Summary
We reported 20 cases of discharged COVID‐19 patients whose RT‐PCR test results showed ‘re‐positive’. After finding ‘re‐positive’, these patients were admitted to hospital for the second time and were followed up until the end of May 2020. We recorded detailed treatment and follow‐up process, and collected relevant data. The possible causes and potential clinical significance of this phenomenon are discussed.
Introduction
Since December 2019, the outbreak of coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has gradually become a major health concern throughout the world (Tang et al., 2020). China has taken a variety of measures to control interpersonal transmission, including isolating and treating patients with COVID‐19, closely tracking and isolating close contacts, restricting people to their homes, and so on (Yanga et al., 2020). After continuous efforts in various fields, the domestic outbreak has been well controlled. Most patients have been cured and discharged, and many provinces have not had new confirmed cases in months. However, some COVID‐19‐cured patients were found to be ‘re‐positive’ in the nucleic acid test after discharge from the hospital. This phenomenon is currently receiving much attention.
Material and method
SARS‐CoV‐2 viral ORF1ab gene and N gene fragment were detected by using a real‐time reverse transcription PCR kit (DAAN GENE, Guangzhou, China) according to the manufacturer's instructions. Briefly, oropharyngeal swab was taken from the patient. Viral RNA was extracted from the swab by using a viral RNA extraction kit (TIANLONG Scientific Company, Xian, China). Five microliters of extracted RNA was added to a 20‐μl reaction mixture of real‐time RT‐PCR. Reactions were incubated at 50°C for 15 min and 95°C for 15 min, followed by 45 cycles at 94°C for 15 s and 55°C for 45 s, and then subjected to melting curve analysis. Cycle threshold value less than 40 of both the ORF1ab gene and the N gene fragment was considered as positive.
All COVID‐19 patients who were discharged from hospitals in Guizhou, China had been followed until May 22, 2020, to assess whether the disease has recurred, and to record the treatment strategy and patient outcome after relapse. Discharge must meet all of the following criteria (China National Health Commission, 2020): (i) The temperature is normal and lasts for more than 3 days, (ii) respiratory symptoms are significantly alleviated, (iii) computed tomography (CT) images present a substantial improvement in acute exudative lesions, and (iv) the results of reverse transcription‐polymerase chain reaction (RT‐PCR) for detecting viral RNA in respiratory specimens are negative for two consecutive times (sampling interval of at least 24 h). The electronic medical record will record the patient's demographic information, laboratory findings and radiological images. At least 28 days after discharge from the hospital, patients were placed in designated locations for centralized isolation and health monitoring. During this period, if the patient's SARS‐CoV‐2 becomes positive again, the discharged patient will be admitted to the hospital again.
Results
Of a total of 147 patients, 20 convalescent cases (13.61%) had viral RNA ‘re‐positive’ in respiratory specimens 7–47 days after discharge. The demographic and clinical data of these patients, such as clinical types, comorbidities, therapeutic drugs, and so on are shown in Table 1. The average age of the 20 patients is 37.2 years, ranging from 4 to 80 years, including 12 males. Seven of these patients had comorbidities, of which hypertension was the most common comorbidity, followed by diabetes. Of these, 14 cases had a travel history to Wuhan or direct contact with patients from Wuhan. There were two cases of critically ill type, three cases of a severe type, 12 cases of moderate type and three cases of the mild type according to the guideline of the National Health Commission. The average time from illness onset to admission was 2.5 days (0–7 days). During the first hospitalization, most patients received a combination of lopinavir/ritonavir and interferon. The average length of the first hospitalization was 18.65 days. After discharge, the patients with COVID‐19 were placed in designated locations for centralized isolation and health monitoring. The average time from the discharge to pharyngeal swabs RT‐PCR ‘re‐positive’ results was 17.25 days, ranging from 7 to 47 days. Although the viral RNA was detected as ‘positive again’, these 20 patients were still asymptomatic, with no reduction in leukocytes or lymphocytes. Compared with chest CT images at the first discharge, no progressive lesions were observed. Despite this, they were re‐admitted to the hospital, mainly receiving chloroquine phosphate, thymalfasin and traditional Chinese medicine (TCM). The average length of re‐hospitalization was 16.1 days. Patients were still scheduled to undergo centralized isolation and health monitoring at least for another 28 days at the designated location after the second discharge. The detailed schedule of PCR and antibody testing for these relapsed patients is shown in Fig. 1. For cases 2, 5, 6, 7, 13, 14, 15 and 19, the phenomenon of alternating PCR positivity and negativity had been observed. Routine blood tests showed no significant abnormalities in leukocyte, lymphocyte and T lymphocyte subsets. Chest CT images showed no active lesions in all patients. Serum antibodies of SARS‐CoV‐2 immunoglobulin (Ig) IgG and IgM tested positive in 19 patients. For case 15, two serum antibody tests were still negative at 63 days after onset.
