Abstract
Background
In the pandemic, testing for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) by real‐time polymerase chain reaction is one of the pillars on which countermeasures are based. Factors limiting the output of laboratories interfere with the effectiveness of public health measures. Conserving reagents by pooling samples in low‐probability settings is proposed but may cause dilution and loss of sensitivity. Blood transfusion services had experience in performance of high throughput nucleic acid testing (NAT) analysis and can support the national health system by screening of the inhabitants for SARS‐COV‐2.
Methods
We evaluated a new approach of a multiple‐swab method by simultaneously incubating multiple respiratory swabs in a single tube. Analytical sensitivity was constant up to a total number of 50 swabs. It was consequently applied in the testing of 50 symptomatic patients (5‐sample pools) as well as 100 asymptomatic residents of a nursing home (10‐sample pools).
Results
The novel method did not cause false‐negative results with nonsignificantly differing cycle threshold values between single‐swab and multiple‐swab NAT. In two routine applications, all minipools containing positive patient samples were correctly identified.
Conclusions
The new method enables countries to increase the total number of testing significantly. The multiple‐swab method is able to screen system relevant groups of employees frequently. The example in Germany shows that blood transfusion services can support general health systems with their experience in NAT and their high‐throughput instruments. Screening of a huge number of inhabitants is currently the only option to prevent a second infection wave and enable exit strategies in many countries.
Abbreviations
- COVID‐19
coronavirus disease 2019
- Ct
cycle threshold
- HCV
hepatitis C virus
- NAT
nucleic acid testing
- PCR
polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- WHO
World Health Organization
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is the causative agent of the novel lung disease coronavirus disease 2019 (COVID‐19). With more than 1.3 million cases and almost 80 000 deaths recorded worldwide by 8 April 2020, 1 cases are still rising sharply in many parts of the world. Nations throughout the world are attempting to slow down the surge in cases by putting extensive countermeasures in place.
Infection may remain asymptomatic or pass with only minor symptoms, making a clinical diagnosis impossible in many cases. 2 , 3 , 4 High viral titers in the upper airways during the first week of symptoms 5 , 6 and presymptomatic transmission 7 likely contributes to the difficulty containing the pandemic. In the struggle against the pandemic, the World Health Organization (WHO) recently urged nations to “test, test, test.” 8 Detection of SARS‐CoV‐2 by nucleic acid testing (NAT), such as polymerase chain reaction (PCR), in a nasopharyngeal or throat swab and/or lower respiratory specimen is the preferred method as recommended by the WHO. 9
The unprecedented demand for NAT reagents and test kits has already led to shortages, obstructing the efforts to combat COVID‐19. Another factor limiting the output of laboratories is the availability of qualified staff. Furthermore, especially in low‐income settings, where the threat by COVID‐19 is no less imminent, cases may go undetected when tests are too expensive.
Blood donor services have been asked in many regions to make their high throughput NAT testing systems available to support patient and population testing for SARS‐Co‐V‐2, in addition to donor screening. To make testing for SARS‐CoV‐2 more efficient, sample pooling has been proposed, and recently applied in a retrospective analysis. 10 Dilution effects leading to a loss in diagnostic sensitivity is a concern in this strategy, when sample solutions are pooled. Here, we evaluated a new alternate multiple‐swab NAT protocol (Frankfurt adjusted COVID‐19 testing method) without any volume dilutions in the pooling process.
