Abstract
Objectives
We aimed to evaluate the diagnostic value of the combination of the monocyte-to-high-density lipoprotein cholesterol ratio (MHR) with the monocyte-to-lymphocyte ratio (MLR) in ischemic stroke patients.
Methods
There were 253 patients who were diagnosed with ischemic stroke and 211 healthy subjects enrolled into this retrospective study.
Result
MHR and MLR were significantly higher in ischemic stroke patients compared with controls. MHR and MLR remained as independent variables for the presence of ischemic stroke. In receiver operating characteristic analyses, the optimal cut-off values for MHR and MLR were 0.28 and 0.19, respectively. The area under the curve for MHR was 0.777 (sensitivity, 66.01%; specificity, 77.25%), and that for MLR was 0.742 (sensitivity, 70.36%; specificity, 67.77%) in ischemic stroke patients. Moreover, the combination MHR and MLR increased the sensitivity compared with MHR or MLR alone.
Conclusion
The present study shows that a high MHR and MLR are each predictive for the risk of ischemic stroke, and together, they exhibit a better diagnostic value compared with each ratio alone.
Keywords: Ischemic stroke, monocyte-to-high-density lipoprotein cholesterol ratio, monocyte-to-lymphocyte ratio, predictive factor, diagnostic value, high-density lipoprotein
Introduction
Ischemic stroke is a leading cause of death and disability worldwide,1 and the burden of stroke appears to be increasing in China.2 Inflammatory mechanisms that are triggered by inflammatory cells play a critical role in the pathology processes of ischemic stroke and its subtypes.3,4
Several studies have shown an independent association between the total white blood cell count and the stroke risk, stroke severity, and mortality.5–8 Monocytes/macrophages and T-lymphocytes are essential in stroke pathogenesis, which increases the production of inflammatory cytokines, infiltration, and lipid core formation, and this results in exacerbation of brain damage9,10 and the formation of atherosclerotic plaque.11 Conversely, high-density lipoprotein cholesterol (HDL-C) has anti-inflammatory, anti-oxidant, and antithrombotic effects by reversing cholesterol transport and preventing endothelial dysfunction.12 Thus, the monocyte-to-high-density lipoprotein cholesterol ratio (MHR) is likely a systematic inflammatory marker that is related to atherosclerosis.
The MHR was recently shown to be a novel prognostic biomarker in cardiovascular diseases,13 ischemic stroke,14 and acute intracerebral hemorrhage (ICH).15 Clinical studies found that a low lymphocyte-to-monocyte ratio (LMR) in the peripheral blood is a useful prognosis biomarker in patients with acute ischemic stroke.16 Additionally, the monocyte-to-lymphocyte ratio (MLR) could represent an inflammatory marker that is related to an increased risk of cardiovascular diseases.17 Recently, MLR has been reported to be an independent risk factor for carotid stenosis.18 However, the association between MHR, MLR, and ischemic stroke has not been evaluated.
The aim of this study was to investigate whether an elevated MHR or MLR can increase the risk of ischemic stroke, and to investigate whether there is a correlation between baseline MHR or MLR and ischemic stroke. Therefore, we evaluated the diagnostic value of MHR or MLR alone and the combination MHR and MHR for ischemic stroke.
Materials and methods
Study subjects
In this retrospective study, 253 ischemic stroke patients were screened and enrolled into the study from January 2017 to December 2018 at Changzhou Cancer Hospital of Soochow University, China. Ischemic stroke was confirmed based on the World Health Organization (WHO)’s definition of stroke,19 which is “rapidly developing signs of focal (or global) disturbance of cerebral function lasting >24 hours (excluding trauma or death).” Computed tomography (CT) and magnetic resonance imaging (MRI) scans were used to diagnose all patients on admission. Neurological severity on admission was identified using of the National Institutes of Health Stroke Scale (NIHSS) score. Two hundred eleven age and sex-matched healthy individuals were selected as the controls. We excluded patients with hemorrhage stroke, transient ischemic attacks, malignant tumor, brain trauma, central nervous system infection, recent surgery, patients who presented with severe systemic disease, dementia, psychiatric disease, renal or hepatic dysfunction, heart failure, chronic inflammatory diseases or chronic infection, or immunologic disorders before stroke. The study was approved by the medical Ethics Committee of Changzhou Tumor Hospital of Soochow University, China. Informed consent was not obtained because this was a retrospective study.
