Abstract
Myelin oligodendrocyte glycoprotein is a major target of the humoral immune response in children affected by inflammatory demyelinating diseases of the central nervous system. Although myelin oligodendrocyte glycoprotein causes autoimmune encephalitis in different animal models, the relevance of this mechanism in human autoimmune diseases of the central nervous system is unclear. We herein report a child with acute disseminated encephalomyelitis possibly triggered by central nervous system infection of primary herpes simplex virus in the presence of antimyelin oligodendrocyte glycoprotein antibody. A healthy 5-year-old Japanese boy suffered from acute disseminated encephalomyelitis. He was positive for antimyelin oligodendrocyte glycoprotein antibody in both the serum and the cerebrospinal fluid, and herpes simplex virus-1 DNA on polymerase chain reaction of the cerebrospinal fluid. We speculated that the central nervous system infection of primary herpes simplex virus disrupted the blood–brain barrier, and antimyelin oligodendrocyte glycoprotein antibody already present in serum was transferred to the cerebrospinal fluid, resulting in the onset of acute disseminated encephalomyelitis. This might be the mechanism underlying postinfectious acute disseminated encephalomyelitis associated with myelin oligodendrocyte glycoprotein antibody.
Keywords: antimyelin oligodendrocyte glycoprotein antibodies, acute disseminated encephalomyelitis, herpes simplex virus infection
Myelin oligodendrocyte glycoprotein is exclusively expressed on the surface of oligodendrocytes in the central nervous system.1 Antimyelin oligodendrocyte glycoprotein antibody is predominantly detected in pediatric acute disseminated encephalomyelitis, optic neuritis, and aquaporin-4 antibody-seronegative neuromyelitis optica spectrum disorder.2 Pediatric acute disseminated encephalomyelitis cases have a higher rate of positive antimyelin oligodendrocyte glycoprotein antibody than adult cases.3 The ratio of preceding infection is higher in patients with demyelinating disorders associated with antimyelin oligodendrocyte glycoprotein antibody than in those with disorders not associated with myelin oligodendrocyte glycoprotein antibody.4 These findings suggest that infection might trigger the immune reaction causing acute disseminated encephalomyelitis associated with antimyelin oligodendrocyte glycoprotein antibody in children.
We encountered a case of acute disseminated encephalomyelitis likely triggered by central nervous system infection of primary herpes simplex virus in the presence of antimyelin oligodendrocyte glycoprotein antibody. This case supports the mechanism involving the passive incurrence of antimyelin oligodendrocyte glycoprotein antibody through the blood–brain barrier from the periphery into the central nervous system potentially playing an important role in the development of acute disseminated encephalomyelitis.
Case Report
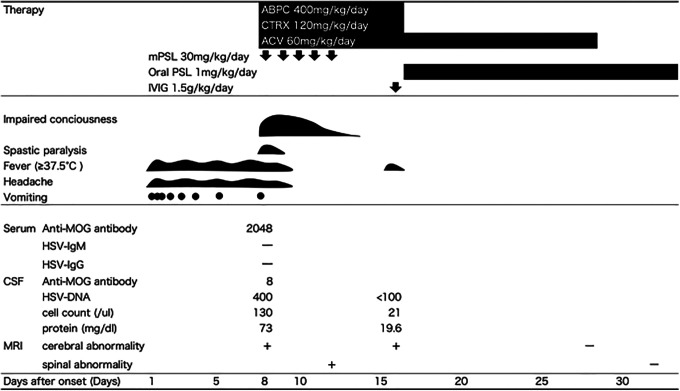
A healthy 5-year-old boy developed a fever, pharyngitis, vomiting, and headache (Figure 1). He was admitted our hospital because of a persistent fever, vomiting, and headache, as well as the new appearance of an impaired consciousness 8 days after the onset.
Figure 1.
Clinical course of the therapy, symptoms, and laboratory data. This graph illustrates the therapy course, the patient’s symptoms, and the laboratory data. ABPC indicates ampicillin; ACV, acyclovir; CSF, cerebrospinal fluid; CTRX, ceftriaxone; HSV, herpes simplex virus; IVIG, intravenous immunoglobulin; MOG, myelin oligodendrocyte glycoprotein; mPSL, methylprednisolone; MRI, magnetic resonance imaging; PSL, prednisolone.
Clinical findings showed a Glasgow Coma Scale of E3V2M5, body temperature of 38.0 °C, neck stiffness, spasticity of the bilateral ankle joints, and hypertonia. A laboratory analysis showed an elevated white blood cell count of 21 280/µL and a C-reactive protein level of 1.5 mg/dL. A cerebrospinal fluid examination showed pleocytosis (130/µL with mononuclear cells 110/μL, neutrophils 20/μL), with a protein concentration of 73.1 mg/dL.
