Abstract
Human immunodeficiency virus (HIV) infection is a known hypercoagulable state with venous thromboembolism with a high mortality rate compared to the general population. The homeostatic balance in HIV infected patients improves with treatment compared to those who are not. A decreased hypercoagulable state noted by low levels of Von Willebrand factor, factor VIII and d-dimer levels along with higher protein C and S activity in patients on treatment suggests that hypercoagulable state is partially correctable with highly active antiretroviral therapy. HIV with heart muscle involvement can present as myocarditis or as dilated cardiomyopathy with left or right ventricular dysfunction. Here we present a case of a 57-year-old man with a known history of HIV infection, noncompliant with medical therapy presenting with dilated cardiomyopathy with biventricular thrombi with reduced protein C, protein S, and Antithrombin III levels.
Keywords: HIV, Hypercoagulable, Ventricular, thrombus, protein c, protein s, antithrombin 3
Introduction
Human immunodeficiency virus (HIV) infection is a well-known hypercoagulable state associated with venous thromboembolism with high mortality risk compared to the general population 1, 2. HIV with heart muscle involvement can present as myocarditis or as dilated cardiomyopathy with left or right ventricular dysfunction 3. Here we present a case of a patient infected with HIV presenting with dilated cardiomyopathy with biventricular thrombi secondary to reduced protein C, protein S, and antithrombin III levels. On review of the literature, we were able to find only one similar presentation where a patient with HIV has cardiomyopathy with biventricular thrombosis 4.
Case report
The patient is a 57-year-old Caucasian male with a known past medical history of human immunodeficiency virus (HIV) noncompliant with medical therapy and hyperlipidemia, who presented to the emergency department with shortness of breath, hypoxia with oxygen saturation of 70%, pleuritic chest pain and a syncopal episode with fall. The patient denied any significant family, surgical, or social history. He was treated for pneumonia six weeks prior to presentation with ceftriaxone 1 gram daily and azithromycin 500mg daily for 5 days, and since then he has been experiencing exertional dyspnea. The patient denied orthopnea or paroxysmal nocturnal dyspnea. He had a syncopal episode at home with fall and hitting the left side of the chest on a barstool causing pleuritic chest pain. Chest pain was non-exertional. The patient's level of activity has significantly diminished over the last six weeks, and he was unable to perform his daily activities without difficulty. The patient admitted that he had previous episodes of syncope that occur with little or no warning signs except for mild dizziness before passing out. He denied any associated chest tightness, palpitations, headache, and stool or urine incontinence. The physical examination was significant for chest wall tenderness with a normal cardiorespiratory exam.
Laboratory findings showed mildly elevated troponin. An echocardiogram demonstrated biventricular dilatation with ejection fraction (EF) of 30% and compelling evidence for the presence of thrombus in the apex of both ventricles and free wall of the right ventricle (as shown in Figure 1– Figure 4). Echocardiogram did not demonstrate any spontaneous echo contrast, suggesting severely diminished ejection fraction and stagnation of blood flow. Orthostatic vitals were normal, and the patient did not experience any arrhythmias on telemetry ruling them out as a cause for syncope. Syncope was later presumed to be likely secondary to a low flow state from reduced EF. The patient denied any prior history of deep vein thrombosis, transient ischemic attack, or stroke. CT chest with contrast did not show any evidence of pulmonary embolism but showed diffuse cardiomegaly ( Figure 5 and Figure 6). Given the presence of biventricular thrombus, the patient was evaluated for the hypercoagulable state. Results showed low Protein C, protein S, and antithrombin III levels. Factor V Leiden and lupus anticoagulant were normal. The laboratory findings are summarized in Table 1.
Figure 1. Echocardiogram (Apical 2 chamber view) showing dilated left ventricle showing apical thrombus.

Figure 2. Echocardiogram (Apical 2 chamber view) showing dilated left ventricle with apical thrombus measurements.

Figure 3. Echocardiogram (Apical 4 chamber view) showing dilated right ventricle with apical thrombus.

Figure 4. Echocardiogram (Apical 4 chamber view) showing dilated right ventricle with apical thrombus measurements.
