Abstract
Many patients with severe coronavirus disease 2019 (COVID‐19) remain unresponsive after surviving critical illness. Although several structural brain abnormalities have been described, their impact on brain function and implications for prognosis are unknown. Functional neuroimaging, which has prognostic significance, has yet to be explored in this population. Here we describe a patient with severe COVID‐19 who, despite prolonged unresponsiveness and structural brain abnormalities, demonstrated intact functional network connectivity, and weeks later recovered the ability to follow commands. When prognosticating for survivors of severe COVID‐19, clinicians should consider that brain networks may remain functionally intact despite structural injury and prolonged unresponsiveness. ANN NEUROL 2020;88:851–854
Introduction
After surviving critical illness, many patients with severe coronavirus disease 2019 (COVID‐19) experience a prolonged disorder of consciousness. Some cases have been attributed to structural brain injury; among patients with COVID‐19 who undergo magnetic resonance imaging (MRI), brain lesions are detected in up to 44%. 1 However, the impact of these lesions on brain function, and their implications for neurologic recovery, remain unknown. Furthermore, other patients with COVID‐19 and a prolonged disorder of consciousness have no evidence of structural brain injury. Thus, although clinicians typically rely on structural neuroimaging for neuroprognostication, its utility in COVID‐19 is unclear, complicating critical decisions about continuation of life‐sustaining treatment.
Functional neuroimaging specifically resting‐state functional MRI (rs‐fMRI) has prognostic significance in disorders of consciousness. 2 , 3 , 4 , 5 Recent studies have shown that rs‐fMRI is more effective in predicting neurologic outcomes than the structural neuroimaging frequently used for neuroprognostication. 6 , 7 As such, rs‐fMRI, which evaluates the connectivity of brain networks by measuring spontaneous oscillations of brain activity, 8 may inform the likelihood of neurologic recovery after COVID‐19. The default mode network (DMN) is a functional brain network thought to be involved in human consciousness. 9 , 10 , 11 A growing body of literature has shown that stronger DMN connectivity in patients with disorders of consciousness predicts better neurologic recovery. 2 , 3 , 4 , 7 Therefore, as part of a clinical MRI protocol, we implemented rs‐fMRI to evaluate DMN connectivity in a critically ill patient with severe COVID‐19 and prolonged unresponsiveness.
Subjects and Methods
Case Description
The patient is a 47‐year‐old man with hypertension and asthma who presented with several days of fevers and dyspnea. He was found to be hypoxic and tested positive for severe acute respiratory syndrome–coronavirus 2. On hospital day 1, he developed progressive respiratory failure, requiring intubation and transfer to the intensive care unit. His course was notable for acute respiratory distress syndrome requiring mechanical ventilation for >40 days, shock, renal failure, and pneumomediastinum. Twenty days after admission, sedation was weaned, but for the next several weeks he fluctuated between coma and a minimally conscious state, in which he intermittently visually tracked an examiner but did not otherwise demonstrate purposeful behaviors (Fig. 1A). Electroencephalography (EEG) on day 39 showed disorganized delta‐theta slowing but no evidence of seizures or epileptiform discharges (see Fig. 1B).
FIGURE 1.
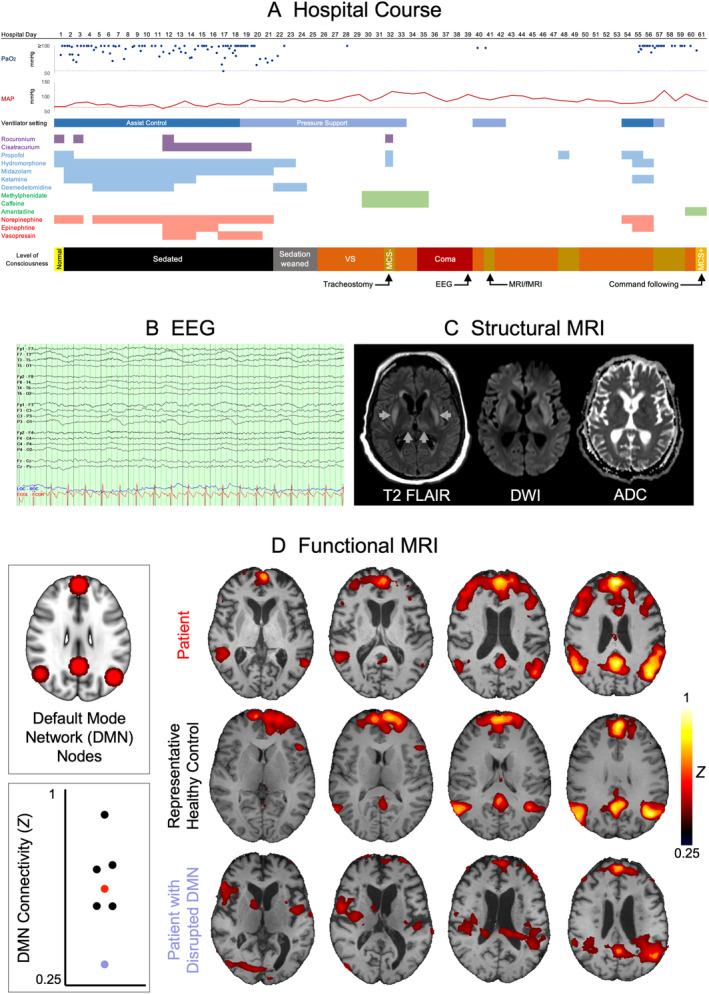
