Abstract
Because of the national emergency triggered by the coronavirus disease 2019 (COVID‐19) pandemic, government‐mandated public health directives have drastically changed not only social norms but also the practice of oncologic medicine. Timely head and neck cancer (HNC) treatment must be prioritized, even during emergencies. Because severe acute respiratory syndrome coronavirus 2 predominantly resides in the sinonasal/oral/oropharyngeal tracts, nonessential mucosal procedures are restricted, and HNCs are being triaged toward nonsurgical treatments when cures are comparable. Consequently, radiation utilization will likely increase during this pandemic. Even in radiation oncology, standard in‐person and endoscopic evaluations are being restrained to limit exposure risks and preserve personal protective equipment for other frontline workers. The authors have implemented telemedicine and multidisciplinary conferences to continue to offer standard‐of‐care HNC treatments during this uniquely challenging time. Because of the lack of feasibility data on telemedicine for HNC, they report their early experience at a high‐volume cancer center at the domestic epicenter of the COVID‐19 crisis.
Keywords: coronavirus disease 2019 (COVID‐19), head and neck cancer, radiation oncology, telehealth, telemedicine
Short abstract
Cancer care is undeniably affected by the coronavirus disease 2019 (COVID‐19) public health emergency, but quality cancer care must persist. A high‐volume cancer center from the domestic epicenter of the COVID‐19 crisis shares its multidisciplinary approach to integrating current public health restrictions and adopting telemedicine to optimize the care of patients with head and neck cancer; it is hoped that this center's experience will provide helpful insights to oncology colleagues.
Introduction
Coronavirus Disease 2019 and Cancer
The World Health Organization declared coronavirus disease 2019 (COVID‐19) a public health emergency on January 30, 2020, 1 and a global pandemic on March 11, 2020. Initially, research from Wuhan, China, identified elderly patients as being at particularly high risk. 2 Patients with cancer, because of their immunosuppressive states, are also known to have high morbidity/mortality risks from community respiratory viruses. 3 Reports from the Chinese Center for Disease Control and the World Health Organization–China Joint Mission found that the case fatality rate was doubled in patients with cancer (5.6% vs 2.3% and 7.6% vs 3.8% respectively). 4 , 5 Cancer was actually found to be a greater risk factor for severe events in comparison with chronic obstructive pulmonary disease, diabetes, hypertension, and old age, with 39% of patients with cancer experiencing severe events (intensive care unit stays requiring ventilation or death) versus 8% of patients without cancer. 6
A study from Wuhan confirmed that patients with cancer were more likely to be infected than the general community (odds ratio, 2.31). 7 The nationwide study estimated that 1% of COVID‐19 patients had cancer, whereas 0.29% of the general population had a cancer diagnosis. 6 Alarmingly, another study from Wuhan estimated that 29% of COVID‐infected patients with cancer had acquired the infection while they were in the hospital to receive their cancer therapies. 8 Lastly, in Italy, 20.3% of 355 COVID‐19 patients who died had active cancer. 9 It is clear that minimizing exposure in a population at high risk of contracting the virus and succumbing to its effects is paramount.
Minimizing Exposure and the Rationale for Telemedicine
Viral titers are known to be highest in nasal mucosal, oral, pharyngeal, and pulmonary secretions, and asymptomatic patients have viral titers comparable to those of symptomatic patients. 10 Any procedure involving these surfaces puts all health care workers (HCWs) at high exposure risk. Studies have shown that viable severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) remains in aerosols for at least 3 hours and is detectable on plastic/stainless steel for up to 72 hours after application. 11 It is not surprising that a case series of 138 consecutive hospitalized patients from Wuhan reported that 29% of patients were HCWs and 17% were hospitalized patients. 12
On March 24, 2020, the Centers for Disease Control and Prevention recommended that all elective ambulatory care visits and elective procedures be delayed. 13 The American College of Surgeons highlighted that the recommendation to minimize, postpone, or cancel elective operations/procedures requires careful consideration of the risks of delay versus the risks of proceeding along with the need for resource conservation (HCWs, personal protective equipment [PPE], and ventilators). 14 Given that cancer care often necessitates treatment without delays, the American Society of Therapeutic Radiation Oncology and the American Society of Clinical Oncology advised that care should be “taken to avoid delays in consultation and treatment which may adversely affect potentially curable patients” and encouraged telemedicine where appropriate. 15 , 16 For patients with head and neck cancer (HNC) who do require intervention, a global panel of otolaryngologists, head and neck surgeons, and HCWs advocated for negative COVID testing within 48 hours of any aerosol‐generating procedures. 17
HNC Treatment, a Priority Through the National Emergency
An online journal club convened in March 2020 to address the global response by radiation oncologists to the pandemic; this encouraged telehealth to minimize virus transmission and offered consensus opinions on appropriate scenarios for radiation omission or delay. 18 Importantly, neither omission nor delay was considered appropriate for any subsite or stage of HNC. Similarly, Fox Chase placed high priority on HNC and recommended immediate treatment for patients 70 years or younger and a careful consideration of risks versus benefits for those older than 70 years. 19 Many have encouraged nontraditional care delivery models using expanded telehealth. 20 With the Centers for Medicare and Medicaid Services issuing temporary regulatory waivers that allow increased flexibility to expand telehealth services, experts estimate that there has been a 10‐fold increase in telemedicine utilization over the past few weeks in the United States. 21
Limited Evidence of Telemedicine for HNC
Once a radiation referral is made, standard practices for evaluation/management (ie, in‐person consultations and endoscopic examinations) are not permissible because of the exposure risks for physicians and PPE shortages. Therefore, we implemented telemedicine consultations and multidisciplinary conferences to maintain our standard of care for HNC during this uniquely challenging time. Randomized evidence supports telemedicine for other disease sites, 22 , 23 , 24 but HNC telemedicine efforts have largely been limited to swallow therapy, nutrition, quality of life, supportive care, and case conferences. 25 , 26 , 27 , 28 , 29 , 30 One study from the Veterans Administration reported that a teleconference HNC preoperative visit spared the average patient 28 hours of travel time and $900 for travel‐related costs. 31 Given the lack of data on the feasibility of HNC telemedicine, we report our early experience, which to our knowledge is the first. We hope that these practices will help others to make this transition and provide insights into the safe, serviceable, and sustainable utilization of telemedicine to deliver high‐quality care.
Materials and Methods
Consensus Process and Telemedicine
A multidisciplinary team of head and neck surgeons and radiation and medical oncologists at our cancer center convened during the early days of the pandemic. Because of the prioritization of nonoperative management, our main focus was radiation therapy (RT). We reviewed the literature with an emphasis on randomized controlled trials, prospective observational studies, systematic reviews, and meta‐analyses to derive contingency plans if outpatient oncology practices were to become constrained.