Data collection
We collected the baseline demographic information and vascular risk factors, including age; gender; risk factors such as hypertension, diabetes mellitus, hyperlipidemia, coronary heart disease (CHD), atrial fibrillation; and medication history. The systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured during physical examination. The biochemical index, including serum glucose and lipid profiles were obtained within 24 hours of admission using Advia 2400 automatic chemistry analyzer (Siemens Healthcare Diagnostics Inc., Dublin, Ireland). Then, the blood counts from all subjects were obtained within 3 hours of admission using a Siemens Advia 2120 automated hematology analyzer (Siemens Healthcare Diagnostics Inc.). MLR or MHR was calculated by dividing the absolute monocyte count by the lymphocyte count or the HDL-C level, respectively.
Statistical analysis
The Kolmogorov–Smirnov test was used to determine whether the data had a normal distribution. Categorical variables were expressed as number and percentage, and we compared the frequency counts between the groups using the Chi-square test. The continuous variables are presented as the mean and standard deviation (SD) in normally distributed data, whereas the median and interquartile range (IQR) were used in non-normally distributed variables. Pairwise comparisons were performed using a t-test and the Mann–Whitney U-test, respectively. Correlation analysis was performed using the Spearman test. Independent variables were determined using logistic regression analysis. All clinical variables with P<0.15 in the univariate analysis were included in the multivariable logistic regression model. Results were expressed as odds ratio (OR) with the corresponding 95% confidence interval (CI). Receiver operating characteristic (ROC) curves were constructed to define the optimum cut-off value of MLR or MHR for predicting the sensitivity and specificity when diagnosing ischemic stroke. The area under the curve (AUC) was determined as a criterion for the accuracy of the test, and the statistically significance differences in the AUC were determined using the MedCalc software version 15.2.2 (MedCalc Software Ltd., Ostend, Belgium, https://www.medcalc.org; 2020). Statistical analysis was performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Statistical significance was set at P < 0.05.
Results
Baseline characteristics and laboratory parameters of study subjects
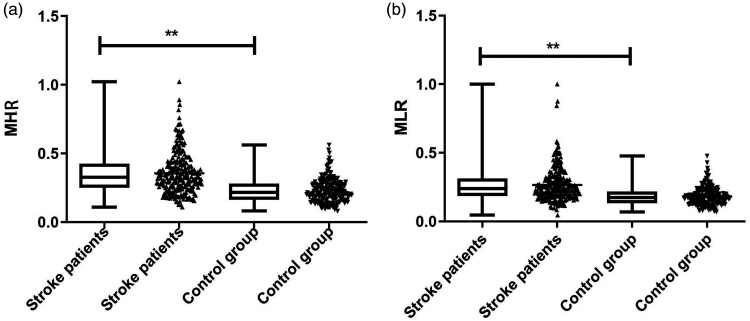
The stroke group comprised 161 men and 92 women with an average age of 67 years (IQR, 59–74 years), while the control group comprised 141 men and 70 women with an average age of 66 years (IQR, 63–70 years) (Table 1). Comparison of the baseline demographic characteristics between the two groups revealed that there were no significant differences in terms of age, gender, hypertension, diabetes, hyperlipidemia, cardiac disease, or medications (Table 1). There were no significant differences between the two groups in terms of blood glucose, cholesterol (CHOL), and low-density lipoprotein cholesterol (LDL-C) levels. However, the triglycerides (TGs) level and the monocyte and lymphocyte counts were significantly higher (p<0.001, p<0.001, p = 0.04, respectively), whereas HDL-C level was significantly lower (p<0.001) in the patient group compared with the control group. MHR and MLR levels were also higher in the patient group compared with the control group (p<0.001 for both) (Table 1, Figure 1).
Table 1.
Characteristics of control subjects and stroke patients.