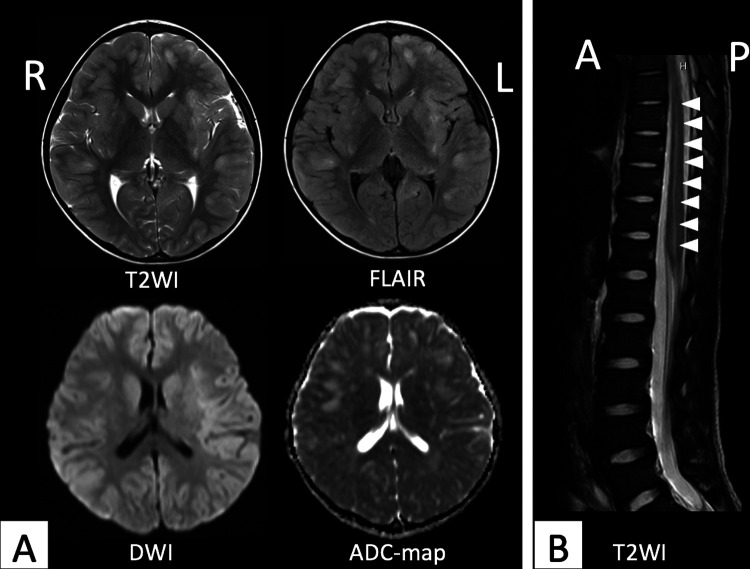
An electroencephalogram showed a 1.5- to 2-Hz diffuse signal consisting of a high-amplitude δ wave predominantly in bilateral frontal head regions, 8 days after the onset. There were no findings suggesting focal encephalitis with periodic lateralized epileptiform discharges, asymmetry of background activity, or focal abnormal discharges. Brain magnetic resonance imaging (MRI) on day 8 after the onset showed multifocal high-intensity lesions in the bilateral cortex and subcortical white matter on corresponding T2-weighted imaging, fluid-attenuated inversion recovery imaging, and diffusion-weighted imaging, and a low-intensity lesion on corresponding apparent diffusion coefficient map (Figure 2A).
Figure 2.
A, Brain MRI findings in day 8 after the onset. The images showed multifocal high-intensity lesions on corresponding axial T2-weighted imaging, fluid-attenuated inversion recovery imaging, and diffusion-weighted imaging, and a low-intensity lesion on corresponding apparent diffusion coefficient map. B, Spinal MRI findings in day 13 after the onset. The image showed a high-intensity lesion (arrow) in the cervical spinal cord on T2-weighted imaging. A indicates anterior; ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery imaging; L, left; MRI, magnetic resonance imaging; P, posterior; R, right; T2WI, T2-weighted imaging.
We suspected the patient had acute disseminated encephalomyelitis based on the findings of an impaired consciousness and the neuroimaging and electroencephalography results and acute meningoencephalitis based on the clinical symptoms, neck stiffness, and cerebrospinal fluid pleocytosis. The diagnosis of acute disseminated encephalomyelitis was based on the International Pediatrics Multiple Sclerosis Study Group criteria.5 We started therapy with intravenous methylprednisolone (30 mg/kg/d) for 5 consecutive days for acute disseminated encephalomyelitis. At the same time, we administered acyclovir 60 mg/kg/d and the antibiotics ampicillin 400 mg/kg/d and ceftriaxone 120 mg/kg/d for acute meningoencephalitis. The fever, vomiting, headache, and spasticity disappeared at 10 days after the onset. We performed spinal MRI on day 13 after the onset and found a high-intensity lesion in the cervical spinal cord on T2-weighted imaging (Figure 2B). His conscious level improved at 14 days after the onset.
Although we confirmed negativity for blood culture and stopped ampicillin and ceftriaxone on day 16 after the onset, he developed a fever again. We added intravenous immunoglobulin at 1.5 g/kg/d and continued acyclovir until day 28 after the onset, as we retrospectively confirmed elevated levels of herpes simplex virus-1 DNA in the cerebrospinal fluid (400 copies/mL; normal: <100 copies/mL) by real-time polymerase chain reaction at the time of admission. All of his symptoms were improved, and we started the administration of oral prednisolone (1 mg/kg/d) on day 18 after the onset. We confirmed negativity for herpes simplex virus-1 DNA in the cerebrospinal fluid and improvement in his brain and spinal MRI lesions on day 29 after the onset. He was discharged from our hospital on day 31 after the onset. He did not have any neurological deficits.