Results
Figure 1 illustrates a preflight checklist that integrates telehealth into the pretreatment, treatment, and posttreatment cancer care paradigm. It also acknowledges important opportunities to uphold COVID‐19 precautions within the workflow. Incorporating telemedicine and public health concerns has been possible only because of multidisciplinary engagement, which has enabled us to continue lifesaving cancer operations and to deliver the best possible care while respecting critical safety concerns for patients and HCWs alike:
On March 23, 2020, the American Academy of Otolaryngology–Head and Neck Surgery stated that the “need to flatten the curve of transmission and preserve critical supplies and equipment for those who need it most necessitates limiting care at this time to time‐sensitive and emergent problems”; thus, surgical and high‐risk aerosol‐generating endoscopic procedures should be limited. A panel of international experts from the American Society for Therapeutic Radiation Oncology, the European Society of Therapeutic Radiation Oncology, and select Asian‐Pacific countries reported that more than half of the panelists (53%) were no longer performing aerosol‐generating procedures (including nasopharyngoscopy) in the radiation oncology department. 32 Our radiation department has stopped all endoscopic procedures. We use multiple forms of cross‐sectional imaging—positron emission tomography (PET)/computed tomography (CT) and/or magnetic resonance imaging—for radiation planning. Figure 2 illustrates endoscopic examinations with corresponding cross‐sectional images (CT, magnetic resonance imaging, and PET/CT) in base of tongue and tonsil patients. When endoscopic examinations are restricted, multimodality imaging can minimize high‐risk exposures, augment staging, and enhance tumor visualization for optimal target delineation.
On March 16, 2020, the American Dental Association recommended postponement of elective procedures; this was echoed by the Centers for Disease Control and Prevention on March 27, 2020, and New York State mandated no elective dental extractions before radiation. Although periodontal health is important, extractions may not prevent osteoradionecrosis. 33 , 34 Thus, we currently recommend dental hygiene education throughout radiation followed by close posttreatment surveillance with dental colleagues once that is permissible again.
Figure 1.
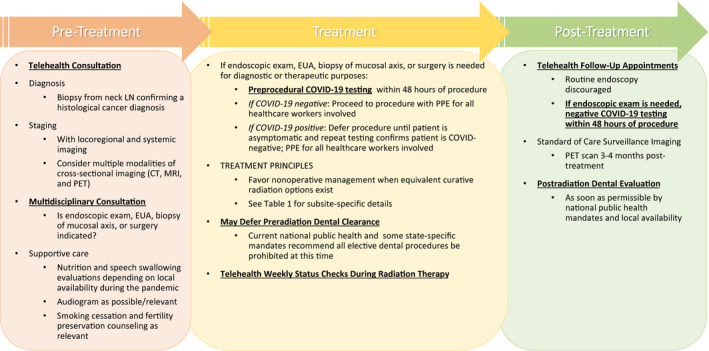
Incorporating telehealth and COVID‐19 precautions into cancer care during the pandemic: preflight checklist. COVID‐19 indicates coronavirus disease 2019; CT, computed tomography; EUA, examination under anesthesia; LN, lymph node; MRI, magnetic resonance imaging; PET, positron emission tomography; PPE, personal protective equipment.
Figure 2.
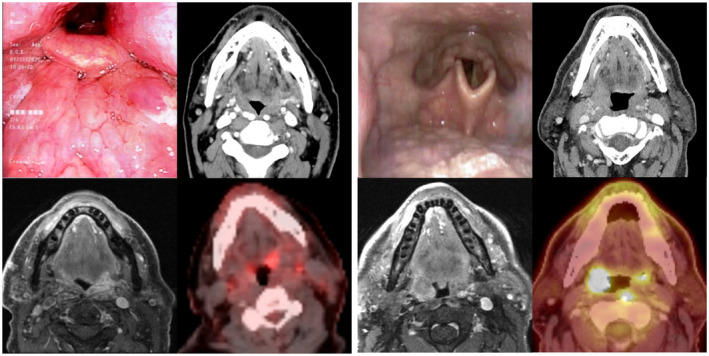
Panels comparing endoscopic examinations with cross‐sectional imaging in (Left) base of tongue and (Right) tonsil patients. The combination of magnetic resonance imaging (lower left images) and positron emission tomography/computed tomography (lower right images) provides valuable tumor localization and staging information that can be overlooked by conventional computed tomography (upper right images) and endoscopic examination alone (upper left images). When endoscopy is not possible, multiple forms of dedicated cross‐sectional imaging can be used for treatment planning.
Site‐Specific Treatment Recommendations and Contingency Plans
Although there have been significant disruptions to usual practices and clear restrictions placed on procedures considered high‐risk during the pandemic, it is important to note that this should not translate into a reflexive abandonment of all best practices.
Table 1 shows guidelines for tier 1 curable HNCs. Because of current restrictions on surgery, nonsurgical management is preferred when surgery and RT have equal outcomes (ie, oropharynx, hypopharynx, and larynx cancers). For RT alone, modest hypofractionation is included for subsites with known benefits (oropharynx, hypopharynx, and larynx). In the United Kingdom, a modestly hypofractionated schedule of 65 to 66 Gy in 30 fractions is a standard for pharyngeal cancers. 59 This amounts to marginally fewer fractions (only 3‐5) in comparison with conventional dosing in the United States, and thus we continue to recommend our standard 2 Gy per fraction. International experts agree that there is insufficient justification to alter standard practices by administering hypofractionated RT or omitting concomitant chemotherapy during risk‐mitigation phases of the pandemic. 32
Table 1.
Treatment Recommendations By Subsite During the Pandemic
| Subsite a | Treatment Recommendations During Pandemic | Rationale and Guiding Principles |
|---|---|---|
| Tier 1: treatment guidelines for curable patients | ||
| Nasopharynx | ||
| T1N0 | RT alone (2.12 Gy/fx to 69.96 Gy or 2 Gy/fx to 70 Gy) | Radiation doses and volumes per NRG‐HN001 |
| All other M0 patients | CRT (2.12 Gy/fx to 69.96 Gy or 2 Gy/fx to 70 Gy) | |
| Nasal cavity and paranasal sinuses | Surgery ± adjuvant RT (2 Gy/fx to 60‐66 Gy) ± concurrent chemotherapy | Surgery remains the standard of care when possible. |
| T1‐T4 | If surgery is not possible or for organ preservation: | If surgery is not possible or is refused by the patient, organ preservation is possible. |
| Definitive CRT: 2 Gy/fx to 70 Gy with concurrent chemotherapy | Definitive RT with proton therapy has shown excellent outcomes with 2‐y LC of 83%. 35 | |
| Consider proton therapy if feasible. | ||
| Oral cavity | Surgery ± adjuvant RT (2 Gy/fx to 60‐66 Gy) ± concurrent chemotherapy | Surgery remains the standard of care when possible. |
| T1‐T4 | If surgery will be delayed or not possible or for organ preservation: | Brachytherapy has been used as part of definitive RT for oral cavity cancers but is also limited under current restrictions on surgical interventions. 36 |
| Definitive RT (2 Gy/fx to 70 Gy) | In the setting of operating room closures, experts agree that radical RT for early oral tongue cancer and radical CRT for locally advanced oral tongue cancer are appropriate. 32 | |
| Consider proton therapy if feasible. | There is limited randomized evidence to support induction chemotherapy, which can delay surgery. 37 , 38 However, because of uncertainty about the safe reinstitution of surgery, potential detriments to the patient's immune system, and the risks of extra exposures, we caution against induction chemotherapy. | |
| Concurrent chemotherapy if T3, T4, or N+ | Definitive CRT with IMRT has been shown to achieve 2‐y LRC of 64%. 39 | |
| Highly recommend for early‐stage disease if surgery is not possible. | Because of superior dosimetry, proton therapy could be considered to deliver high doses with less toxicity. | |
| Oropharynx and unknown primary | Given equivalent outcomes with surgery and radiation, RT is favored because of public health mandates and pandemic precautions. | |
| p16‐positive | ||
| T1N0‐T2N0 | Definitive RT (2.12 Gy/fx to 69.96 Gy or 2 Gy/fx to 70 Gy) | |
| Any T3, T4, or N+ | Definitive CRT (2 Gy/fx to 70 Gy) + concurrent platinum‐based chemotherapy (prefer high‐dose cisplatin) | Because of the failure of deintensification, definitive CRT remains the standard of care for node‐positive, p16+ OPC off trial. 40 |