| Characteristic | Patient group n (%) | Control group n (%) | Statistical quantity | p |
|---|---|---|---|---|
| Numbers | 253 | 211 | ||
| Age, median (IQR) | 67 (59–74) | 66 (63–70) | Z = −0.780 | 0.435 |
| Sex n, (%) | ||||
| Male | 161 (63.64) | 141 (66.82) | χ2 = 0.515 | 0.495 |
| Female | 92 (36.37) | 70 (33.18) | ||
| Stroke risk factors | ||||
| Hypertension | 218 (86.17) | 176 (83.41) | χ2 = 0.681 | 0.436 |
| Diabetes | 71 (28.06) | 50 (23.70) | χ2 = 1.138 | 0.291 |
| Hyperlipidemia | 63 (24.90) | 58 (27.49) | χ2 = 0.399 | 0.596 |
| Cardiac disease | ||||
| Atrial fibrillation | 20 (7.91) | 14 (6.64) | χ2 = 0.273 | 0.601 |
| Atrial flutter | 2 (0.8) | 1 (0.47) | χ2 = 0.180 | 0.672 |
| CAHD | 5 (1.97) | 3 (1.42) | χ2 = 0.209 | 0.648 |
| Revascularization | 1 (0.4) | 1 (0.47) | χ2 = 0.017 | 0.898 |
| Medications | ||||
| Antihypertensive | 152 (60.08) | 112 (53.08) | χ2 = 2.298 | 0.130 |
| Lipid-lowering | 5 (1.98) | 3 (1.42) | χ2 = 0.209 | 0.648 |
| Statins | 6 (2.37) | 3 (1.42) | χ2 = 0.546 | 0.460 |
| Hypoglycemic agent | 47 (18.58) | 31 (14.69) | χ2 = 1.242 | 0.265 |
| Insulin | 3 (1.19) | 2 (0.95) | χ2 = 0.061 | 0.805 |
| Aspirin | 23 (9.09) | 18 (8.53) | χ2 = 0.045 | 0.832 |
| Warfarin | 8 (3.16) | 3 (1.42) | χ2 = 1.505 | 0.220 |
| β-blocker | 3 (1.19) | 2 (0.95) | χ2 = 0.061 | 0.805 |
| BP, mmHg | ||||
| Systolic | 152.43±22.998 | 149.91±15.069 | t = 1.371 | 0.171 |
| Diastolic | 87.60±11.814 | 85.79±9.741 | t = 1.778 | 0.076 |
| Lipids, mM/L | ||||
| Cholesterol-total | 4.69 (4.245–5.31) | 4.85 (4.29–5.26) | Z = −0.788 | 0.431 |
| Triglycerides | 1.41 (1.02–2.1) | 1.2 (0.88–1.67) | Z = −3.713 | <0.001** |
| LDL-Cholesterol | 2.93 (2.52–3.39) | 2.96 (2.53–3.24) | Z = −0.566 | 0.571 |
| HDL-Cholesterol | 1.14 (0.98–1.33) | 1.36 (1.2–1.58) | Z = −8.050 | <0.001** |
| Fasting GLU, mM/L | 5.58 (5.015–6.635) | 5.46 (5.1–6.44) | Z = −0.165 | 0.869 |
| Monocytes, ×109/L | 0.37 (0.30 − 0.47) | 0.29 (0.24–0.36) | Z = −7.875 | <0.001 |
| Lymphocytes, ×109/L | 1.56 (1.21–1.995) | 1.69 (1.4–2.1) | Z = −2.916 | 0.04* |
| MHR | 0.327 (0.249–0.426) | 0.216 (0.161–0.279) | Z = −10.271 | <0.001** |
| MLR | 0.239 (0.184–0.312) | 0.174 (0.133–0.218) | Z = −8.973 | <0.001** |
| NIHSS | 3 (2.7) | – | – | – |
Data are presented as the median (IQR, 25–75%) or mean ± standard deviation, number (percentage).
p value was assessed using the Mann–Whitney U test, t-test, or the Chi-Square test; *p < 0.05, **p < 0.001.
CAHD, coronary atherosclerotic heart disease; HDL, high-density lipoproteins; LDL, low-density lipoproteins; GLU, glucose; MHA, monocyte to high density lipoprotein cholesterol ratio; MLR, monocyte to lymphocyte ratio; IQR, interquartile range; BP, blood pressure; MHR, monocyte to high-density lipoprotein cholesterol ratio; NIHSS, National Institutes of Health Stroke Scale.
Figure 1.
MHR or MLR levels in different groups. MHR level (a) and MLR level (b) in ischemic stroke patients and healthy controls. Statistical comparisons were made using the Mann–Whitney U-test (**p < 0.001).
MLR, monocyte to lymphocyte ratio; MHR, monocyte to high-density lipoprotein cholesterol ratio.
Independent risk predictors in ischemic stroke
To confirm the independent variables for ischemic stroke, univariate and multivariate logistic regression analysis were performed (Table 2). In univariate logistic regression analysis showed that TG, HDL-C, glucose, monocytes, lymphocyte, MHR, and MLR were possible potential risk factors for ischemic stroke. The results showed that MHR or MLR was an independent predictor for the presence of ischemic stroke with an adjusted OR of 1.043 (95% CI, 1.011–1.076; p = 0.007) and 1.093 (95% CI, 1.057–1.130; p < 0.001), respectively. In addition, TC and HDL were independent predictors of ischemic stroke (Table 2).
Table 2.