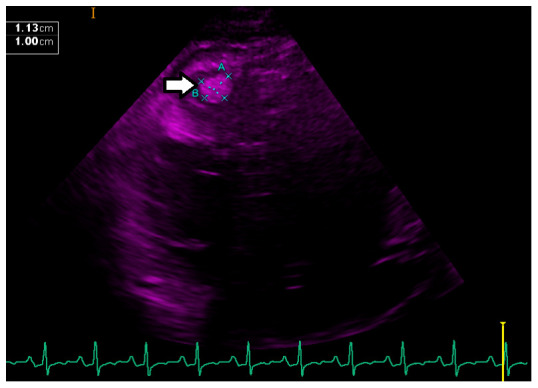
Figure 5. CT of chest with IV contrast showing left ventricular and right ventricular enlargement.

Figure 6. CT of chest without contrast showing left ventricular and right ventricular enlargement.
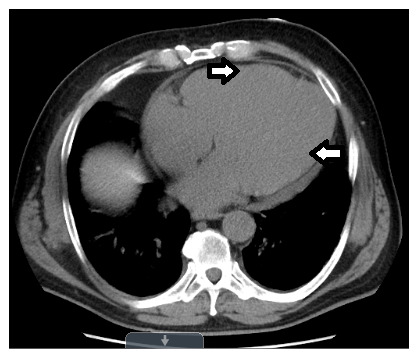
Table 1. Summary of laboratory findings.
| Patient value (normal range) | |
|---|---|
| Sodium | 132 mol/L (136–145) |
| Glucose | 64 mg/dl (74–99) |
| Blood Urea Nitrogen | 31 mg/dl (6–20) |
| Creatinine | 1.09 mg/dl (0.7–1.20) |
| White Blood Cell Count | 13.4 k/ul (4.5–0.8) |
| Absolute Neutrophil Count | 8.65 k/ul (1.5–7) |
| Hemoglobin | 16.5 g/dl (13.5–18.0) |
| Platelets | 346 k/ul (150–450) |
| HIV Antibodies | Positive |
| CD4 T Cell Count | 815cells/mcl (365–1437) |
| CD8 T Cell Count | 614 cells/mcl (117–846) |
| CD4/CD8 Ratio | 1.3 (>0.9) |
| Hepatitis A Antibody | Negative |
| Hepatitis B Surface and Core Antigen | Negative |
| Hepatitis C Antibody | Negative |
| Alkaline Phosphatase | 152 u/l (40–129) |
| Aspartate Aminotransferase | 270 u/l (10–50) |
| Alanine Aminotransferase | 458 u/l (10–50) |
| NT pro B-type Natriuretic Peptide | 4485 pg/ml (0–125) |
| Troponin (0 hr, 6 hr, 12 hr) | 89, >90, >106 ng/l (<15) |
| Protein C Activity | 46% (70–140) |
| Protein S Activity | 38% (45–125) |
| Antithrombin III Activity | 77% (80–120) |
| Factor V Leiden | Negative |
| Lupus Anticoagulant | Negative |
| Anti-Cardiolipin Antibodies | Normal |
| Anti-Beta 2 Glycoprotein Antibodies | Normal |
| Prothrombin Gene Mutation | Negative |
Cardiology initially considered cardiac catheterization to delineate the patient’s coronary anatomy for the potential need for revascularization, but instead decided to perform stress myocardial perfusion study to prevent thromboembolic events and strokes that can be associated with the procedure. The nuclear stress test was negative for reversible ischemia. There was no evidence of fixed defects on the stress test, suggesting no evidence of prior myocardial infarction or scar tissue within the myocardium. The patient was started on Lasix 20mg oral daily, metoprolol succinate 25mg oral daily, and low-dose Lisinopril 2.5mg oral daily. Novel anticoagulants (NOACs) are not recommended for mural thrombus; therefore, the patient was started on a therapeutic dose of low molecular weight heparin 60mcg subcutaneous twice daily and bridged with warfarin 5mg oral daily. Heparin was discontinued once his International Normalized Ratio (INR) was therapeutic. One week after the admission, the patient was ready to be discharged. His symptoms had significantly improved with no further syncopal episodes or exertional dyspnea. His ambulatory oxygen saturations were normal on room air. The patient was advised to follow up with infectious diseases to initiate highly active antiretroviral therapy (HAART) therapy for his HIV, cardiology and hematology for continued care.