| p16‐negative | There is no role for modest hypofractionation in patients receiving chemotherapy because of the high likelihood of a cure and increased risks of toxicity with higher doses per fraction. 41 | |
| T1N0‐T2N0 | Definitive RT (2.12 Gy/fx to 69.96 Gy preferred or 2 Gy/fx to 70 Gy) | |
| Any T3, T4, or N+ | Definitive CRT (2 Gy/fx to 70 Gy) + concurrent platinum‐based chemotherapy | We do not recommend twice daily hyperfractionation schedules because they increase the number of visits to a clinic and thus increase exposures. |
| Larynx | Given equivalent outcomes with surgery and radiation, RT is favored because of public health mandates and pandemic precautions. | |
| T1N0 glottic larynx | Definitive RT (2.25 Gy/fx to 63 Gy) | |
| T2N0 glottic larynx | Definitive RT (2.25 Gy/fx to 65.25 Gy) | There is no role for modest hypofractionation in patients receiving chemotherapy because of the high likelihood of a cure and increased risks of toxicity with higher doses per fraction. |
| T1‐T2N0 supraglottic or subglottic larynx | Definitive RT (2 Gy/fx to 70 Gy; consider 2.12 Gy/fx to 69.96Gy) | We do not recommend twice daily hyperfractionation schedules because they increase the number of visits to a clinic and thus increase exposures. |
| T3, T4, or N+ glottic larynx; all other larynx | Definitive CRT (2 Gy/fx to 70 Gy) + concurrent platinum‐based chemotherapy | |
| Hypopharynx | ||
| T1N0‐T2N0 | Definitive RT (2.12 Gy/fx to 69.96 Gy preferred or 2 Gy/fx to 70 Gy) | Given equivalent outcomes with surgery and radiation, RT is favored because of public health mandates and pandemic precautions. |
| Any T3, T4, or N+ | Definitive CRT (2 Gy/fx to 70 Gy) + concurrent platinum‐based chemotherapy | There is no role for modest hypofractionation in patients receiving chemotherapy because of the high likelihood of a cure and increased risks of toxicity with higher doses per fraction. 41 |
| We do not recommend twice daily hyperfractionation schedules because they increase the number of visits to a clinic and thus increase exposures. | ||
| Tier 2: treatment guidelines where LRC is important | ||
| Recurrent HNC in need of reirradiation | The Multi‐Institutional Reirradiation Collaborative performed RPA to identify favorable patients for definitive reirradiation and found that hyperfractionation does not improve outcomes and may increase toxicity. 42 , 43 Absence of organ dysfunction and doses > 50 Gy are important determinants of LRC. 44 | |
| Postoperative patients | Conventionally fractionated RT (2 Gy/fx to 60‐66 Gy) | |
| No surgery: >2 y from RT or good KPS | Conventionally fractionated RT (2 Gy/fx to 70 Gy) | |
| No surgery and rapid recurrence from first course | Quad Shot (3.7 Gy/fx twice daily × 2 consecutive days = 1 cycle; may repeat cycle every 3‐4 wk for up to 4 total cycles) | |
| Metastatic HNC in need of local therapy | The RTOG Quad Shot regimen is a well‐validated treatment that achieves palliative responses in two‐thirds of patients with a grade 3 toxicity rate of only approximately 10%, even in the reirradiation setting. 45 Quad Shot can be administered with concurrent systemic therapy, is effective in multiple histologies, and is effective across all H&N subsites. | |
| Prior RT | Quad Shot (3.7 Gy/fx twice daily × 2 consecutive days = 1 cycle; may repeat cycle every 3‐4 wk for up to 4 total cycles) | |
| No prior RT | Quad Shot (3.7 Gy/fx twice daily × 2 consecutive days = 1 cycle; may repeat cycle every 3‐4 wk for up to 4 total cycles) | We favor the RTOG Quad Shot over the HYPO trial regimen (6 Gy/fx at 2/wk to 30 Gy with optional 6‐Gy boost for small disease [≤3 cm] in suitable patients) because of its potent efficacy, minimal toxicity, and universal applicability to palliative, metastatic, and reirradiation settings. 46 , 47 Furthermore, Quad Shot allows higher BED and repeat cycles versus hypofractionation. There is less daily exposure in comparison with hypofractionation. Quad Shot has been reported to be less toxic than 30 Gy/10 fx and 20 Gy/5 fx. 48 Lastly, Quad Shot is ideal during the crisis because it decreases exposures for both patients and staff and has a built‐in break between cycles. |
| Other primary cancer metastatic to H&N | Quad Shot (3.7 Gy/fx twice daily × 2 consecutive days = 1 cycle; may repeat cycle every 3‐4 wk for up to 4 total cycles) | Consider the histology, patient's performance status, and systemic therapies in selecting an appropriate treatment regimen. |
| Other palliative regimens: 30 Gy/10 fx, 20 Gy/5 fx, 8 Gy/1 fx | ||
| Tier 3: severe restrictions or limitations in radiation oncology operations | ||
| Larynx | ||
| T1N0 glottic larynx | Definitive RT (3.12‐3.28 Gy/fx to 50‐52.5 Gy; 16 fractions) | The Christie and Royal Marsden Hospital reported 93% 5‐y LC with 3 wk of RT for T1 glottic larynx cancer. 49 |
| T1‐T2N0 glottic | Definitive RT (2.55 Gy/fx to 51 Gy; 20 fractions) | Princess Margaret reported 81.7% 5‐y LC. 50 |
| Larynx | Definitive RT (2.75 Gy/fx to 55 Gy; 20 fractions) | St. James's Institute of Oncology reported 85.6% 5‐y LC. 51 |
| Oropharynx | ||
| T1‐T2N0‐N1 oropharynx | Definitive IMRT (2.2 Gy/fx to 66 Gy; 30 fractions) | In RTOG 0022, modestly accelerated hypofractionated IMRT without chemotherapy achieved 91% 2‐y LRC with reduced xerostomia in comparison with prior RTOG studies. 52 |
| p16+ T1N1‐T2N2b or T3N0‐T3N2b with ≤10‐pack‐y smoking history | Definitive CRT (2 Gy/fx to 60 Gy; 30 fractions) + concurrent platinum‐based chemotherapy | The de‐escalated CRT arm from HN002 will serve as an experimental arm of HN005. 53 |
| Locally advanced HNC | ||
| T1N0‐T4N3 SqCC of oral cavity, oropharynx, hypopharynx, or larynx | Definitive CRT (2.75 Gy/fx to 55 Gy; 20 fractions) + concurrent carboplatin | Hypofractionation accelerated CRT has been used in the United Kingdom with 79% 2‐y LC, 74% 2‐y OS, and only 9% of patients having grade 3 mucositis ≥4 wk from the onset of symptoms. 54 |
| T1‐T4N2‐N3 SqCC of oral cavity, oropharynx, hypopharynx, larynx, or unknown primary | Definitive CRT (2.75 Gy/fx to 55 Gy; 20 fractions) + concurrent cisplatin (high dose or weekly) or cetuximab | In a randomized trial of definitive chemoradiation in patients with advanced nodal disease, a minority of patients were treated with this hypofractionated schedule. 54 |
| T3‐T4N0 or any N+ SqCC of oropharynx, hypopharynx, or larynx | Definitive RT (2.55 Gy/fx to 51 Gy; 20 fractions) | A randomized trial from Princess Margaret Hospital used this regimen of hypofractionated RT alone as the standard of care. 55 |
| Compensation for Radiation Treatment Gaps | ||
|---|---|---|
| Treatment Type | Compensation | Rationale and Guiding Principles |
| RT alone | Add 1 fraction for every 1 wk of treatment gap | Treatment duration is well known to affect LC in patients treated with definitive RT. Under the assumption of 80% 2‐y LC, a 5‐d increase in duration was associated with a 3.5% reduction in LC. 56 Although there is evidence that treatment duration affects outcomes, there is no high‐level evidence that additional radiation compensates for treatment breaks. If clinical status and dosimetry permit, consider adding extra treatments as possible. |