Univariate logistic regression analysis for outcome.
| Variables |
Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age | 0.999 | 0.981–1.017 | 0.922 | – | – | |
| Sex | 0.869 | 0.592–1.276 | 0.473 | – | – | – |
| Systolic (mmHg) | 1.007 | 0.997–1.016 | 0.922 | – | – | – |
| Diastolic (mmHg) | 1.015 | 0.998–1.033 | 0.171 | – | – | – |
| CHOL (mM/L) | 0.940 | 0.766–1.153 | 0.552 | – | ||
| LDL (mM/L) | 1.137 | 0.881–1.468 | 0.324 | – | – | – |
| TG (mM/L) | 1.573 | 1.266–1.953 | <0.001 | 1.323 | 1.039–1.686 | 0.023 |
| HDL (mM/L) | 0.057 | 0.027–0.12 | <0.001 | 0.232 | 0.078–0.688 | 0.008 |
| Glucose (mM/L) | 1.114 | 1.007–1.232 | 0.035 | – | – | – |
| Monocyte, 109/L | 1.081 | 1.059–1.105 | <0.001 | – | – | – |
| Lymphocyte, 109/L | 0.698 | 0.509–0.95 | 0.025 | – | – | – |
| MHR | 1.107 | 1.082–1.133 | <0.001 | 1.043 | 1.011–1.076 | 0.007 |
| MLR | 1.119 | 1.088–1.152 | <0.001 | 1.093 | 1.057–1.130 | <0.001 |
OR, odds ratio; CI, confidence interval; CHOL, cholesterol; LDL, low-density lipoprotein; TG, triglyceride; HDL, high-density lipoprotein; MLR, monocyte to lymphocyte ratio; MHR, monocyte to high-density lipoprotein cholesterol ratio.
Correlation between MHR, MLR, and NIHSS SCORE
To confirm the association between stroke severity and the MHR and MLR levels, the NIHSS score was recorded at admission to evaluate the neurological injury severity. There was a weak positive correlation between the NIHSS score and the MHR (r = 0.159, p = 0.012, Figure 2a) and the MLR (r = 0.147, p = 0.019, Figure 2b) in stroke patients.
Figure 2.
Correlations between MHR or MLR and NIHSS score.
MLR, monocyte to lymphocyte ratio; MHR, monocyte to high-density lipoprotein cholesterol ratio; NIHSS, NIHSS, National Institutes of Health Stroke Scale.
Diagnostic efficacy of MHR or/and MLR in ischemic stroke
Based on the ROC curve analysis, the optimal cut-off value of the MHR and MLR levels for diagnosing ischemic stroke was 0.2816 and 0.1958, respectively. The AUC of MHR was calculated as 0.777 (95% CI, 0.736–0.814, p < 0.001), with a sensitivity of 66.01% and a specificity of 77.25%. The AUC of MLR was also calculated as 0.742 (95%CI, 0.699–0.781, P < 0.001), with a sensitivity of 70.36% and a specificity of 67.77% (Table 3, Figure 3). Figure 3 shows that MHR in combination with MLR (AUC, 0.802; 95% CI, 0.763–0.837), with a sensitivity of 73.91% and a specificity of 74.41%, which showed a better performance in predicting ischemic stroke compared with MHR (p=0.0155) and MLR (P < 0.001) alone. A significant increase in sensitivity was observed based on the combination of MHR and MLR compared with the MHR (Z = 2.420, P = 0.0155) or MLR (Z = 4.030, P < 0.001) index alone (Table 3, Figure 3).
Table 3.
Diagnostic value of the presence in ischemic stroke.
| MHR – MHR+MLR | |||||||||
| Difference between areas | 0.0251 | ||||||||
| Standard Errora | 0.0104 | ||||||||
| 95%CI | 0.00477–0.0454 | ||||||||
| Z statistic | 2.420 | ||||||||
| P value | P = 0.0155 | ||||||||
| MLR – MHR+MLR | |||||||||
| Difference between areas | 0.0601 | ||||||||
| Standard Errora | 0.0149 | ||||||||
| 95% confidence interval | 0.0309–0.0893 | ||||||||
| Z statistic | 4.030 | ||||||||
| P value | P = 0.0001 | ||||||||
|
AUC (95%CI) |
SEN (%) |
SPE (%) |
Yden |
Cut-off |
+LR |
−LR |
+PV |
−NPV |
|
| MHR | 0.777 (0.736–0.814) | 66.01 | 77.25 | 0.4326 | 0.2816 | 2.9 | 0.44 | 77.77 | 65.5 |
| MLR | 0.742 (0.699–0.781) | 70.36 | 67.77 | 0.3813 | 0.1958 | 2.18 | 0.44 | 72.4 | 65.6 |
| MHR+MLR | 0.802 (0.763–0.837) | 73.91 | 74.41 | 0.4832 | 0.49978 | 2.89 | 0.35 | 77.6 | 70.4 |
aDeLong et al., 198843
MHR or/and MLR ROC analysis of the between stroke and control group.