(A) The patient's hospital course is depicted relative to days since admission. The patient's partial pressure of oxygen (PaO2) is depicted in dark blue (with the oxygenation goal of >55mmHg proposed by the Acute Respiratory Distress Syndrome Network depicted as a light blue dotted line), and his mean arterial pressure (MAP) is depicted in dark red (with the goal of >65mmHg depicted as a pink dotted line). The timing of paralytics is depicted in purple, sedatives in blue, stimulants in green, and vasopressors in red. The patient's level of consciousness is depicted over time; he was in a coma when not opening his eyes, in a vegetative state (VS; also known as the unresponsive wakefulness syndrome) when opening his eyes but not showing purposeful responses, in a minimally conscious state minus (MCS−) when demonstrating visual pursuit, and in a minimally conscious state plus (MCS+) when demonstrating visual pursuit and following commands. (B) Electroencephalographic (EEG) results are shown from a representative 12‐second recording. (C) Structural magnetic resonance imaging (MRI) results are shown, including T2‐weighted fluid‐attenuated inversion recovery (FLAIR), diffusion‐weighted imaging (DWI), and apparent diffusion coefficient (ADC) sequences. Basal ganglia and thalamic T2 hyperintensities are indicated by arrows. (D) Resting‐state fMRI results are shown for the patient, a representative healthy control, and another patient with diminished default mode network (DMN) connectivity (ie, a negative control). DMN nodes, used as seeds in the analysis, are shown in red. Overall DMN connectivity, defined as the average correlation values within the DMN nodes, is compared across subjects; the patient is represented in red, the healthy controls in black, and the negative control in purple. fMRI = functional MRI.
On day 40, while mechanically ventilated via tracheostomy, he underwent structural and functional brain MRI, using a 3T Skyra MRI scanner (Siemens Healthcare, Erlangen, Germany) and 32‐channel head coil. Structural MRI showed symmetric T2 hyperintensity of the bilateral basal ganglia, medial thalami, and parahippocampal gyri, and diffusion restriction of the basal ganglia (see Fig. 1C), a pattern seen in hypoxic–ischemic brain injury (although there was never cardiac or respiratory arrest) as well as toxic, metabolic, infectious, and inflammatory conditions.
He underwent high‐resolution rs‐fMRI using a 10‐minute blood oxygen level–dependent sequence with an echo time of 30.3 milliseconds, a repetition time of 1250 milliseconds, and simultaneous multislice acquisition (acceleration factor = 4). He was not under sedation at the time of the scan. We assessed DMN connectivity by conducting a seed‐to‐voxel analysis, using 10mm spherical seeds at 4 nodes of the DMN—the medial prefrontal cortex, the posterior cingulate cortex, and bilateral inferior parietal lobules—as previously described. 12 We used freely available CONN software (https://www.nitrc.org/projects/conn). Acquisition parameters and analytic code are provided at www.github.com/ComaRecoveryLab/COVID-19_rsfMRI. We compared the patient's rs‐fMRI results to those of 5 healthy control subjects scanned with identical parameters as part of an ongoing research study (https://clinicaltrials.gov/ct2/show/NCT03504709).
Results
Unexpectedly, rs‐fMRI revealed robust functional connectivity within the patient's DMN (see Fig. 1D). DMN connectivity—defined as the average correlation within the 4 nodes of the DMN—was comparable between the patient and healthy controls. Illustrating that DMN connectivity is not found indiscriminately in all patients with disorders of consciousness, diminished DMN connectivity was observed in another unresponsive individual scanned with identical parameters (see Fig. 1D).
Twenty days later, on hospital day 61, the patient began following commands and continued to do so intermittently for at least the next week. Specifically, he blinked his eyes to command, opened his mouth to command, and on day 66 followed 4 of 4 vocalization commands during standardized behavioral assessment with the Coma Recovery Scale–Revised. 13 By this time, he also consistently demonstrated gaze tracking to visual and auditory stimuli.