| CRT | If the total treatment duration will be >8 wk: | There are no strong data to suggest that treatment breaks influence outcomes for patients receiving concurrent chemotherapy. |
| Reimage the patient first if the break is >1 month. | There are suggestions that a 1‐wk gap is associated with 14% to 20% reductions in LC. 57 Twice daily fractionation is easily implementable compensation because it does not require replanning and can add a dose without extending the total treatment duration. | |
|
Look at the histology and site to determine the need for additional fractions of radiation:
|
In RTOG 1016, cisplatin patients with total treatment durations of up to 58 days completed the planned course without additional fractions and with no change in total dose or dose fractionation. 58 | |
Abbreviations: BED, biologically effective dose; CRT, chemoradiotherapy; CT, computed tomography; fx, fraction(s); H&N, head and neck; HNC, head and neck cancer; IMRT, intensity‐modulated radiation therapy; KPS, Karnofsky performance status; LC, local control; LRC, locoregional control; OPC, oropharyngeal cancer; OS, overall survival; PET, positron emission tomography; RPA, recursive partitioning analysis; RT, radiation therapy; RTOG, Radiation Therapy Oncology Group; SqCC, squamous cell carcinoma.
Seventh edition of the American Joint Committee on Cancer staging system.
Although head and neck operations have decreased significantly, urgent essential cases may proceed after a thorough and rigorous preoperative review and negative preoperative COVID‐19 testing. Patients who test positive may be reassessed for surgery at a later date if time permits. Essential surgeries that have been prioritized include advanced or rapidly progressive oral cavity, sinonasal, salivary, and skin cancers and sarcomas; aggressive thyroid cancers; and other cancers that threaten vital functions. Because of the potential for false‐negative COIVD‐19 tests, all operating room members are equipped with PPE appropriate for the risk level for virus aerosolization. When surgery is not possible, definitive nonsurgical treatments should be pursued. There are institutional series reporting success with definitive RT for select nasal and oral cavity cancers. 35 , 39 We do not recommend routine use of induction chemotherapy. There is limited randomized evidence to support induction chemotherapy for locally advanced oral cavity cancers, but there is no local control or survival benefit. Induction may shrink the tumor and allows an approximately 12‐week delay in surgery. 37 , 38 However, there are potentially significant risks because it creates additional exposures for patients and HCWs for a treatment that delays definitive therapy without proven benefit. Furthermore, the immune system could become compromised, and this could potentially increase mortality from COVID‐19 infection. In addition, these patients are heavily symptomatic and require significant help from caregivers, and this could lead to more possible exposures.
Table 1 also shows guidelines for patients with tier 2 HNCs, that is, those with recurrent or metastatic disease for whom locoregional control remains important. Multiple publications have consistently identified the importance of the interval from prior RT, organ dysfunction, functional status, and surgical salvage to reirradiation outcomes. 42 , 43 , 44 Patients who have good functional status, are more than 2 years from prior RT, or have had salvage surgery should receive conventional fractionation. The Radiation Therapy Oncology Group Quad Shot regimen (1 cycle = 3.7 Gy twice daily × 4 fractions over 2 days; repeated every 3‐8 weeks for a total of 3‐4 cycles) is used for recurrent and metastatic HNC across all disease subsites and histologies. 45 , 46 Quad Shot is ideal because cycles may be repeated to achieve nearly definitive doses and can be given concurrently with chemotherapy with minimal toxicity.
Table 1 also encompasses tier 3 if RT becomes restricted. Under these circumstances, there are considerably hypofractionated treatment schedules for larynx, oropharynx, hypopharynx, and oral cavity cancers. 49 , 50 , 51 , 52 , 53 , 54 , 55 , 60 Even under extreme duress, we do not advocate the indiscriminate use of these alternative dosing schedules for subsites to which they have not explicitly been applied. Although 2.34 Gy per fraction seems to be only modest hypofractionation in comparison with 2.12 Gy per fraction for nasopharyngeal cancer, 2.34 Gy per fraction in a prospective trial was deemed unsuitable because of high in‐field brain necrosis. 61 Therefore, we do not recommend above 2.12 Gy per fraction for nasopharyngeal cancer unless the radiation treatment plan permits this fractionation without causing significant complications.
Four cycles of Quad Shot (59.2 Gy in 16 fractions over 8 treatment days) delivers a dose equivalent to 67.59 Gy in 2 Gy per fraction; this is nearly identical to the conventional treatment of 70 Gy in 2 Gy per fraction over 35 treatment days. The tumor biologically effective dose of Quad Shot is 81.10 Gy, whereas the tumor biologically effective dose is 84 Gy for conventional RT. Thus, Quad Shot can deliver nearly curative doses with a fraction of the clinic visits in comparison with other hypofractionated regimens (8 vs 20 treatments). Quad Shot has been used in nasal cavity, nasopharynx, oral cavity, oropharynx, hypopharynx, and larynx cancers. 45 It can be considered in extreme circumstances when there is urgency to treat and there is a drastic shortage of radiation resources. Quad Shot has a built‐in treatment break during which tumor regression and normal tissue recovery occur. It is ideal for controlling bleeding, painful, rapidly progressive, putrid masses.
We have also instituted a daily radiation oncology huddle called the Radiation Oncology S.W.A.T. team; led by one of our head and neck radiation oncologists (S.M.M.), it determines which patients require COVID‐19 testing beyond the institutional policy. In HNC, it is often difficult to distinguish between symptoms due to cancer, treatment, or infection. Because of risks to patients, HCWs, and the community, it is advisable to err on the side of caution and test any patient with cancer with a cough, fever, shortness of breath, flulike symptoms, or high‐risk exposure. An official polymerase chain reaction swab test is performed, and if it is positive, our policy has been to stop treatment; the patient may resume treatment 10 days later if he or she is asymptomatic. Official French guidelines advise avoiding the admission of COVID‐19 patients into oncology or RT departments or isolating them from other patients as quickly as possible upon the discovery of infection; the guidelines also recommend discontinuing systemic anticancer treatments until the complete resolution of symptoms (at the clinician's discretion). 62 In an already vulnerable population, continuation of treatment may increase COVID‐19 mortality. However, if the risks of delaying RT outweigh the uncertain risks of COVID‐19 mortality, we are also considering treating all urgent positive cases at the end of the day on 1 linear accelerator to minimize contamination risks.
