysis of nine patients treated in Korea. Korean J Radiol 2020;21:494‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong HYF, Lam HYS, Fong AH, et al. Frequency and distribution of chest radiographic findings in COVID‐19 positive patients. Radiology 2020. 10.1148/radiol.2020201160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang X, Fang J, Zhu Y, et al. Clinical characteristics of non‐critically ill patients with novel coronavirus infection (COVID‐19) in a Fangcang hospital. Clin Microbiol Infect 2020. 10.1016/j.cmi.2020.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burke RM, Midgley CM, Dratch A, et al. Active monitoring of persons exposed to patients with confirmed COVID‐19 ‐ United States, January‐February 2020. MMWR Morb Mortal Wkly Rep 2020;69:245‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Du Z, Wang L, Cauchemez S, et al. Risk for transportation of 2019 novel coronavirus disease from Wuhan to other cities in China. Emerg Infect Dis 2020;26:1049‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li C, Ji F, Wang L, et al. Asymptomatic and human‐to‐human transmission of sars‐cov‐2 in a 2‐family cluster, Xuzhou, China. Emerg Infect Dis 2020;26. 10.3201/eid2607.200718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med 2020;382:1199‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qian G, Yang N, Ma AHY, et al. A COVID‐19 transmission within a family cluster by presymptomatic infectors in China. Clin Infect Dis 2020. 10.1093/cid/ciaa316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Riou J, Althaus CL. Pattern of early human‐to‐human transmission of Wuhan 2019 novel coronavirus (2019‐ncov), December 2019 to January 2020. Euro Surveill 2020;25:2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019‐ncov infection from an asymptomatic contact in Germany. N Engl J Med 2020;382:970‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steven S, Yen Ting L, Chonggang X, et al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis 2020;26. 10.3201/eid2607.200282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019‐ncov outbreak originating in Wuhan, China: a modelling study. Lancet 2020;395:689‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yuan J, Li M, Lv G, et al. Monitoring transmissibility and mortality of COVID‐19 in Europe. Int J Infect Dis 2020;95:311‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China Life Sci 2020;63:706‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun Q, Qiu H, Huang M, et al. Lower mortality of COVID‐19 by early recognition and intervention: experience from Jiangsu province. Ann Intensive Care 2020;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Team CC‐R. Severe outcomes among patients with coronavirus disease 2019 (COVID‐19) ‐ United States, February 12‐March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:343‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Du RH, Liu LM, Yin W, et al. Hospitalization and critical care of 109 decedents with COVID‐19 pneumonia in Wuhan, China. Ann Am Thorac Soc 2020. 10.1513/AnnalsATS.202003-225OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao J, Rudd A, Liu R. Challenges and potential solutions of stroke care during the coronavirus disease 2019 (COVID‐19) outbreak. Stroke 2020;51:1356‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: a scoping review. Infect Dis Poverty 2020;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ahn DG, Shin HJ, Kim MH, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID‐19). J Microbiol Biotechnol 2020;30:313‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jordan RE, Adab P, Cheng KK. COVID‐19: risk factors for severe disease and death. BMJ 2020;368:m1198. [DOI] [PubMed] [Google Scholar]
- 49. Jung SM, Akhmetzhanov AR, Hayashi K, et al. Real‐time estimation of the risk of death from novel coronavirus (COVID‐19) infection: inference using exported cases. J Clin Med 2020;9:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of COVID‐19 ‐ studies needed. N Engl J Med 2020;382:1194‐6. [DOI] [PubMed] [Google Scholar]
- 51. Dittus RS, Roberts SD, Wilson JR. Quantifying uncertainty in medical decisions. J Am Coll Cardiol 1989;14:23A‐8A. [DOI] [PubMed] [Google Scholar]
- 52. Sarasin FP. Decision analysis and the implementation of evidence‐based medicine. QJM 1999;92:669‐71. [DOI] [PubMed] [Google Scholar]
- 53. Team CC‐R . Characteristics of health care personnel with covid‐19 — United States, February 12–April 9, 2020. Morb Mortal Wkly Rep 2020;69:477‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu Y, Gayle AA, Wilder‐Smith A, et al. The reproductive number of COVID‐19 is higher compared to SARS coronavirus. J Travel Med 2020;27:taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kassirer JP, Pauker SG. The toss‐up. N Engl J Med 1981;305:1467‐9. [DOI] [PubMed] [Google Scholar]