AUC, Area under the curve; SEN, sensitivity; SPE, specificity; Yden, Youden index; +LR, positive likelihood ratio; −LR, negative likelihood ratio; +PV, positive predictive value; −PV, negative predictive value; MLR, monocyte to lymphocyte ratio; MHR, monocyte to high-density lipoprotein cholesterol ratio; CI, confidence interval.
Figure 3.
MHR or/and MLR ROC analysis of the between stroke and control group.
MLR, monocyte to lymphocyte ratio; MHR, monocyte to high-density lipoprotein cholesterol ratio; ROC, receiver operating characteristics.
Discussion
In the present study, we found that MHR and MLR were significantly increased in the stroke group compared with the healthy control group. The MHR value (above 0.28) and MLR value (above 0.1958) were defined as independent risk factors for the incidence of ischemic stroke events. The combination of MHR and MLR showed a higher sensitivity for diagnosis of ischemic stroke. These results indicate that elevated MHR and MLR levels may be associated with ongoing inflammation in the pathophysiology of ischemic stroke. To the best of our knowledge, this is the first study to determine the relationship between these parameters in ischemic stroke.
MHR is a prognostic marker of cardiovascular diseases13 and other inflammatory diseases for that reason. Many studies have evaluated MHR as a prognostic marker indicating inflammation and oxidative stress in systemic diseases.20,21 However, to date, only a few studies have attempted to investigate the relationship between MHR and cerebrovascular disease. In our study, the MHR level was high, and it was suggested as an independent risk factor for ischemic stroke patients. A similar study by Bolayir et al.14 demonstrated a high level value of MHR for IS patient. You et al.15 showed that an increased MHR level was associated with the 3-month outcome in patients with ICH.
An increasing amount of evidence suggests that inflammatory and oxidative stress mechanisms are involved at all stages of ischemic stroke progression development. Monocytes and activated macrophages can infiltrate and adhere to the inside of the arterial wall by producing various types of pro-inflammatory and pro-oxidant mediators (including inflammatory cell, pro-inflammatory cytokines/adhesion molecules, chemokines, and growth factors) and these inflammatory mediators interact with endothelial cells and thereby cause in an inflammatory cascade and foam cell formation by taking up oxidized low-density lipoproteins (LDLs) and other lipids.3,11 An animal study showed that the circulating Ly6C(hi) monocyte levels were increased in a chemokine (C-C motif) receptor 2 (CCR2)-dependent manner after permanent cerebral ischemia.22 Circulating monocytes and macrophage foam cells express high levels of tissue factor protein in inflammation or in atherosclerotic plaque states, and thereby contribute to the pro-coagulant activity.23 When tissue factors come into contact with circulating blood, thrombosis results in occlusion of the related artery.24 Additionally, a variety of cytokines trigger the inflammatory response to ischemic brain injury. The production of pro-inflammatory cytokines (such as tumor necrosis factor [TNF]-α, interleukin [IL]-1, and IL-6) was detected within the brain and in the blood in the early phase after stroke onset. IL-1 activates astrocytes and metalloproteinase (MMP)-9 and causes the release of vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1.25 Chemokines also play a crucial role in leukocyte recruitment and arrest, which is a critical step in atherosclerosis development. Macrophage migration inhibitory factor (MIF) has been shown to be a more pro-inflammatory, and thus, pro-atherogenic chemokine, while CXCL12 seems to have a more protective function.26 Overexpression of monocyte chemoattractant protein-1 (MCP-1) (also known as CCL-2) leads to exacerbated brain injury via the recruitment of inflammatory cells including IL-6, IL-1β, TNF-α, and granulocyte-colony stimulating factor (G-CSF), reactive astrogliosis, and the post ischemic migration of neutrophil granulocytes and T-cells.27,28 Reactive oxygen species (ROS) are generated as metabolic by-products by a variety of enzyme systems such as nitric oxide (NO) synthase (NOS), xanthine oxidases, the cyclooxygenases, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase isoforms, and metal-catalyzed reactions.29,30 When ROS-induced oxidized LDLs crossed the damaged endothelium into the intima, monocytes can differentiate into macrophages, which then take up oxidized LDL and subsequently become foam cells. These lipid-containing foam cells in the arterial wall can evolve into atherosclerotic plaques or atheromas. Oxidative stress is defined the key factor that mediates stroke-related damage.29
Serum HDL-C levels in our study groups were significantly lower compared with controls. These findings are consistent with previous findings, which showed low baseline HDL-C levels at admission in patients with atherosclerotic stroke.31 Recent studies indicate that the HDL-C molecules can modulate monocyte activation, adhesion, and macrophage migration, and control the proliferation of progenitor cells that differentiate to monocytes.32–34 HDL has anti-inflammatory effects in macrophages or adipocytes, which are regulated via cholesterol transporters (such as ATP-binding cassette A-1, ATP-binding cassette G-1, scavenger receptor B-1 [SR-B1])35,36 and the transcriptional regulator ATF3, against the Toll-like receptor (TLR)-induced inflammation response.37 HDLs also have antioxidant activity via HDL-associated antioxidant enzymes, such as paraoxonase 1 (PON1) and platelet-activating factor acetylhydrolase (PAF-AH), resulting in inhibition of LDL oxidation and intracellular oxidative stress in endothelial cells as well as removal of potentially cytotoxic lipid hydroperoxides from the circulation.32 HDL also promotes endothelial repair and antithrombotic properties via increasing NO bioavailability in the vessel.