The bottom of Table 1 outlines recommended compensation for treatment gaps. Treatment duration affects local control in larynx cancer receiving definitive RT. 63 In general, a 5‐day increase in duration causes an estimated 3.5% absolute reduction in local control. 56 Thus, we recommend adding 1 fraction for every 1‐week radiation gap. The impact of treatment gaps in the setting of concurrent chemotherapy is controversial, with no strong data to suggest that breaks affect outcomes in chemoradiotherapy patients. 57 If there has been a gap of at least 1 week and the total treatment duration will exceed 8 weeks, tumor site and histology should be considered in the decision to add additional fractions. Twice daily fractionation is easily implementable, does not require replanning, and adds a dose without extending treatment duration. Consider treatment with the same plan twice daily (preferably 6 hours apart) on Fridays to accelerate the remaining course once RT is resumed. It is also reasonable to consider not adding treatments if there is concern about exceeding dose tolerances to critical structures (eg, brachial plexus). Notably, in Radiation Therapy Oncology Group 1016, for human papillomavirus–positive tumors, chemoradiotherapy patients with breaks and total treatment durations of up to 58 days (vs a median duration of 39 days) completed the planned course without additional fractions and no change in the total dose or dose fractionation. 58 As an alternative to delivering extra treatments, early interval follow‐up with first surveillance CT with contrast 4 to 6 weeks after treatment could be performed to confirm a response. If there is no progression, standard posttreatment PET at 3 to 4 months should be performed, and additional treatment could be considered as needed. If the treatment gap is more than 1 month, diagnostic imaging should be considered. If there is progression and it is safe to deliver, 1 cycle of Quad Shot can be administered as a boost to compensate for patients with a treatment gap longer than 1 month. If it is difficult to coordinate additional diagnostic imaging, a resimulation CT scan or cone‐beam CT on the linear accelerator can help to determine whether the tumor has progressed during the break.
Summary of Our Early Telemedicine Experience for HNC
On March 17, 2020, we instituted telemedicine for all patient appointments. We report our experience from the first (March 17, 2020, to March 20, 2020) and second weeks (March 23, 2020, to March 27, 2020). In this 2‐week interval, there were 48 consultations, 117 status checks, and 36 follow‐up appointments performed across 7 radiation oncology clinics. During the in‐person consults, only 1 nasopharyngoscopy (the last permitted) was performed. Patients were very grateful for telemedicine visits, and no telemedicine‐consultation, status‐check, or follow‐up patients subsequently requested an in‐person visit.
Table 2 details descriptive characteristics of the consultations. Notably, the majority of consults (>70%) were for tier 1 curative‐intent patients, with the remainder for tier 2 recurrent or metastatic cases. Even with the exponential rise of COVID‐19 cases and consequent strains on the local health care system, there was no need to activate tier 3 measures. There was an increase in definitive radiation consults (from 52% to 61%) after the Centers for Disease Control and Prevention recommended delaying nonessential surgeries. Radiation was recommended for >75%, with concurrent chemotherapy in 56%. One‐fourth of the patients enrolled in clinical trials. There were no workflow delays with a median time to simulation of 7 days (range, 0‐15 days); most delays were initiated by patients or were for necessary additional imaging workup. Those patients not recommended to undergo RT (23%) were redirected for further workup as appropriate.
Table 2.
Descriptive Characteristics of Telehealth Visits
| Week 1, No. (%) | Week 2, No. (%) | Total, No. (%) | |
|---|---|---|---|
| HNC consultations in radiation oncology | |||
| Consults | |||
| Telemedicine | 21 (84) | 19 (83) | 40 (83) |
| In person (protocol‐mandated, patient request) | 4 (16) | 4 (17) | 8 (17) |
| Endoscopic examination | 1 (4) | 0 (0) | 1 (2) |
| Total No. of consults | 25 | 23 | 48 |
| Subsite | |||
| Nasal cavity/paranasal sinuses | 3 (12) | 0 (0) | 3 (6) |
| Nasopharynx | 0 (0) | 0 (0) | 0 (0) |
| Oropharynx | 7 (28) | 8 (35) | 15 (31) |
| Hypopharynx | 2 (8) | 1 (4) | 3 (6) |
| Larynx | 4 (16) | 0 (0) | 4 (8) |
| Oral cavity | 4 (16) | 3 (13) | 7 (15) |
| Unknown primary | 2 (8) | 2 (9) | 4 (8) |
| Other (salivary, thyroid, sarcoma, etc) | 3 (12) | 9 (38) | 12 (25) |
| Disease status | |||
| Tier 1: primary | 16 (64) | 18 (78) | 34 (71) |
| Tier 2: recurrent | 4 (16) | 3 (13) | 7 (15) |
| Tier 2: metastatic | 5 (20) | 2 (9) | 7 (15) |
| Tier 3: severely limited RT resources | 0 (0) | 0 (0) | 0 (0) |
| Radiation intent | |||
| Definitive | 13 (52) | 14 (61) | 27 (56) |
| Adjuvant | 6 (24) | 7 (30) | 13 (27) |
| Palliative | 6 (24) | 2 (9) | 8 (17) |
| Radiation history | |||
| No prior H&N RT | 17 (68) | 19 (83) | 36 (75) |
| History of prior H&N RT | 8 (32) | 4 (17) | 12 (25) |
| Age | |||
| <70 y | 20 (80) | 19 (83) | 39 (81) |
| Radiation recommended | 18 (72) | 19 (83) | 37 (77) |
| Referred to proton center | 7 (39) | 5 (26) | 12 (32) |
| Treatment at MSK Manhattan or regional facility | 11 (61) | 14 (74) | 25 (68) |
| Time to SIM, median (range), d | 8 (0‐15) | 7 (0‐12) | 7 (0‐15) |
| Dental clearance obtained/recommended | 5/8 (63) | 3/12 (25) | 8/20 (40) |
| Enrolled in a clinical trial | 1 (6) | 6 (32) | 12 (25) |
| Dose fractionation | |||
| Conventional fractionation (2 Gy/fx to 60‐70 Gy) | 14 (78) | 17 (89) | 31 (84) |
| Palliative hypofractionation regimen | 4 (22) | 2 (11) | 6 (16) |
| Radiation not recommended | 7 (28) | 4 (17) | 11 (23) |
| Surgery recommended first | 2 (28) | 2 (50) | 4 (36) |
| Further workup recommended | 2 (28) | 1 (25) | 3 (28) |
| Patient declined, hospice, or RT closer to home | 2 (28) | 1 (25) | 3 (28) |
| Other | 1 (14) | 0 (0) | 1 (9) |
| Concurrent chemotherapy | |||
| Yes | 10 (56) | 15 (79) | 25 (68) |
| No | 8 (44) | 4 (21) | 12 (32) |
| HNC status checks | |||
| Telemedicine | 36 (73) | 63 (93) | 99 (85) |
| In person | 13 (27) | 5 (7) | 18 (15) |
| HNC follow‐up appointments | |||
| Telemedicine | 8 (100) | 28 (100) | 36 (100) |
| In person | 0 (0) | 0 (0) | 0 (0) |
Abbreviations: fx, fraction; H&N, head and neck; HNC, head and neck cancer; MSK, Memorial Sloan Kettering; RT, radiation therapy; SIM, simulation for radiation therapy.