2. MATERIAL AND METHODS
2.1. Multiple‐swab method
We applied a novel multiple‐swab protocol to NAT of respiratory swabs for SARS‐CoV‐2: The new method was tested for following dry swabs (dry swab, Roche, uni‐swab sample; dry swab, Sarstedt, neutral swabs; and dry swab Classiq swab,™ dry swab). Other dry swabs are possible but currently not tested by the authors. It is recommended in Germany to take specimens first from the pharyngeal region followed by the nasal region. Samples can be taken with two swabs or with one swab. Due to experimental data (not shown), the time between sample collection and performing NAT should be not longer than 48 hours. Swabs should be protected from ultraviolet light. Respiratory swabs were first incubated in a reference tube containing 4.3 mL of guanidinium hydrochloride buffer (cobas medium, Roche) solution for 5 minutes with constant agitation. Consequently, all swabs used for the multiple‐swab method are removed and collectively placed in one new single‐media tube containing 2 mL of guanidinium hydrochloride buffer, the multiple‐swab tube, under constant agitation for 5 minutes (Figure 1). Up to 10 swabs can be placed at one time point into the multiple‐swab tube. Other buffer reagents, for example, guanidinium thiocyanate mixed with phosphate‐buffered saline (1:1) is also possible. All swabs were performed in a laminar flow hood. Before transferring the swabs into a new tube, they were wiped softly off the tube wall. The swabs are then removed from the multiple‐swab tube, which proceeds to NAT. The complete process of performing multiple‐swab tubes was controlled by an in‐house information technology software. In brief, all original swabs and the archive tubes were labeled with a primary barcode sticker and scanned after labeling. In transferring the swabs into the archive tubes, all barcodes were scanned a second time. Errors were detected by comparing the barcodes from the swab and the archive tube. In the next step, the multiple‐swab tube is labeled with an MS‐T sticker. In transferring the swabs from the archive tube to the multiple‐swab tube, all archive tubes as well as the multiple‐swab tube were scanned again. The original swabs were discarded after inoculation of the multiple‐swab tube. If the multiple‐swab tube showed a positive or invalid test result, further testing was performed from the archive tube. Archive tubes were stored at 2 to 8°C until NAT analysis from the multiple‐swab tube is completed. In case of a negative NAT result in the multiple‐swab tube, each sample in the multiple‐swab tube received a negative result. If the NAT result of the multiple‐swab tube was positive, individual SARS‐CoV‐2 NATs are carried out from the archive tubes.
FIGURE 1.
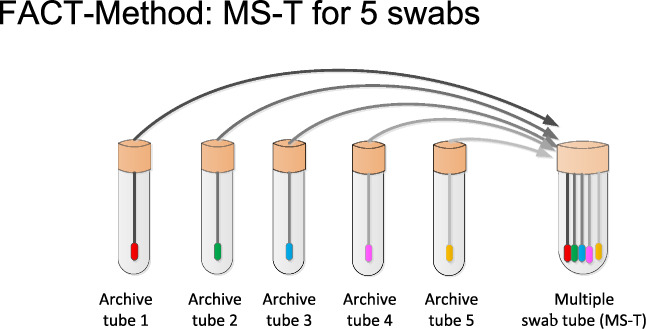
Multiple‐swab NAT method. Swabs were incubated first in a reference tube followed by a 5‐minute incubation in the minipool tube. SARS‐CoV‐2 virus concentration did not differ significantly between both samples
2.2. NAT by RT‐PCR
NAT was performed by Roche cobas SARS‐CoV‐2 on a central server (Cobas 6800 or Cobas 8800, Roche Diagnostics) instrument. The sample input volume was 400 μL. Amplification was done in a multiplex CE‐certified assay in the ORF 1a/b region as well as in the E‐gene. All samples were tested in accordance to the instruction for use from the manufacturer.
2.3. Quantification of inactivated SARS CoV‐2 standard
For the first series of experiments, an inactivated SARS CoV‐2 standard was used, with a final concentration of 104 copies/mL. The standard was quantified with a quantitative real‐time PCR described in detail by Toptan et al. 11
2.4. Evaluation
The concept was assessed in five setups, and the diagnostic value was evaluated in practical application in symptomatic patients as well as in a screening procedure in asymptomatic employees.
2.5. Proof‐of‐concept setup, second experiment
In Germany, each screening laboratory must participate in official proficiency panel tests to demonstrate correct testing. Therefore, unknown samples were sent to each laboratory. These samples were solved in sterile water. In the next step, sterile swabs were placed into each sample and tested by the individual‐swab method as well as by the multiple‐swab method, with five samples containing one sample from the proficiency panel and four negative control swab samples. With this experiment we can show that independent external control samples were tested correctly (positive samples were positive, and negative samples were negative) either with the single‐swab method or with the multiple‐swab method. It is an important experiment that samples with different virus concentrations were tested correctly with both methods.