Table 1.
The demographic and clinical data of 20 patients.
| Case No. | Sex | Age (years) | Contact or return from Wuhan | Clinical type | Comorbidity | Drugs in the whole course of the disease |
|---|---|---|---|---|---|---|
| Case 1 | F | 21 | Yes | Ordinary | No | Lopinavir/ritonavir, interferon, chloroquine phosphate, Arbidol, thymalfasin and TCM |
| Case 2 | M | 47 | Yes | Critical ill | Hypertension, DM | Lopinavir/ritonavir, interferon, chloroquine phosphate and thymalfasin |
| Case 3 | F | 4 | Yes | Ordinary | No | Interferon, Arbidol and TCM |
| Case 4 | F | 45 | Yes | Mild | No | Lopinavir/ritonavir, interferon, chloroquine phosphate, thymalfasin and TCM |
| Case 5 | M | 37 | No | Ordinary | No | Lopinavir/ritonavir, interferon, chloroquine phosphate, Arbidol, thymalfasin and TCM |
| Case 6 | F | 56 | Yes | Ordinary | No | Lopinavir/ritonavir, interferon, chloroquine phosphate, Arbidol and TCM |
| Case 7 | M | 80 | Yes | Critical ill | CHD | Lopinavir/ritonavir, interferon, Arbidol, thymalfasin, convalescent plasma and TCM |
| Case 8 | M | 5 | No | Mild | No | Interferon and TCM |
| Case 9 | M | 40 | Yes | Ordinary | No | Lopinavir/ritonavir, interferon, chloroquine phosphate and thymalfasin |
| Case 10 | M | 24 | Yes | Ordinary | No | Lopinavir/ritonavir, interferon, chloroquine phosphate, Arbidol, thymalfasin and TCM |
| Case 11 | F | 16 | No | Ordinary | No | Lopinavir/ritonavir, interferon, chloroquine phosphate, Arbidol, ribavirin and TCM |
| Case 12 | M | 35 | Yes | Ordinary | Hypertension | Lopinavir/ritonavir, interferon, Arbidol, thymalfasin and TCM |
| Case 13 | F | 35 | Yes | Ordinary | No | Lopinavir/ritonavir, interferon, chloroquine phosphate, thymalfasin and TCM |
| Case 14 | M | 37 | Yes | Severe | Gout | Lopinavir/ritonavir, interferon, chloroquine phosphate, Arbidol, thymalfasin and TCM |
| Case 15 | F | 31 | No | Mild | No | Lopinavir/ritonavir, interferon, Arbidol and TCM |
| Case 16 | M | 28 | No | Ordinary | No | Lopinavir/ritonavir, interferon, Arbidol, thymalfasin and TCM |
| Case 17 | F | 58 | Yes | Severe | Hypertension | Lopinavir/ritonavir, interferon, chloroquine phosphate, thymalfasin and TCM |
| Case 18 | M | 42 | Yes | Ordinary | Hypertension | Lopinavir/ritonavir, interferon, chloroquine phosphate and thymalfasin |
| Case 19 | F | 53 | Yes | Severe | Hypertension, DM | Lopinavir/ritonavir, interferon, Arbidol, chloroquine phosphate and TCM |
| Case 20 | M | 49 | Yes | Ordinary | No | Lopinavir/ritonavir, interferon, Arbidol, thymalfasin and TCM |
Abbreviations: F, female; M, male; TCM, Traditional Chinese medicine; DM, diabetes mellitus; CHD, coronary heart disease.
Fig 1.