Present/Future Challenges for Telemedicine During the Pandemic
Although the efficient transition from telemedicine consultation to RT planning confirms the feasibility of tele‐oncology, rapidly changing recommendations and local conditions throughout the pandemic will require adaptability. Even since our initiation of telehealth, governmental, professional society, and organizational mandates have already changed in response to progressive distress in the health care system. By the second week of telemedicine, dental clearance before RT went down from 63% to 25%.
Although there is a call to deliver fewer treatments to minimize exposures during the pandemic, 18 , 64 it is currently not our practice even though we work at a high‐volume cancer center at the epicenter of the domestic COVID‐19 crisis. Hypofractionated regimens (>2 Gy per fraction) can be pursued as outlined. 63 , 65 However, if care becomes rationed and further reductions are needed, tier 3 in Table 1 illustrates the appropriate clinical settings for extreme hypofractionation.
As limitations, needs, and public health directives evolve during the pandemic, there will arise new challenges to oncology practices. Frequent communication with multidisciplinary colleagues is critical to anticipate barriers, prepare contingency plans, evaluate their efficacy, and refine/revise operations as needed. Our entire multidisciplinary tumor board meets weekly via Zoom. These meetings ensure communication, compensation, and consensus for optimal patient care during the pandemic. Although these practices have helped us to continue to provide patients with HNC with the highest quality of care, they were informed by principles that are applicable to any oncology specialty.
In conclusion, patients with HNC typically present with rapidly progressive tumors (that can become incurable with delays) and symptomatic emergencies (bleeding and difficulty with breathing). They represent a high‐risk subset in whom early intervention can prevent progression and limit burdens on already strained health care systems. Because some typical management practices are simply not permissible during a pandemic, frequent multidisciplinary communications are critical for the highest quality care within the confines of current limitations. In the midst of their own emergencies, oncology departments from Italy, Taiwan, and China shared their processes, including expanded PPE use, staff reorganization, screening of patients as well as staff, and triage procedures. 66 , 67 , 68 , 69 , 70 , 71 After Hurricane Maria in Puerto Rico, experts created a framework centered on measures to “prepare, communicate, operate, compensate” to mitigate the medical impact of a disaster. 72 With the current global magnitude of the crisis and the immediate challenges of testing and PPE shortages in the United States, telemedicine has been the backbone of our strategy to protect against infection and continue the fight against cancer.
A notable difference between the United States and Asia is that cancer treatment in our country is delivered on an outpatient basis. 73 With current projections, the public health social distancing mandates and restrictions on health care operations may remain in place for months. It would be unconscionable to stop cancer care for the duration of the pandemic, and the burden falls on outpatient oncologists to fill these gaps. It is necessary to make trade‐offs (cancer cure vs decreasing risks of COVID‐19 infection) with the understanding that we do not know the full consequences of some of our actions. In general, there has been a lack of research in the United States (and elsewhere) looking at how to decrease our interventional and surveillance practices. One study from Kaiser Permanente found that adherence to a routine surveillance schedule did not confer any survival advantage and was of limited utility because nearly all clinically detected recurrences were elicited by patient symptoms that prompted earlier presentation to the clinician. 74 Because the current situation demands changes to our practice, perhaps this is also an opportunity to better investigate these issues so that we are better prepared for the next wave of this crisis or any other crisis. As we work together to care for patients with cancer, telemedicine is a practical and practicable response to the call to “be safe, be smart, be kind.”
Funding Support
No specific funding was disclosed.
Conflict of Interest Disclosures
Nadeem Riaz reports grants from Pfizer, Bristol‐Myers Squibb, AstraZeneca, and REPARE and consulting fees from REPARE, Illumina, and Mirati Therapeutics outside the submitted work. C. Jillian Tsai reports consulting for Varian outside the submitted work. Yao Yu reports travel funds from Elekta outside the submitted work. Nancy Y. Lee reports grants from Pfizer, Merck, Merck Serono, and AstraZeneca and consulting for Pfizer, Merck, Merck Serono, and Lilly outside the submitted work. The other authors made no disclosures.
Kang JJ, Wong RJ, Sherman EJ, Rybkin A, McBride SM, Riaz N, Tsai CJ, Yu Y, Chen L, Zakeri K, Gelblum DY, Gillespie EF, Cohen MA, Cracchiolo JR, Ganly I, Patel S, Singh B, Boyle JO, Roman BR, Morris LG, Shaha AR, Dunn LA, Ho AL, Fetten JV, Shah JP, Pfister DG, Lee NY. The 3 Bs of cancer care amid the COVID‐19 pandemic crisis: “Be safe, be smart, be kind”—A multidisciplinary approach increasing the use of radiation and embracing telemedicine for head and neck cancer. Cancer. 2020:126:4092‐4104. 10.1002/cncr.33031
References
- 1. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. doi:10.1016/S0140‐6736(20)30566‐3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 2009;10:589‐597. [DOI] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. Published online February 24, 2020. doi:10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Report of the WHO‐China Joint Mission on Coronavirus Disease 2019 (COVID‐19). Accessed March 23, 2020. https://www.who.int/docs/default‐source/coronaviruse/who‐china‐joint‐mission‐on‐covid‐19‐final‐report.pdf
- 6. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu J, Ouyang W, Chua MLK, et al. SARS‐CoV‐2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. Published online March 25, 2020. doi:10.1001/jamaoncol.2020.0980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID‐19–infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. Published online March 26, 2020. doi:10.1016/j.annonc.2020.03.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. Published online March 23, 2020. doi:10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 10. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177‐1179. doi:10.1056/NEJMc2001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. Published online March 17, 2020. doi:10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. doi:10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. US Centers for Disease Control and Prevention . Healthcare facilities: preparing for community transmission. Accessed March 23, 2020. https://www.cdc.gov/coronavirus/2019‐ncov/healthcare‐facilities/guidance‐hcf.html
- 14. American College of Surgeons . COVID‐19 guidelines for triage of cancer surgery patients. Accessed March 23, 2020. https://www.facs.org/covid‐19/clinical‐guidance/elective‐case/cancer‐surgery
- 15. American Society for Therapeutic Radiation Oncology . COVID‐19 recommendations and information. Accessed March 23, 2020. https://www.astro.org/Daily‐Practice/COVID‐19‐Recommendations‐and‐Information/Summary