Cell-based immunoassays revealed positivity for antimyelin oligodendrocyte glycoprotein antibody with a titer of 1:2048 (normal <1:128) in the serum, 1:8 (normal <1:2) in the cerebrospinal fluid, and negativity for antiaquaporin-4 antibody. Other specific to the autoimmune encephalitis antibodies such as anti-N-methyl-d-aspartate receptor antibody or antileucine-rich glioma-inactivated 1 antibody were not measured. Myelin basic protein level was elevated (15 714 pg/mL, normal <102 pg/mL) and oligoclonal bands were negative at the time of admission (day 8). At the same time, herpes simplex virus-1 DNA polymerase chain reaction in the cerebrospinal fluid revealed positivity, but the serum values for herpes simplex virus-1 immunoglobulin M (0.09; normal: <0.80) and immunoglobulin G (0.1; normal: <2.0) indicated negativity.
Antimyelin oligodendrocyte glycoprotein antibody assays for antimyelin oligodendrocyte glycoprotein antibody were performed at Tohoku University. The cell-based assay for myelin oligodendrocyte glycoprotein antibody detection in living cells has previously been described.2 Goat antihuman immunoglobulin G was used as a secondary antibody.
Discussion
We herein report a patient who had herpes simplex virus meningoencephalitis coexisting with acute disseminated encephalomyelitis associated with antimyelin oligodendrocyte glycoprotein antibody. We confirmed primary herpes simplex virus infection based on the clinical findings of a fever, pharyngitis, meningitis, and virological findings of serological negativity for both herpes simplex virus immunoglobulin M and immunoglobulin G and positivity for herpes simplex virus polymerase chain reaction in his cerebrospinal fluid. Detection of herpes simplex virus DNA in the cerebrospinal fluid by polymerase chain reaction has become the gold-standard test for the diagnosis of herpes simplex virus infection involving the central nervous system because the sensitivity of cerebrospinal fluid polymerase chain reaction in HSE is 96%, and its specificity is 99%.6
Antimyelin oligodendrocyte glycoprotein antibody is predominantly detected in pediatric acute disseminated encephalomyelitis, optic neuritis, and aquaporin-4 antibody-seronegative neuromyelitis optica spectrum disorder.2 Pediatric acute disseminated encephalomyelitis cases have a higher rate of positive antimyelin oligodendrocyte glycoprotein antibody than adult cases.3 In the present pediatric patient, antimyelin oligodendrocyte glycoprotein antibody was already present at a high level in the peripheral blood during the acute phase of herpes simplex virus primary infection before the elevation of herpes simplex virus antibody. In his cerebrospinal fluid, oligoclonal bands were negative. These findings suggest that the blood–brain barrier was disrupted by the primary herpes simplex virus infection, and antimyelin oligodendrocyte glycoprotein antibodies already present in the peripheral blood were then transferred to the cerebrospinal fluid, resulting in the development of acute disseminated encephalomyelitis.
In animal models, pathogenic antimyelin oligodendrocyte glycoprotein antibody can be present in the circulation with no adverse effects induced unless it enters the central nervous system via a broken blood–brain barrier, which can be induced by inflammatory responses.1 Experiments in animal models generating antimyelin oligodendrocyte glycoprotein antibody showed the development of an acute disseminated encephalomyelitis-like disease after infection with encephalomyelitis virus.7 This mechanism has not been reported in humans. However, we believe that the same mechanism as was reported in the animal model of acute disseminated encephalomyelitis associated with antimyelin oligodendrocyte glycoprotein antibody was involved in the present patient.
The clinical characteristics of postinfectious demyelinating diseases with antimyelin oligodendrocyte glycoprotein antibody are reviewed in Table 1.8–12 All cases had an onset of postinfectious demyelinating disease with antimyelin oligodendrocyte glycoprotein antibody of 3 to 12 days (median 8.5 days) within 2 weeks from the primary infection. However, the interval between the primary infection and onset of demyelinating disease was too short to determine whether or not antimyelin oligodendrocyte glycoprotein antibody was produced by the primary infection because the measured antimyelin oligodendrocyte glycoprotein antibody was suspected to be immunoglobulin G fractionation, not immunoglobulin M. We speculate that antimyelin oligodendrocyte glycoprotein antibody transferred through the blood–brain barrier from the periphery into the central nervous system rather than being produced within the central nervous system. However, we did not analyze antimyelin oligodendrocyte glycoprotein antibody before the onset of acute disseminated encephalomyelitis, which is one limitation of this report.
Table 1.