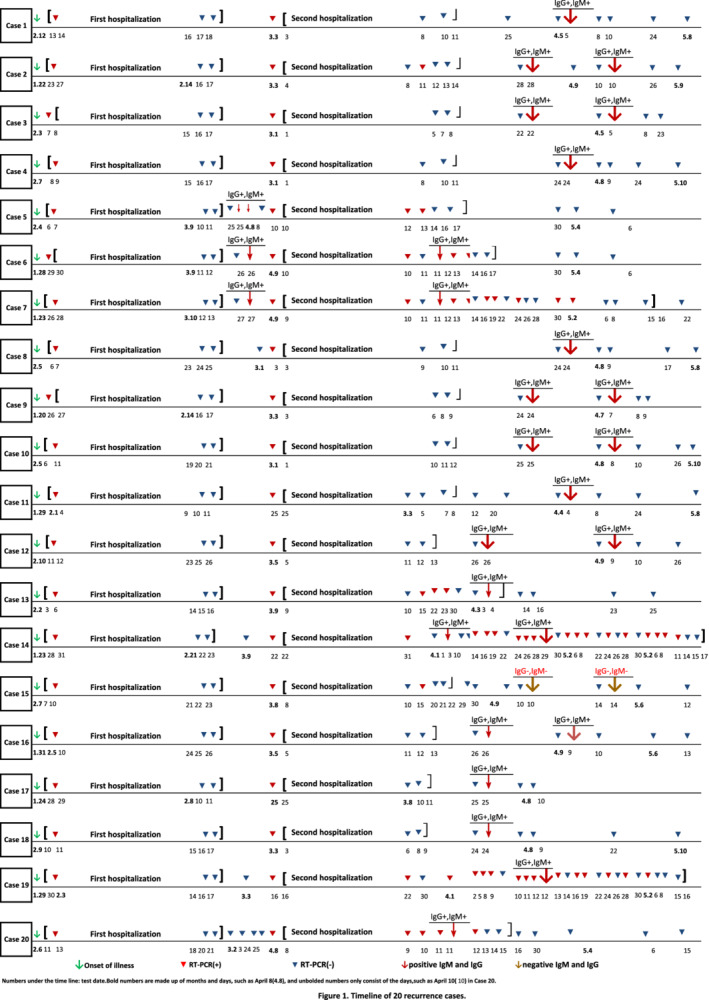
Timeline of 20 recurrence cases. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
In our report, the incidence of ‘re‐positive’ RT‐PCR test results of COVID‐19 cured patients was 13.61%, which was close to the reported incidence (14%) of a hospital in Guangdong Province, China. What causes this phenomenon? Some scholars believe that the reasons might include the following aspects (Lan et al., 2020; Ling et al., 2020a). First, the virus severely damages the immune function of patients, especially when accompanied by comorbidities in patients, so the virus was not completely cleared. Indeed, the proportion of severe cases among our relapsed patients is higher than the national average (25% vs 14%), and all five severe cases had comorbidities (Wu & McGoogan, 2020). Interestingly, intermittent and recurrent viral shedding was found in four of the five patients. Second, the effect of previous antiviral drugs might be limited, so virus replication was not completely inhibited. Zhou and colleagues (2020) did not observe a reduction in the viral shedding duration after lopinavir/ritonavir treatment in their study. Young and colleagues (2020) also observed that a decrease in viral load indicated by the cycle threshold value from nasopharyngeal swabs appeared similar between the lopinavir‐ritonavir treatment group and the control group. These patients received chloroquine phosphate, thymalfasin and TCM during the second hospitalization. The average length of re‐hospitalization was 16.1 days, which was shorter than the first admission. As of May 22, no patient had been found to turn positive again for SARS‐CoV‐2. Although controversial, chloroquine was found to be effective in preventing the replication of this virus in vitro (Wang et al., 2020). Thymalfasin and TCM are recognized to enhance the body's immunity and resistance, thus helping to clean up the virus. Third, the nucleic acid clearance rate of the virus could be affected by glucocorticoid therapy. Ling and colleagues (2020b) found that the duration of viral RNA detection for oropharyngeal swabs and faeces in the corticosteroid treatment group was longer than that in the non‐corticosteroid treatment group, which were 15 days vs. 8 days and 20 days vs. 11 days respectively. It was unclear whether our patients used glucocorticoids or the exact doses, so the effect of this drug on the prognosis was uncertain here. Fourth, the duration of SARS‐CoV‐2 RNA detection has not been well characterized, and its duration may be longer than that of MERS‐CoV RNA and SARS‐CoV RNA (Xu et al., 2005; Corman et al., 2016; Oh et al., 2016). The duration of viral shedding of nasopharyngeal aspirates was extended by at least 24 days after the onset of symptoms in a study in Singapore (Young et al., 2020). Zhou and colleagues (2020) found that the longest observed duration of viral shedding among survivors was 37 days. Some of our ‘relapsed’ patients, such as cases 1, 4, 8, 10, 11, 12 and 18, might have been discharged shortly after the first treatment, so their natural course of viral shedding had not been observed completely eliminated. Fifth, the criteria of hospital discharge may be too loose so that patients were discharged without being cured. In order to avoid the ‘re‐positive’ phenomenon, virus antibody detection or testing of anal swabs had been required before discharge in some provinces of China. However, viral RNA was still detected after both SARS‐CoV‐2‐specific IgM and IgG antibodies turned positive in five patients (cases 6, 7, 14, 19 and 20). Even more bizarrely, two serum antibodies were still negative 63 days after the illness onset in case 15, but her viral RNA had been undetectable for a period of time. Sixth, the natural recombination of RNA viruses is an element of the natural evolution of coronaviruses, and the relapse and delay of the disease may be just the characteristic manifestations of this new virus. SARS‐CoV‐2 might be able to coexist with humans through continuous recombination so that a proportion of recovered patients may become virus carriers to eliminate virus intermittently. For cases 5, 6, 7, 8, 14, 19 and especially 20, after more than two times of negative nucleic acid test results, ‘re‐positive’ phenomenon was observed again. Although we have the longest observation time for the ‘relapse’ patients so far, it still needs a longer time to determine whether this phenomenon exists. Seventh, false‐negative RT‐PCR test