2.6. Multiple‐swab testing up to 50 swabs per tube (figure 1)
In these test series, different numbers of multiple‐swab testing were evaluated against individual swab testing. Therefore, dry swabs were incubated into an inactivated SARS‐CoV‐2 standard solution (103 copies/mL) for 1 minute. Thereafter, the swabs were placed into archive tubes (one swab per tube). After 5 minutes, different multiple‐swab pools (pools with 10 swabs, 20 swabs, 30 swabs, 40 swabs, and 50 swabs) were performed. In each pool, one swab that was contaminated with SARS‐CoV‐2 standard material was taken and combined with the outstanding number of swabs without SARS‐CoV‐2 (with 9 negative swabs for pools of 10, 19 negative swabs for pools of 20, 29 negative swabs for pools of 30, 39 negative swabs for pools of 40, and 49 negative swabs for pools of 50). In the next step, SARS‐CoV‐2 NAT was performed for the archive samples as well as for the different multiple‐swab samples. Table 5 shows the cycle threshold (Ct) values of the individual testing of the archive sample as well as the Ct values of the different multiple‐swab samples.
TABLE 5.
Validation of the multiple‐swab method in tubes containing 10, 20, 30, 40, and 50 swabs
| Multiple‐swab number | ORF region | E‐gene | ||
|---|---|---|---|---|
| Individual‐swab NAT | Multiple‐swab NAT | Individual‐swab NAT | Multiple‐swab NAT | |
| 10 | 32.49 | 33.74 | 31.84 | 33.22 |
| 20 | 32.65 | 32.93 | 32.11 | 32.59 |
| 30 | 33.72 | 33.21 | 33.40 | 33.01 |
| 40 | 33.00 | 32.94 | 32.65 | 32.63 |
| 50 | 33.83 | 32.60 | 33.35 | 32.16 |
Abbreviation: NAT, nucleic acid testing.
2.7. Statistics
Paired t tests were calculated for Ct values between the single‐swab method and the multiple‐swab method. P values <.05 were found to be statistically significant.
3. RESULTS
First, to evaluate for suitability of different mini‐pool sizes, swabs were contaminated with a defined SARS‐CoV‐2 virus concentration of 1 × 104 copies/mL, and then placed in a series of 10 tubes with lysis buffer for 5 minutes each. Ct values in each tube were determined, which is proportional to copy numbers. The results were examined for significant increase in Ct values in the succession of tubes, which would signify loss of sensitivity. We did not observe a significant difference in the semiquantitative viral load between the first tube (representing individual sample testing) and the tenth tube. The largest observed difference in Ct value was 1.73 and 2.23 for ORF 1a and E‐gene, respectively (Table 1), which we consider not significant.
TABLE 1.
Incubation of a SARS‐CoV‐2 contaminated swab with 104 copies/mL sequentially into 10 sample tubes with lysis buffer
| ORF region | E‐gene | |
|---|---|---|
| Tube 1 | 30.37 | 29.91 |
| Tube 2 | 30.59 | 30.29 |
| Tube 3 | 30.6 | 30.26 |
| Tube 4 | 31.32 | 30.95 |
| Tube 5 | 32.52 | 32.36 |
| Tube 6 | 32.19 | 32.01 |
| Tube 7 | 32.84 | 32.83 |
| Tube 8 | 32.61 | 32.68 |
| Tube 9 | 32.21 | 32.01 |
| Tube 10 | 32.1 | 32.14 |
Note: The difference in Ct values between Tube 1 and Tube 10 was 1.73 and 2.23 for the ORF 1a region and the E‐gene, respectively. The experiment was done with Roche media kits. Each tube contains 4.3 mL Roche media buffer.
Next, we evaluated a five‐sample minipool in a proof‐of‐concept setup. Samples from a proficiency panel test provider (INSTAND) with predetermined concentrations of SARS‐CoV‐2 were used. Four of the samples were incubated in a solution containing SARS‐CoV‐2, and one was incubated in solution not containing virus. Each of the swabs was transferred to a five‐sample multiple‐swab tube, in accordance with the protocol described above. Respiratory swabs from SARS‐CoV‐2–negative volunteers were used to complete the pools. Results of single‐swab sample tubes and multiple‐swab sample tubes were compared. We determined that all multiple‐swab tubes containing a SARS‐CoV‐2–positive sample were correctly identified in the multiple‐swab protocol, independent of the virus concentration in the original sample. All multiple‐swab tubes containing no SARS‐CoV‐2–positive sample were also true negative. When comparing Ct values in single‐swab and multiple‐swab tubes (Table 2), the largest observed gap between Ct values was 0.87 (Sample 4, in the E‐gene as well as ORF 1a region), which we consider not significant.