Clinical Characteristics of Postinfectious ADEM With Anti-MOG Antibody.
| Reference | Patients | Type of demyelinating disease | Days until the onset of demyelinating disease after prior infection (days) | Etiology of prior infection | MOG antibody (serum) | AQ4 | ||
|---|---|---|---|---|---|---|---|---|
| Age (years) | Gender | Titer | (days) | |||||
| Our case | 5 | M | ADEM | 8 | HSV | 2048 | 8 | N |
| Nakamura et al8 | 36 | M | ADEM | 8 | EBV | 1024 | 8 | N |
| Nakamura et al9 | 41 | M | Optic neuritis, meningoganglionitis | 12 | HSV | 1024 | 32 | N |
| Vieira et al10 | 9 | F | Optic neuritis, myelitis | 10 | HHV6 | 32 | 10 | N |
| Amano et al11 | 32 | M | Myelitis | 9 | Influenza type A | 65 536 | 8 | N |
| Shiga et al12 | 69 | M | Myelitis | 3 | VZV | 128 | 4 | N |
Abbreviations: ADEM, acute disseminated encephalomyelitis; AQ4, aquaporin-4 antibody; EBV, Epstein-Barr virus; F, female; HHV6, human herpes virus 6; HSV, herpes simplex virus; M, male; MOG, myelin oligodendrocyte glycoprotein; VZV, varicella zoster virus.
Conclusion
Serologic myelin oligodendrocyte glycoprotein antibodies were present at the time of primary herpes simplex virus infection in the central nervous system. We hypothesized that primary herpes simplex virus infection in the central nervous system might possibly disrupt the blood–brain barrier, and antimyelin oligodendrocyte glycoprotein antibodies, which had been already present in the peripheral blood, transferred to the central nervous system and triggered central demyelination. This case might suggest the mechanism underlying postinfectious acute disseminated encephalomyelitis associated with myelin oligodendrocyte glycoprotein antibody.
Acknowledgments
The authors thank the family for their cooperation.
Footnotes
Author Contributions: RS, KO treated the patient, designed this study, and wrote the manuscript. KO, TM, and KI supervised patient management and critically reviewed the manuscript. KK and TT performed antimyelin oligodendrocyte glycoprotein antibody assays for the antimyelin oligodendrocyte glycoprotein antibody at Tohoku University and advised us on any correlations between the clinical findings and the data. All authors approved the final manuscript as submitted. RS, KO, TM, and KI substantially contributed to conception or design. RS, KO, KK, and TT contributed to acquisition, analysis, or interpretation of data. RS, KO, TM, and KI drafted the manuscript. All authors critically revised the manuscript for important intellectual content and agree to be accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kazuo Okanari, MD  https://orcid.org/0000-0001-5270-7356
https://orcid.org/0000-0001-5270-7356
Ethical Approval: Informed consent was received from the patient’s parents for publication of the case report.
References
- 1. Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15(2):89–102. [DOI] [PubMed] [Google Scholar]
- 2. Sato DK, Callegaro D, Lana-Peixoto MA, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. 2014;82(6):474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ketelslegers IA, Van Pelt DE, Bryde S, et al. Anti-MOG antibodies plead against MS diagnosis in an acquired demyelinating syndromes cohort. Mult Scler. 2015;21(12):1513–1520. [DOI] [PubMed] [Google Scholar]
- 4. Ramanathan S, Reddel SW, Henderson A, et al. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm. 2014;1(4):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krupp LB, Tardieu M, Amato MP, et al. International pediatric multiple sclerosis study group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261–1267. [DOI] [PubMed] [Google Scholar]
- 6. Steiner I, Schmutzhard E, Sellner J, et al. EFNS-ENS Guidelines for the Use of PCR technology for the diagnosis of infections of the nervous system. Eur J Neurol. 2012;19(10):1278–1291. [DOI] [PubMed] [Google Scholar]
- 7. Burrer R, Buchmeier MJ, Wolfe T, et al. Exacerbated pathology of viral encephalitis in mice with central nervous system-specific autoantibodies. Am J Pathol. 2007;170(2):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakamura Y, Nakajima H, Tani H, et al. Anti-MOG antibody-positive ADEM following infectious mononucleosis due to a primary EBV infection: a case report. BMC Neurol. 2017;17(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakamura M, Iwasaki Y, Takahashi T, et al. A case of MOG antibody-positive bilateral optic neuritis and meningoganglionitis following a genital herpes simplex virus infection. Mult Scler Relat Disord. 2017;17:148–150. [DOI] [PubMed] [Google Scholar]
- 10. Vieira JP, Sequeira J, Brito MJ. Postinfectious anti-myelin oligodendrocyte glycoprotein antibody positive optic neuritis and myelitis. J Child Neurol. 2017;32(12):996–999. [DOI] [PubMed] [Google Scholar]
- 11. Amano H, Miyamoto N, Shimura H, et al. Influenza-associated MOG antibody-positive longitudinally extensive transverse myelitis: a case report. BMC Neurol. 2014;14(1):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiga Y, Kamimura T, Shimoe Y, Takahashi T, Kaneko K, Kuriyama M. Anti-myelin oligodendrocyte glycoprotein (MOG) antibody-positive varicella-zoster virus myelitis presenting as longitudinally extensive transverse myelitis: a case report. Rinsho Shinkeigaku. 2017;57(10):579–583. [DOI] [PubMed] [Google Scholar]