Therefore, monocytes have a pro-inflammatory effect, but HDL-C functions as a reverse factor during this process. Elevated MHR, which is a composite marker of the monocyte count and HDL-C levels, may be a useful surrogate marker for systemic inflammation and endothelial dysfunction.
Recent studies have reported that decreased LMR is associated with the stroke-induced immunosuppression and a poor prognosis for acute ischemic stroke patients who are treated with thrombolysis.38,39 In this study, because of the increased monocyte and lower lymphocyte counts, an increased MLR was suggested to be an independent risk factor, and it could represent a peripheral marker for ischemic stroke patients. LMR has been proposed to be a surrogate marker for inflammation in distinct populations, and it also has a prognostic and predictive value.
Chen et al.40 showed that a decrease in the percentage of peripheral CD4 naïve T cells is an independent risk factor for ischemic stroke in patients on hemodialysis. A study reported stroke-induced lymphopenia that is associated with a reduction in HMGB1 release in the peripheral blood.41 In a LDLr−/− mouse atherosclerosis model, CD8+ T-cells played a protective role in advanced atherosclerosis by limiting accumulation of Th1 (CD4+) cells and macrophages in the lesions.42 Therefore, elevated monocyte and decreased lymphocyte counts may be a useful biomarker of ischemic stroke. Based on these findings from our study, the circulating MHR or/and MLR ratio was defined as a surrogate marker of both systemic inflammation and oxidative stress because both systemic inflammation and oxidative stress may be involved in the pathophysiology of ischemic stroke.
Additionally, the combined usefulness of MHR and MLR for predicting IS revealed a significant increase in the sensitivity (73.91%) compared with MHR or MLR alone. These results indicate that MHR in combination with MLR was superior to either MHR or MLR alone for diagnosing ischemic stroke.
Our study has several limitations. First, this study has a relatively small sample size and a retrospective study design. Second, we did not show a correlation between higher MHR, MLR, and the stroke subtype. Finally, only admission MHR and MLR were calculated in the current study, while the inflammatory reprocess are ongoing process. Thus, more detailed prospective studies will be needed in the future.