TABLE 2.
Comparative Ct value results in a proof‐of‐concept approach with external samples of a proficiency test
| Sample ID | ID‐NAT | MS‐NAT | ID‐NAT | MS‐NAT | |
|---|---|---|---|---|---|
| ORF region | ORF region | Sample ID | E‐gene | E‐gene | |
| 1 | 28.02 | 27.25 | 1 | 28.51 | 28.01 |
| 2 | 30.60 | 30.40 | 2 | 31.42 | 31.23 |
| 3 | 33.41 | 33.22 | 3 | 34.40 | 34.55 |
| 4 | 36.33 | 35.46 | 4 | 37.98 | 37.11 |
| 5 | Negative | Negative | 5 | Negative | Negative |
Note: Ct values did not differ significantly between the individual sample and multiple‐swab tube.
Abbreviations: Ct, cycle threshold; ID‐NAT, individual donor nucleic acid testing; MS‐NAT, multiple swab nucleic acid testing.
To evaluate the test in patients with a moderate likelihood of SARS‐CoV‐2 infection, 50 samples routinely sent in for SARS‐CoV‐2 testing of patients with clinical symptoms were randomly assigned to 10 five‐sample mini‐pools. Both the reference tube and the multiple‐swab tube underwent NAT testing. Each of the four pools containing a positive sample was correctly identified with the multiple‐swab method. Multiple‐swab tubes containing no positive sample were also correctly identified to be negative in multiple‐swab tubes of five swabs. Table 3 shows the comparative presentation of the Ct values from both methods. P value for individual sample and multiple‐swab tube NAT was 0.299 and 0.354 for the ORF region and E‐gene, respectively, which we consider not statistically significant.
TABLE 3.
Testing of the novel multiple‐swab protocol in testing of 50 symptomatic patients
| Pool number | ORF region | E‐gene | ||
|---|---|---|---|---|
| Individual‐swab NAT | Multiple‐swab NAT | Individual‐swab NAT | Multiple‐swab NAT | |
| 1 | 29.07 | 29.21 | 30.07 | 30.61 |
| 4 | 33.85 | 40.00 | 36.28 | 36.34 |
| 5 | 21.24 | 19.03 | 21.72 | 19.65 |
| 6 | 26.12 | 26.23 | 26.65 | 27.11 |
Note: P value was evaluated with the paired t test. The P value was 0.299 and 0.354 between the combined Ct values from the individual‐sample NAT and multiple‐swab tube NAT of the ORF 1a region and the E‐gene, respectively. Comparative results of the four 5‐sample minipools containing a SARS‐CoV‐2 positive sample (multiple‐swab Tubes 1, 4, 5, and 6) are shown.
Abbreviations: Ct, cycle threshold; NAT, nucleic acid testing; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
In a second real‐life application, 100 samples from asymptomatic residents of a nursing home were randomly assigned to 10 multiple‐swab tubes containing 10 swabs each. All 5 multiple‐swab tubes containing a total of 8 positive swabs were correctly identified. All 5 multiple‐swab tubes containing no positive swab sample were also true negative. Ct values did not differ significantly between multiple‐swab tubes and the single‐swab tubes testing (P value for the ORF region and E‐gene were 0.44 and 0.46, respectively) (Table 4).
TABLE 4.
Testing of the multiple‐swab protocol in a nursing home
| Pool number | ORF region | E‐gene | ||
|---|---|---|---|---|
| Individual‐swab NAT | Multiple‐swab NAT | Individual‐swab NAT | Multiple‐swab NAT | |
| 1 | 19.54 | 21.53 | 20.21 | 22.23 |
| 2 | 31.24 | 30.26 | 32.89 | 32.19 |
| 3 | 25.00 | 22.86 | 25.90 | 23.68 |
| 4 | 20.95 | 21.33 | 21.49 | 22.11 |
| 9 | 31.35 | 31.58 | 33.64 | 33.52 |
Note: P‐value was evaluated with the paired T‐Test. The P‐value was .44 and .46 between the combined Ct values from the individual sample NAT and multiple swab tube NAT of the ORF 1a/b region and the E‐gene, respectively. All samples were tested in a multiple‐swab format of 10 swabs per minipool. Comparative results of the five multiple‐swab tubes containing SARS‐CoV‐2 positive sample (multiple‐swab Tubes 1‐4 and 9) are shown.