- 16. American Society of Clinical Oncology . ASCO coronavirus resources. Accessed March 23, 2020. https://www.asco.org/asco‐coronavirus‐information
- 17. Givi B, Schiff BA, Chinn SB, et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID‐19 pandemic. JAMA Otolaryngol Head Neck Surg. Published online March 31, 2020. doi:10.1001/jamaoto.2020.0780 [DOI] [PubMed] [Google Scholar]
- 18. Simcock R, Thomas TV, Mercy CE, et al. COVID‐19: global radiation oncology's targeted response for pandemic preparedness. Clin Transl Radiat Oncol. 2020;22:55‐68. doi:10.1016/j.ctro.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kutikov A, Weinberg DS, Edelman MJ, et al. A war on two fronts: cancer care in the time of COVID‐19. Ann Intern Med. Published online March 27, 2020. doi:10.7326/M20‐1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID‐19 pandemic: agility and collaboration toward a common goal. J Natl Compr Canc Netw. Published online March 20, 2020. doi:10.6004/jnccn.2020.7560 [DOI] [PubMed] [Google Scholar]
- 21. Webster P. Virtual health care in the era of COVID‐19. Lancet. 2020;395:1180‐1181. doi:10.1016/S0140‐6736(20)30818‐7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beaver K, Williamson S, Sutton C, et al. Comparing hospital and telephone follow‐up for patients treated for stage‐I endometrial cancer (ENDCAT trial): a randomised, multicentre, non‐inferiority trial. BJOG. 2017;124:150‐160. doi:10.1111/1471‐0528.14000 [DOI] [PubMed] [Google Scholar]
- 23. Cleeland CS, Wang XS, Shi Q, et al. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. J Clin Oncol. 2011;29:994‐1000. doi:10.1200/JCO.2010.29.8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steffen LE, Boucher KM, Damron BH, et al. Efficacy of a telehealth intervention on colonoscopy uptake when cost is a barrier: the Family CARE cluster randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2015;24:1311‐1318. doi:10.1158/1055‐9965.EPI‐15‐0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wall LR, Ward EC, Cartmill B, et al. Prophylactic swallowing therapy for patients with head and neck cancer: a three‐arm randomized parallel‐group trial. Head Neck. 2020;42:873‐885. doi:10.1002/hed.26060 [DOI] [PubMed] [Google Scholar]
- 26. Collins A, Burns CL, Ward EC, et al. Home‐based telehealth service for swallowing and nutrition management following head and neck cancer treatment. J Telemed Telecare. 2017;23:866‐872. doi:10.1177/1357633X17733020 [DOI] [PubMed] [Google Scholar]
- 27. van den Brink JL, Moorman PW, de Boer MF, et al. Impact on quality of life of a telemedicine system supporting head and neck cancer patients: a controlled trial during the postoperative period at home. J Am Med Inform Assoc. 2007;14:198‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfeifer MP, Keeney C, Bumpous J, et al. Impact of a telehealth intervention on quality of life and symptom distress in patients with head and neck cancer. J Community Support Oncol. 2015;13:14‐21. doi:10.12788/jcso.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Head BA, Keeney C, Studts JL, et al. Feasibility and acceptance of a telehealth intervention to promote symptom management during treatment for head and neck cancer. J Support Oncol. 2011;9:e1‐e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stalfors J, Estrom S, Bjork‐Erikkson T, et al. Accuracy of tele‐oncology compared with face‐to‐face consultation in head and neck cancer case conferences. J Telemed Telecare. 2001;7:338‐343. [DOI] [PubMed] [Google Scholar]
- 31. Beswick DM, Vashi A, Song Y, et al. Consultation via telemedicine and access to operative care for patients with head and neck cancer in a Veterans Health Administration population. Head Neck. 2016;38:925‐929. doi:10.1002/hed.24386 [DOI] [PubMed] [Google Scholar]
- 32. Thomson DJ, Palma D, Guckenberger M, et al. Practice recommendations for risk‐adapted head and neck cancer radiotherapy during the COVID‐19 pandemic: an ASTRO‐ESTRO consensus statement. Int J Radiat Oncol Biol Phys. Published online April 14, 2020. doi:10.1016/j.ijrobp.2020.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Owosho AA, Tsai CJ, Lee RS, et al. The prevalence and risk factors associated with osteoradionecrosis of the jaw in oral and oropharyngeal cancer patients treated with intensity‐modulated radiation therapy (IMRT): the Memorial Sloan Kettering Cancer Center experience. Oral Oncol. 2017;64:44‐51. doi:10.1016/j.oraloncology.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang DT, Sandow PR, Morris CG, et al. Do pre‐irradiation dental extractions reduce the risk of osteoradionecrosis of the mandible? Head Neck. 2007;29:528‐536. [DOI] [PubMed] [Google Scholar]
- 35. Fan M, Kang JJ, Lee A, et al. Outcomes and toxicities of definitive radiotherapy and reirradiation using 3‐dimensional conformal or intensity‐modulated (pencil beam) proton therapy for patients with nasal cavity and paranasal sinus malignancies. Cancer. 2020;126:1905‐1916. doi:10.1002/cncr.32776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inoue T, Inoue T, Teshima T, et al. Phase III trial of high and low dose rate interstitial radiotherapy for early oral tongue cancer. Int J Radiat Oncol Biol Phys. 1996;36:1201‐1204. doi:10.1016/s0360‐3016(96)00420‐8 [DOI] [PubMed] [Google Scholar]
- 37. Licitra L, Grandi C, Guzzo M, et al. Primary chemotherapy in resectable oral cavity squamous cell cancer: a randomized controlled trial. J Clin Oncol. 2003;21:327‐333. doi:10.1200/JCO.2003.06.146 [DOI] [PubMed] [Google Scholar]
- 38. Bossi P, Lo Vullo S, Guzzo M, et al. Preoperative chemotherapy in advanced resectable OCSCC: long‐term results of a randomized phase III trial. Ann Oncol. 2014;25:462‐466. [DOI] [PubMed] [Google Scholar]
- 39. Sher DJ, Thotakura V, Balboni TA, et al. Treatment of oral cavity squamous cell carcinoma with adjuvant or definitive intensity‐modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2011;81:e215‐e222. doi:10.1016/j.ijrobp.2011.02.023 [DOI] [PubMed] [Google Scholar]
- 40. Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low‐risk human papillomavirus–positive oropharyngeal cancer (De‐ESCALaTE HPV): an open‐label randomised controlled phase 3 trial. Lancet. 2019;393:51‐60. doi:10.1016/S0140‐6736(18)32752‐1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Caudell JJ. Long‐term update of NRG Oncology RTOG 0522: a randomized phase III trial of concurrent radiation and cisplatin with or without cetuximab in locoregionally advanced head and neck cancer. Talk presented at: Multidisciplinary Head and Neck Cancers Symposium; February 27‐29, 2020; Scottsdale, AZ. [Google Scholar]
- 42. Ward MC, Riaz N, Caudell JJ, et al. Refining patient selection for reirradiation of head and neck squamous carcinoma in the IMRT era: a multi‐institution cohort study by the MIRI collaborative. Int J Radiat Oncol Biol Phys. 2018;100:586‐594. doi:10.1016/j.ijrobp.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Caudell JJ, Ward MC, Riaz N, et al. Volume, dose, and fractionation considerations for IMRT‐based reirradiation in head and neck cancer: a multi‐institution analysis. Int J Radiat Oncol Biol Phys. 2018;100:606‐617. doi:10.1016/j.ijrobp.2017.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Riaz N, Hong JC, Sherman EJ, et al. A nomogram to predict loco‐regional control after re‐irradiation for head and neck cancer. Radiother Oncol. 2014;111:382‐387. doi:10.1016/j.radonc.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fan D, Kang JJ, Fan M, et al. Last‐line local treatment with the Quad Shot regimen for previously irradiated head and neck cancers. Oral Oncol. 2020;104:104641. doi:10.1016/j.oraloncology.2020.104641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Corry J, Peters LJ, Costa ID, et al. The ‘QUAD SHOT’—a phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother Oncol. 2005;77:137‐142. doi:10.1016/j.radonc.2005.10.008 [DOI] [PubMed] [Google Scholar]