Discussion
For this patient with persistently altered consciousness after surviving severe COVID‐19, the biomarkers typically used for neuroprognostication were either equivocal (eg, EEG) or concerning (eg, structural brain MRI). However, rs‐fMRI showed intact brain network connectivity, suggesting that the neurologic prognosis may not be as grim as the conventional prognostic biomarkers implied. In the setting of these discordant data and the resulting prognostic uncertainty, the family believed that the patient would want life‐sustaining treatment continued. Ultimately, his level of consciousness improved to the point of following commands, which indicated not only neurologic recovery, but also a greater likelihood of ongoing functional improvement. 14 , 15
The long‐term outcomes for patients with disorders of consciousness following severe COVID‐19, including the patient presented here, are unknown. Longitudinal cohort studies in this population will be crucial for understanding their disease course and developing prognostic biomarkers. Nonetheless, families and clinicians are often compelled to decide whether to continue life‐sustaining treatment in persistently unresponsive patients, before such long‐term research can be completed. Therefore, any available data that may inform prognosis in this novel disease are critical. Clinical functional neuroimaging may not be feasible in every medical center. However, when prognosticating, clinicians should consider the possibility that patients with disorders of consciousness following severe COVID‐19, despite structural brain injury, may have intact functional brain networks suggestive of a more optimistic neurologic prognosis. It is therefore important to exercise caution before presuming a poor neurologic outcome based on conventional biomarkers, and to acknowledge prognostic uncertainty in discussions with families.
Author Contributions
D.F., Z.D.T., Y.G.B., and B.L.E. contributed to the conception and design of the study. All authors contributed to the acquisition and analysis of data. D.F., Z.D.T., Y.G.B., and B.L.E. contributed to drafting the text and preparing the figure.
Potential Conflicts of Interest
Nothing to report.
Acknowledgment
This study was supported by the James S. McDonnell Foundation COVID‐19 Recovery of Consciousness Consortium, the NIH National Institute of Neurological Disorders and Stroke (R21NS109627, R25NS06574309, K23NS094538, RF1NS115268), the NIH Director's Office (DP2HD101400), the NIH National Institute on Aging (R21AG067562), and the Tiny Blue Dot Foundation.
References
- 1. Kandemirli S, Dogan L, Sarikaya Z, et al. Brain MRI findings in patients in the intensive care unit with COVID‐19 infection. Radiology 2020;20:201697. 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koenig MA, Holt JL, Ernst T, et al. MRI default mode network connectivity is associated with functional outcome after cardiopulmonary arrest. Neurocrit Care 2014;20:348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Norton L, Hutchison RM, Young GB, et al. Disruptions of functional connectivity in the default mode network of comatose patients. Neurology 2012;78:175–181. [DOI] [PubMed] [Google Scholar]
- 4. Song M, Yang Y, He J, et al. Prognostication of chronic disorders of consciousness using brain functional networks and clinical characteristics. Elife 2018;7:e36173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silva S, De Pasquale F, Vuillaume C, et al. Disruption of posteromedial large‐scale neural communication predicts recovery from coma. Neurology 2015;85:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pugin D, Hofmeister J, Gasche Y, et al. Resting‐state brain activity for early prediction outcome in postanoxic patients in a coma with indeterminate clinical prognosis. AJNR Am J Neuroradiol 2020;41:1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sair HI, Hannawi Y, Li S, et al. Early functional connectome integrity and 1‐year recovery in comatose survivors of cardiac arrest. Radiology 2018;287:247–255. [DOI] [PubMed] [Google Scholar]
- 8. Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005;102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosazza C, Andronache A, Sattin D, et al. Multimodal study of default‐mode network integrity in disorders of consciousness. Ann Neurol 2016;79:841–853. [DOI] [PubMed] [Google Scholar]
- 10. Fernández‐Espejo D, Soddu A, Cruse D, et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol 2012;72:335–343. [DOI] [PubMed] [Google Scholar]
- 11. Buckner RL, DiNicola LM. The brain's default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci 2019;20:593–608. [DOI] [PubMed] [Google Scholar]
- 12. Threlkeld ZD, Bodien YG, Rosenthal ES, et al. Functional networks reemerge during recovery of consciousness after acute severe traumatic brain injury. Cortex 2018;106:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giacino JT, Kalmar K, Whyte J, The JFK. Coma Recovery Scale‐Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004;85:2020–2029. [DOI] [PubMed] [Google Scholar]
- 14. Thibaut A, Bodien YG, Laureys S, Giacino JT. Minimally conscious state “plus”: diagnostic criteria and relation to functional recovery. J Neurol 2020;267:1245–1254. [DOI] [PubMed] [Google Scholar]
- 15. Giacino J, Kalmar K. The vegetative and minimally conscious states: a comparison of clinical features and functional outcome. J Head Trauma Rehabil 1997;12:36–51. [Google Scholar]