In conclusion, MHR and/or MLR is a simple, easy, convenient, cost-effective tool in clinical practice. An increased MHR and/or MLR was an independent predictor of the presence of ischemic stroke. Moreover, MHR in combination with MLR increased the predictive value compared with the individual markers in patients with ischemic stroke. Our findings provide a new perspective that MHR and MLR levels may be novel biomarkers for ischemic stroke diagnosis and a possible therapeutic target for stroke.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Huiling Liu https://orcid.org/0000-0003-1423-8282
References
- 1.Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology 2015; 45: 161–176. DOI: 10.1159/000441085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480,687 adults. Circulation 2017; 135: 759–771. DOI: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 3.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 2010; 87: 779–789. DOI: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuttolomondo A, Di Raimondo D, Pecoraro R, et al. Inflammation in ischemic stroke subtypes. Curr Pharm Des 2012; 18: 4289–4310. [DOI] [PubMed] [Google Scholar]
- 5.Wu TH, Chien KL, Lin HJ, et al. Total white blood cell count or neutrophil count predict ischemic stroke events among adult Taiwanese: report from a community-based cohort study. BMC Neurol 2013; 13: 7. DOI: 10.1186/1471-2377-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehme AK, Kumar AD, Lyerly MJ, et al. Persistent leukocytosis-is this a persistent problem for patients with acute ischemic stroke? J Stroke Cerebrovasc Dis 2014; 23: 1939–1943. DOI: 10.1016/j.jstrokecerebrovasdis.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furlan JC, Vergouwen MD, Fang J, et al. White blood cell count is an independent predictor of outcomes after acute ischaemic stroke. Eur J Neurol 2014; 21: 215–222. DOI: 10.1111/ene.12233. [DOI] [PubMed] [Google Scholar]
- 8.Zia E, Melander O, Bjorkbacka H, et al. Total and differential leucocyte counts in relation to incidence of stroke subtypes and mortality: a prospective cohort study. J Intern Med 2012; 272: 298–304. DOI: 10.1111/j.1365-2796.2012.02526.x. [DOI] [PubMed] [Google Scholar]
- 9.Chiba T, Umegaki K. Pivotal roles of monocytes/macrophages in stroke. Mediators Inflamm 2013; 2013: 759103. DOI: 10.1155/2013/759103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu L, Jian Z, Stary C, et al. T cells and cerebral ischemic stroke. Neurochem Res 2015; 40: 1786–1791. DOI: 10.1007/s11064-015-1676-0. [DOI] [PubMed] [Google Scholar]
- 11.Jaipersad AS, Lip GY, Silverman S, et al. The role of monocytes in angiogenesis and atherosclerosis. J Am Coll Cardiol 2014; 63: 1–11. DOI: 10.1016/j.jacc.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Nagao M, Nakajima H, Toh R, et al. Cardioprotective effects of high-density lipoprotein beyond its anti-atherogenic action. J Atheroscler Thromb 2018; 25: 985–993. DOI: 10.5551/jat.RV17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganjali S, Gotto AM, Jr, Ruscica M, et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J Cell Physiol 2018; 233: 9237–9246. DOI: 10.1002/jcp.27028. [DOI] [PubMed] [Google Scholar]
- 14.Bolayir A, Gokce SF, Cigdem B, et al. Monocyte/high-density lipoprotein ratio predicts the mortality in ischemic stroke patients. Neurol Neurochir Pol 2018; 52: 150–155. DOI: 10.1016/j.pjnns.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 15.You S, Zhong C, Zheng D, et al. Monocyte to HDL cholesterol ratio is associated with discharge and 3-month outcome in patients with acute intracerebral hemorrhage. J Neurol Sci 2017; 372: 157–161. DOI: 10.1016/j.jns.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Ren H, Liu X, Wang L, et al. Lymphocyte-to-monocyte ratio: a novel predictor of the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis 2017; 26: 2595–2602. DOI: 10.1016/j.jstrokecerebrovasdis.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Ji H, Li Y, Fan Z, et al. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: a syntax score assessment. BMC Cardiovasc Disord 2017; 17: 90. DOI: 10.1186/s12872-017-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuo B, Zhu S, Meng X, et al. Monocyte/lymphocyte ratio is associated with carotid stenosis in ischemic stroke: a retrospective analysis. Brain Behav 2019; 9: e01429. DOI: 10.1002/brb3.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 1976; 54: 541–553. [PMC free article] [PubMed] [Google Scholar]
- 20.Katipoglu Z, Mirza E, Oltulu R, et al. May monocyte/HDL cholesterol ratio (MHR) and neutrophil/lymphocyte ratio (NLR) be an indicator of inflammation and oxidative stress in patients with keratoconus? Ocul Immunol Inflamm 2020; 28: 632–636. DOI: 10.1080/09273948.2019.1611876. [DOI] [PubMed] [Google Scholar]
- 21.Canpolat U, Cetin EH, Cetin S, et al. Association of monocyte-to-HDL cholesterol ratio with slow coronary flow is linked to systemic inflammation. Clin Appl Thromb Hemost 2016; 22: 476–482. DOI: 10.1177/1076029615594002. [DOI] [PubMed] [Google Scholar]