- 47. Porceddu SV, Rosser B, Burmeister BH, et al. Hypofractionated radiotherapy for the palliation of advanced head and neck cancer in patients unsuitable for curative treatment—"Hypo Trial". Radiother Oncol. 2007;85:456‐462. doi:10.1016/j.radonc.2007.10.020 [DOI] [PubMed] [Google Scholar]
- 48. Chen AM, Vaughan A, Narayan S, Vijayakumar S. Palliative radiation therapy for head and neck cancer: toward an optimal fractionation scheme. Head Neck. 2008;30:1586‐1591. doi:10.1002/hed.20894 [DOI] [PubMed] [Google Scholar]
- 49. Gowda RV, Henk JM, Mais KL, et al. Three weeks radiotherapy for T1 glottic cancer: the Christie and Royal Marsden Hospital experience. Radiother Oncol. 2003;68:105‐111. doi:10.1016/S0167‐8140(03)00059‐8 [DOI] [PubMed] [Google Scholar]
- 50. Warde P, O’Sullivan B, Bristow RG, et al. T1/T2 glottic cancer managed by external beam radiotherapy: the influence of pretreatment hemoglobin on local control. Int J Radiat Oncol Biol Phys. 1998;41:347‐353. doi:10.1016/s0360‐3016(98)00062‐5 [DOI] [PubMed] [Google Scholar]
- 51. Ermis E, Teo M, Dyker KE, Fosker C, Sen M, Prestwich RJ. Definitive hypofractionated radiotherapy for early glottic carcinoma: experience of 55Gy in 20 fractions. Radiat Oncol. 2015;10:203. doi:10.1186/s13014‐015‐0505‐6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eisbruch A, Harris J, Garden AS, et al. Multi‐institutional trial of accelerated hypofractionated intensity‐modulated radiation therapy for early‐stage oropharyngeal cancer (RTOG 00‐22). Int J Radiat Oncol Biol Phys. 2010;76:1333‐1338. doi:10.1016/j.ijrobp.2009.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yom SS, Torres‐Saavedra P, Caudell JJ, et al. NRG‐HN002: a randomized phase II trial for patients with p16‐positive, non–smoking‐associated, locoregionally advanced oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2019;105:684‐685. [Google Scholar]
- 54. Mehanna H, Wong WL, McConkey CC, et al. PET‐CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med. 2016;374:1444‐1454. doi:10.1056/NEJMoa1514493 [DOI] [PubMed] [Google Scholar]
- 55. Cummings B, Keane T, Pintilie M, et al. Five year results of a randomized trial comparing hyperfractionated to conventional radiotherapy over four weeks in locally advanced head and neck cancer. Radiother Oncol. 2007;85:7‐16. doi:10.1016/j.radonc.2007.09.010 [DOI] [PubMed] [Google Scholar]
- 56. Robertson C, Robertson AG, Hendry JH, et al. Similar decreases in local tumor control are calculated for treatment protraction and for interruptions in the radiotherapy of carcinoma of the larynx in four centers. Int J Radiat Oncol Biol Phys. 1998;40:319‐329. [DOI] [PubMed] [Google Scholar]
- 57. James ND, Williams MV, Summers ET, et al. The management of interruptions to radiotherapy in head and neck cancer: an audit of the effectiveness of national guidelines. Clin Oncol (R Coll Radiol). 2008;20:599‐605. [DOI] [PubMed] [Google Scholar]
- 58. Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus–positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non‐inferiority trial [published correction appears in Lancet. 2020;395:784]. Lancet. 2019;393:40‐50. doi:10.1016/S0140‐6736(18)32779‐X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nutting CM, Morden JP, Harrington KJ, et al. Parotid‐sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127‐136. doi:10.1016/S1470‐2045(10)70290‐4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chan AK, Sanghera P, Choo BA, et al. Hypofractionated accelerated radiotherapy with concurrent carboplatin for locally advanced squamous cell carcinoma of the head and neck. Clin Oncol (R Coll Radiol). 2011;23:34‐39. [DOI] [PubMed] [Google Scholar]
- 61. Bakst RL, Lee N, Pfister DG, et al. Hypofractionated dose‐painting intensity modulated radiation therapy with chemotherapy for nasopharyngeal carcinoma: a prospective trial. Int J Radiat Oncol Biol Phys. 2011;80:148‐153. doi:10.1016/j.ijrobp.2010.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. You B, Ravaud A, Canivet A, et al. The official French guidelines to protect patients with cancer against SARS‐CoV‐2 infection. Lancet Oncol. 2020;21:619‐621. doi:10.1016/S1470‐2045(20)30204‐7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yamazaki H, Nishiyama K, Tanaka E, et al. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64:77‐82. [DOI] [PubMed] [Google Scholar]
- 64. Achard V, Tsoutsou P, Zilli T. Radiotherapy in the time of the coronavirus pandemic: when less is better. Int J Radiat Oncol Biol Phys. Published online March 18, 2020. doi:10.1016/j.ijrobp.2020.03.008 [Google Scholar]
- 65. Lee N, Harris J, Garden AS, et al. Intensity‐modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: Radiation Therapy Oncology Group phase II trial 0225. J Clin Oncol. 2009;27:3684‐3690. doi:10.1200/JCO.2008.19.9109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cortiula F, Pettke A, Bartoletti M, Puglisi F, Helleday T. Managing COVID‐19 in the oncology clinic and avoiding the distraction effect. Ann Oncol. 2020;31:553‐555. doi:10.1016/j.annonc.2020.03.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Filippi AR, Russi E, Magrini SM, Corvo R. Letter from Italy: first practical indications for radiation therapy departments during COVID‐19 outbreak. Int J Radiat Oncol Biol Phys. Published online March 19, 2020. doi:10.1016/j.ijrobp.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen YL, Hsu FM, Tsai CJ, Cheng JCH. Efforts to reduce the impacts of COVID‐19 outbreak on radiation oncology in Taiwan. Adv Radiat Oncol. Published online April 6, 2020. doi:10.1016/j.adro.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen W, Su XY, Wang VJ, et al. Novel coronavirus international public health emergency: guidance on radiation oncology facility operation. Adv Radiat Oncol. Published online April 1, 2020. doi:10.1016/j.adro.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chan JYK, Wong EWY, Lam W. Practical aspects of otolaryngologic clinical services during the 2019 novel coronavirus epidemic an experience in Hong Kong. JAMA Otolaryngol Head Neck Surg. Published online March 20, 2020. doi:10.1001/jamaoto.2020.0488 [DOI] [PubMed] [Google Scholar]
- 71. Krengli M, Ferrara E, Mastroleo F, Brambilla M, Ricardi U. Running a radiation oncology department at the time of coronavirus: an Italian experience. Adv Radiat Oncol. Published March 20, 2020. doi:10.1016/j.adro.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gay HA, Santiago R, Gil B, et al. Lessons learned from Hurricane Maria in Puerto Rico: practical measures to mitigate the impact of a catastrophic natural disaster on radiation oncology patients. Pract Radiat Oncol. 2019;9:305‐321. [DOI] [PubMed] [Google Scholar]
- 73. Halpern MT, Yabroff KR. Prevalence of outpatient cancer treatment in the United States: estimates from the Medical Panel Expenditures Survey (MEPS). Cancer Invest. 2008;26:647‐651. [DOI] [PubMed] [Google Scholar]
- 74. Masroor F, Corpman D, Carpenter DM, et al. Association of NCCN‐recommended posttreatment surveillance with outcomes in patients with HPV‐associated oropharyngeal squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2019;145:903‐908. doi:10.1001/jamaoto.2019.1934 [DOI] [PMC free article] [PubMed] [Google Scholar]