Abbreviations: Ct, cycle threshold; NAT, nucleic acid testing; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
In a fourth evaluation, the multiple‐swab method was tested for screening of 3110 asymptomatic employees. All samples were investigated in multiple‐swab tubes containing 10 swabs per tube (311 tubes). In total, 2 multiple‐swab tubes achieved a SARS‐CoV‐2–positive NAT screening result. By testing the archive tubes, two asymptomatic employees were identified as SARS‐CoV‐2 positive. Ct values were between 36 and 37 and represent a low virus concentration. Ct values were identical for the multiple‐swab tube and for the archive tube.
Finally, the multiple‐swab method was extended to 50 swabs per tube. As shown in Table 5, Ct values were comparable between the single‐swab tubes and the multiple‐swab tubes up 50 swabs per tube.
4. DISCUSSION
Increased test efficiency is eagerly awaited for SARS‐CoV‐2, as effective strategies to slow down the pandemic depend on early detection of cases, while a finite supply of reagents, qualified personnel, and high costs interfere. To preserve reagents and reduce hands‐on time and expenses, sample pooling is being proposed for settings with a low pretest probability. 10 , 12 This pooling strategy was implemented in blood donor screening for transfusion‐transmitted virus infections like hepatitis C virus (HCV) or HIV‐1 worldwide. 13 , 14 , 15 For virus infection with a very high doubling time like HCV, the loss of the analytical sensitivity is low and acceptable for blood components. The minipool NAT strategy is a success full story that improves blood safety to a maximum. 16 , 17 For symptomatic patients with SARS‐CoV‐2, the virus load is usual high, which enables the option to implement a minipool method with dilution of sample volume or extracted volume. But the implementation of screening of asymptomatic people will be a challenge because, on the one hand, a method with a very high diagnostic sensitivity is needed, and on the other hand, an easy high throughput system should be present to screen a very high number of samples. Both criteria are fulfilled by use of the multiple‐swab method.
Here, we present a novel alternate multiple‐swab protocol that is based on incubation of a respiratory swab first in a single‐sample tube and then again in a multiple‐swab tube. We detected no significant difference in the amount of virus detectable by NAT in the single‐sample and multiple‐swab tubes. Therefore, by applying this protocol in the diagnostic process, no loss of diagnostic or analytical sensitivity would be observed, dismissing a main concern that might hinder implementation. We presume that our multiple‐swab method can be implemented for all NAT methods and all dry swabs. We applied the protocol in two routine scenarios, where the novel protocol was able to reduce the total number of required NAT tests by up to 80%, without loss of diagnostic sensitivity.
By putting this method into practice in the current SARS‐CoV‐2 pandemic, the number of samples that can be tested with a given amount of NAT reagents could immediately be increased in a subcohort with a low pretest probability, when it is not likely that pools must be resolved and samples tested individually, which would void the initial benefit. This could be especially useful when screening professional groups that are exposed to the virus while also posing a risk of spreading it, such as health care workers and emergency responders, or groups at risk, such as the elderly. This approach would not be efficient in a setting with high pretest probability, where it would be likely that the individual samples would have to be retested. Here, a single‐sample test or a smaller pool size would be advisable.
Mathematic models have recently also addressed the beneficial effects of conventional pooled testing strategies. A preprint paper by Hanel and Thurner 12 evaluated the optimal pool size for varying infection level in the population, and suggested an optimal pool size of 11 for an infection level of 1%, which could lead to a fourfold gain in efficiency. However, with rising infection levels, this gain also decreases. Further research on the optimal test size in varying population frequencies of infection is needed with the novel protocol.