- 22.Chu HX, Kim HA, Lee S, et al. Evidence of CCR2-independent transmigration of Ly6C(hi) monocytes into the brain after permanent cerebral ischemia in mice. Brain Res 2016; 1637: 118–127. DOI: 10.1016/j.brainres.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 23.Meisel SR, Xu XP, Edgington TS, et al. Differentiation of adherent human monocytes into macrophages markedly enhances tissue factor protein expression and procoagulant activity. Atherosclerosis 2002; 161: 35–43. [DOI] [PubMed] [Google Scholar]
- 24.Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol 2018; 38: 709–725. DOI: 10.1161/ATVBAHA.117.309846. [DOI] [PubMed] [Google Scholar]
- 25.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 2012; 32: 1677–1698. DOI: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Der Vorst EP, Doring Y, Weber C. Chemokines and their receptors in atherosclerosis. J Mol Med (Berl) 2015; 93: 963–971. DOI: 10.1007/s00109-015-1317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schilling M, Strecker JK, Schabitz WR, et al. Effects of monocyte chemoattractant protein 1 on blood-borne cell recruitment after transient focal cerebral ischemia in mice. Neuroscience 2009; 161: 806–812. DOI: 10.1016/j.neuroscience.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Strecker JK, Minnerup J, Gess B, et al. Monocyte chemoattractant protein-1-deficiency impairs the expression of IL-6, IL-1beta and G-CSF after transient focal ischemia in mice. PLoS One 2011; 6: e25863. DOI: 10.1371/journal.pone.0025863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng YC, Sheen JM, Hu WL, et al. Polyphenols and oxidative stress in atherosclerosis-related ischemic heart disease and stroke. Oxid Med Cell Longev 2017; 2017: 8526438. DOI: 10.1155/2017/8526438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonaventura A, Liberale L, Vecchie A, et al. Update on inflammatory biomarkers and treatments in ischemic stroke. Int J Mol Sci 2016; 17: 1967. DOI: 10.3390/ijms17121967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh PS, Yang CM, Lin SH, et al. Low levels of high-density lipoprotein cholesterol in patients with atherosclerotic stroke: a prospective cohort study. Atherosclerosis 2013; 228: 472–477. DOI: 10.1016/j.atherosclerosis.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Tabet F, Rye KA. High-density lipoproteins, inflammation and oxidative stress. Clin Sci (Lond) 2009; 116: 87–98. DOI: 10.1042/CS20080106. [DOI] [PubMed] [Google Scholar]
- 33.Murphy AJ, Chin-Dusting JP, Sviridov D, et al. The anti inflammatory effects of high density lipoproteins. Curr Med Chem 2009; 16: 667–675. [DOI] [PubMed] [Google Scholar]
- 34.Yvan-Charvet L, Pagler T, Gautier EL, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science 2010; 328: 1689–1693. DOI: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song GJ, Kim SM, Park KH, et al. SR-BI mediates high density lipoprotein (HDL)-induced anti-inflammatory effect in macrophages. Biochem Biophys Res Commun 2015; 457: 112–118. DOI: 10.1016/j.bbrc.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 36.Umemoto T, Han CY, Mitra P, et al. Apolipoprotein AI and high-density lipoprotein have anti-inflammatory effects on adipocytes via cholesterol transporters: ATP-binding cassette A-1, ATP-binding cassette G-1, and scavenger receptor B-1. Circ Res 2013; 112: 1345–1354. DOI: 10.1161/CIRCRESAHA.111.300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Nardo D, Labzin LI, Kono H, et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat Immunol 2014; 15: 152–160. DOI: 10.1038/ni.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park MG, Kim MK, Chae SH, et al. Lymphocyte-to-monocyte ratio on day 7 is associated with outcomes in acute ischemic stroke. Neurol Sci 2018; 39: 243–249. DOI: 10.1007/s10072-017-3163-7. [DOI] [PubMed] [Google Scholar]
- 39.Ren H, Han L, Liu H, et al. Decreased lymphocyte-to-monocyte ratio predicts poor prognosis of acute ischemic stroke treated with thrombolysis. Med Sci Monit 2017; 23: 5826–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen R, Hu J, Xiang F, et al. Decreased percentage of peripheral naive T cells is independently associated with ischemic stroke in patients on hemodialysis. Int Urol Nephrol 2017; 49: 2051–2060. DOI: 10.1007/s11255-017-1691-y. [DOI] [PubMed] [Google Scholar]
- 41.Gu L, Xiong X, Wei D, et al. T cells contribute to stroke-induced lymphopenia in rats. PLoS One 2013; 8: e59602. DOI: 10.1371/journal.pone.0059602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Duijn J, Kritikou E, Benne N, et al. CD8+ T-cells contribute to lesion stabilization in advanced atherosclerosis by limiting macrophage content and CD4+ T-cell responses. Cardiovasc Res 2019; 115: 729–738. DOI: 10.1093/cvr/cvy261. [DOI] [PubMed] [Google Scholar]
- 43.DeLong ER, DeLong DM and Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837-845. was added in the reference list. [PubMed]