Discussion
HIV infection is a well-known hypercoagulable state with a frequency of thrombotic events recognized in the range of 0.19% to 7.63% per year 1. Compared to the general population of the same age, the risk of arterial and venous thrombosis in HIV infected patients is increased two to tenfold. One possible explanation could be due to the presence of multiple comorbidities and baseline increased inflammatory/hypercoagulable state. Risk factors like low CD4 cell count, especially in the presence of clinical acute immunodeficiency syndrome (AIDS), protein S deficiency, and protein C deficiency, have demonstrated the strongest association with venous thromboembolism. Other less significant and controversial risk factors include protease inhibitor therapy, opportunistic infections, and positive antiphospholipid antibodies, including anticardiolipin antibodies and lupus anticoagulant 1, 5. Thrombophilic abnormalities in total platelet count, protein C, and S activity are directly correlated to CD4 count 6, and their frequency increases as the patient progresses to AIDS 7. HIV infection with venous thromboembolism has a high mortality rate compared to the general population 2.
The homeostatic balance in HIV-infected patients varies if they are on HAART therapy vs. not being on treatment. The indicators of hypercoagulable state like the activity of the Von Willebrand factor, levels of Factor VIII, and D dimer are lower in patients on treatment. Also, anticoagulant proteins like protein C and S activity are higher in patients on therapy, suggesting the hypercoagulable state is partially correctable with HAART. Although patients on HAART therapy have a lower prevalence of coagulation abnormalities, many show the persistent procoagulant state as evident by increased endothelial cell activation and high APCsr when compared to the general population. Continued coagulation markers abnormalities have been observed in HIV-infected individuals before and after the initiation of HAART 8. Even patients who are newly started on HAART therapy showed marked improvement in the coagulation profile but still different from the general population, indicating a persistent abnormal homeostatic balance 9. In addition, it has been previously observed that most HIV positive patients with low protein S levels also had mutations in exon 15 of PROS 1 gene warranting further investigation 10.
Cardiac dysfunction in HIV-infected patients evidenced by congestive heart failure is a well-documented finding. Even in asymptomatic HIV patients, an echocardiographic and echo-doppler examination has shown evidence of early signs of impaired systolic and diastolic function, suggesting an early involvement of the heart in HIV disease 11. Cardiomyopathy associated with HIV may be related to the autoimmune process induced by HIV in conjugation with other cardio tropic virus or could be direct action on the heart muscle 12. It is shown that the incidence of dilated cardiomyopathy in HIV is 17.6% 13. HIV with heart muscle involvement can present as myocarditis or dilated cardiomyopathy with left or right ventricular dysfunction, the pathogenesis of which seems to relate to the coinfection with other infectious agents 3. Isolated right and left heart dysfunction have no direct correlation to the CD4 count and does not carry adverse prognostic implications 14.
Long term anticoagulation may be beneficial in HIV infected patients to prevent future thromboembolic events as most of the contributing risk factors are often irreversible. Since there is a possibility of interactions between warfarin and antiretroviral therapy, health care providers should be watchful of consequent dangerously high or low INRs when giving warfarin to patients undergoing antiretroviral therapy 1. It is shown that newer anticoagulants can be used with antiretroviral therapy without any noticeable interactions 15.
Conclusion
In our patient, a bi-ventricular thrombus is likely the result of the hypercoagulable state along with severe ventricular dysfunction with dilated cardiomyopathy due to underlying HIV infection. Systemic or pulmonary embolization was not seen in our patient, as reported in the past as an associated finding 16. Physicians caring for patients with HIV should always consider thrombotic and thromboembolic events in the differential besides known malignancies and opportunistic infections when treating patients with unexplained dyspnea or hypoxia, especially in young males.