In summary, an efficient multiple‐swab strategy is urgently required. The method presented in this paper can be implemented immediately worldwide and thus could represent an essential component in the fight against the SARS‐CoV‐2 pandemic, especially by starting exit strategies by ending the local social breakdown. The cooperation between the Institute of Virology as well as the German Red Cross blood donor service is an excellent example of a fruitful collaboration in the general national health system. The experience of blood donor screening by NAT and the expertise of the virologists enabled the development of the multiple‐swab method and thus laid the foundation stone for early diagnosis of SARS‐CoV‐2 in an entire population by a general screening. The German Red Cross blood donor services are in principle able to screen 1 million inhabitants per day with the multiple‐swab method containing 50 samples per tube. With such a strategy, asymptomatic people can be identified at an early stage of the disease to prevent the spreading of SARS‐CoV‐2.
CONFLICT OF INTEREST
A patent application has been filed for the method presented in this paper.
Schmidt M, Hoehl S, Berger A, et al. Novel multiple swab method enables high efficiency in SARS‐CoV‐2 screenings without loss of sensitivity for screening of a complete population. Transfusion. 2020;60:2441–2447. 10.1111/trf.15973
Preprint 10.1101/2020.04.28.20074187 has posted on medRxiv: https://medrxiv.org/cgi/content/short/2020.04.28.20074187v1
A portable graphical link to your paper (QR code) can be obtained here: https://connect.medrxiv.org/qr/2020.04.28.20074187.
REFERENCES
- 1. The World Health Organization (WHO) . Coronavirus disease (COVID‐19) outbreak situation. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed March 31, 2020.
- 2. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoehl S, Rabenau H, Berger A, et al. Evidence of SARS‐CoV‐2 infection in returning travelers from Wuhan, China. N Engl J Med. 2020;382:1278–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Song J‐Y, Yun J‐G, Noh J‐Y, Cheong HJ, Kim WJ. Covid‐19 in South Korea ‐ challenges of subclinical manifestations. N Engl J Med. 2020;382:1858–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581:465–469. [DOI] [PubMed] [Google Scholar]
- 7. Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic transmission of SARS‐CoV‐2 — Singapore, January 23–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The World Health Organization (WHO) . WHO director‐general's opening remarks at the media briefing on COVID‐19 ‐ 16 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19–16-march-2020. Accessed March 31, 2020.
- 9. The World Health Organization (WHO) . Laboratory testing for coronavirus disease (COVID‐19) in suspected human cases: interim guidance 19 March 2020. Accessed April 9, 2020.
- 10. Hogan CA, Sahoo MK, Pinsky BA. Sample pooling as a strategy to detect community transmission of SARS‐CoV‐2. JAMA. 2020;323:1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Toptan T, Hoehl S, Westhaus S, et al. Optimized qRT‐PCR approach for the detection of intra‐ and extra‐cellular SARS‐CoV‐2 RNAs. bioRxiv. 2020:2020.04.20.052258. http://www.biorxiv.org/content/10.1101/2020.04.20.052258v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hanel R, Thurner S. Boosting test‐efficiency by pooled testing strategies for SARS‐CoV‐2. 2020;22:3. https://arxiv.org/abs/2003.09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roth WK, Buhr S, Drosten C, et al. NAT and viral safety in blood transfusion. Vox Sang. 2000;78(Suppl 2):257–259. [PubMed] [Google Scholar]
- 14. Roth WK. History and future of nucleic acid amplification technology blood donor testing. Transfus Med Hemother. 2019;46:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmidt M, Brixner V, Ruster B, et al. NAT screening of blood donors for severe acute respiratory syndrome coronavirus can potentially prevent transfusion associated transmissions. Transfusion. 2004;44:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hourfar MK, Jork C, Schottstedt V, et al. Experience of German red cross blood donor services with nucleic acid testing: results of screening more than 30 million blood donations for human immunodeficiency virus‐1, hepatitis C virus, and hepatitis B virus. Transfusion. 2008;48:1558–1566. [DOI] [PubMed] [Google Scholar]
- 17. Roth WK, Busch MP, Schuller A, et al. International survey on NAT testing of blood donations: expanding implementation and yield from 1999 to 2009. Vox Sang. 2012;102:82–90. [DOI] [PubMed] [Google Scholar]