Consent
Written informed consent for the publication of the case report and any associated images was obtained from the patient.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 2 approved with reservations]
References
- 1. Bibas M, Biava G, Antinori A: HIV-associated venous thromboembolism. Mediterr J Hematol Infect Dis. 2011;3(1):e2011030. 10.4084/MJHID.2011.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fultz SL, McGinnis KA, Skanderson M, et al. : Association of venous thromboembolism with human immunodeficiency virus and mortality in veterans. The American journal of medicine. 2004;116(6):420–3. 10.1016/j.amjmed.2003.10.011 [DOI] [PubMed] [Google Scholar]
- 3. Khunnawat C, Mukerji S, Havlichek D, Jr, et al. : Cardiovascular manifestations in human immunodeficiency virus-infected patients. Am J Cardiol. 2008;102(5):635–42. 10.1016/j.amjcard.2008.04.035 [DOI] [PubMed] [Google Scholar]
- 4. Nkoke C, Kuate LM, Luchuo EB, et al. : Biventricular thrombi in dilated cardiomyopathy in a patient with human immunodeficiency virus infection: a case report. BMC Res Notes. 2015;8(1):168. 10.1186/s13104-015-1140-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kiser KL, Badowski ME: Risk factors for venous thromboembolism in patients with human immunodeficiency virus infection. Pharmacotherapy. 2010;30(12):1292–302. 10.1592/phco.30.12.1292 [DOI] [PubMed] [Google Scholar]
- 6. Khare S, Kushwaha R, Kumar A, et al. : Prothrombotic state in HIV: A study on protein C, protein S, homocysteine and correlation with CD4 counts. Indian J Med Microbiol. 2018;36(2):201–206. 10.4103/ijmm.IJMM_15_414 [DOI] [PubMed] [Google Scholar]
- 7. Lijfering WM, Sprenger HG, Georg RR, et al. : Relationship between progression to AIDS and thrombophilic abnormalities in HIV infection. Clinical Chemistry. 2008;54(7):1226–33. 10.1373/clinchem.2008.103614 [DOI] [PubMed] [Google Scholar]
- 8. Jong E, Louw S, Meijers JC, et al. : The hemostatic balance in HIV-infected patients with and without antiretroviral therapy: partial restoration with antiretroviral therapy. AIDS Patient Care STDS. 2009;23(12):1001–7. 10.1089/apc.2009.0173 [DOI] [PubMed] [Google Scholar]
- 9. Jong E, Louw S, Gorp ECM: The effect of initiating combined antiretroviral therapy on endothelial cell activation and coagulation markers in South African HIV-infected individuals. Thromb Haemost. 2010;104(6):1228–34. 10.1160/TH10-04-0233 [DOI] [PubMed] [Google Scholar]
- 10. Di Stefano M, D’Andrea G, Zoboli F, et al. : Preliminary Data From the Study of Coagulative Profile of HIV Infected Individuals Suggest a Role For Point Mutations in the Gene in Protein S Deficiency in Individuals Undergoing Highly Antiretroviral Therapy. Open AIDS J. 2018;12:6–10. 10.2174/1874613601812010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barbaro G, Barbarini G, Di Lorenzo G: Early impairment of systolic and diastolic function in asymptomatic HIV-positive patients: a multicenter echocardiographic and echo-doppler study. AIDS Res Hum Retroviruses. 1996;12(16):1559–63. 10.1089/aid.1996.12.1559 [DOI] [PubMed] [Google Scholar]
- 12. Barbaro G, Di Lorenzo G, Grisorio B, et al. : Cardiac involvement in the acquired immunodeficiency syndrome: a multicenter clinical-pathological study. AIDS Res Hum Retroviruses. 1998;14(12):1071–7. 10.1089/aid.1998.14.1071 [DOI] [PubMed] [Google Scholar]
- 13. Jain N, Reddy DH, Verma SP, et al. : Cardiac abnormalities in HIV-positive patients: results from an observational study in India. J Int Assoc Provid AIDS Care. 2014;13(1):40–6. 10.1177/1545109712456740 [DOI] [PubMed] [Google Scholar]
- 14. Currie PF, Jacob AJ, Foreman AR, et al. : Heart muscle disease related to HIV infection: prognostic implications. BMJ. 1994;309(6969):1605–7. 10.1136/bmj.309.6969.1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Natarajan P, Joolhar F, Thangarasu S, et al. : Embolizing Massive Right Atrial Thrombus in a HIV-Infected Patient. J Investig Med High Impact Case Rep. 2018;6:2324709618802871. 10.1177/2324709618802871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Missault L, Koch A, Colardyn F, et al. : Biventricular thrombi in dilated cardiomyopathy: massive simultaneous pulmonary and systemic embolisation. Eur Heart J. 1994;15(5):713–4. 10.1093/oxfordjournals.eurheartj.a060574 [DOI] [PubMed] [Google Scholar]