results could have been produced due to instabilities in sample collecting, processing and testing. In a patient in our hospital, three consecutive pharyngeal swabs tests were negative for SARS‐CoV‐2 RNA, but RT‐PCR for alveolar lavage fluid was positive. As we all know, pharyngeal swab sampling robots have been developed and have entered clinical trials, which is expected to improve the quality of sample collection. Wei Zhang et al. found that the SARS‐CoV‐2 RNA positive rate of oropharyngeal swabs has limitations, and it is recommended to use serological testing to increase the positive detection rate of patients with COVID‐19 (Zhang et al., 2020). Last but not least, does ‘re‐positive’ RT‐PCR test result mean re‐infection? The patients in our report were asked to continue centralized isolation management and health monitoring in a secondary hospital for at least 28 days after discharge. Therefore, they did not come into contact with any person with respiratory symptoms. Generally, the human body will produce corresponding antibodies after been infected with the virus, and these antibodies could play a protective role to avoid re‐infecting the virus within a period of time after curing. Therefore, it is believed that the ‘re‐positive’ phenomenon is caused by the original virus residue, not by reinfection.
Do patients with ‘re‐positive’ RT‐PCR test results have to receive antiviral therapy again? Similar to cases reported by Lan and colleagues (2020), these 20 patients continued to be asymptomatic and no abnormal routine blood tests or progressive chest CT findings after tested ‘re‐positive’ of viral RNA. Given that the current efficacy of antiviral drugs, drug‐related side effects, and the state of well‐being of patients are uncertain, we believe that enhancing the body's resistance and waiting for observation may be the best treatment for the ‘re‐positive’ patients, although chloroquine phosphate was used in some of our patients during the second hospitalization. However, for relapsed patients, active medical therapy should be performed.
Whether such ‘re‐positive’ patients can easily become a potential source of infection is a key question. Although there are no reports that they transmitted the virus to others, from a safety point of view, these discharged patients should be required to be isolated and monitored for more than 28 days. Although there have been some cases or case series which reported recurrence of positive SARS‐CoV‐2 RNA in COVID‐19 (Chen et al., 2020; Jiang et al., 2020; Li et al., 2020; Peng et al., 2020; Xing et al., 2020; Ye et al., 2020), to the best of our knowledge, this is the first observational study of long‐term follow‐up of so many ‘re‐positive’ patients, and we will continue to follow these patients.
References
- Chen, D. , Xu, W. , Lei, Z. , Huang, Z. , Liu, J. , Gao, Z. , and Peng, L. (2020) Recurrence of positive SARS‐CoV‐2 RNA in COVID‐19: a case report. Int J Infect Dis 93: 297–299. 10.1016/j.ijid.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China National Health Commission . (2020) Diagnosis and treatment of 2019‐nCoV pneumonia in China. In Chinese. Accessed January 27, February 5,and February 19.
- Corman, V.M. , Albarrak, A.M. , Omrani, A.S. , Albarrak, M.M. , Farah, M.E. , Almasri, M. , et al. (2016) Viral shedding and antibody response in 37 patients with Middle East respiratory syndrome coronavirus infection. Clin Infect Dis 62: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, M. , Li, Y. , Han, M. , Wang, Z. , Zhang, Y. , and du, X. (2020) Recurrent PCR positivity after hospital discharge of people with coronavirus disease 2019 (COVID‐19). J Infect 81: 147–178. 10.1016/j.jinf.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, L. , Xu, D. , Ye, G. , Xia, C. , Wang, S. , Li, Y. , and Xu, H. (2020) Positive RT‐PCR test results in patients recovered from COVID‐19. JAMA 323: 1502–1503. 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X.J. , Zhang, Z.W. , and Zong, Z.Y. (2020) A case of a readmitted patient who recovered from COVID‐19 in Chengdu, China. Crit Care 24: 152. 10.1186/s13054-020-02877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, Y. , Xu, S. , Lin, Y. , et al. (2020b) The persistence and clearance of viral RNA in 2019 novel coronavirus disease survivors. Chin Med J (Engl) 133: 1039–1043. 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling, Z. , Kui, L. , and Guo, L.H. (2020a) Cause analysis and treatment strategies of “recurrence” with novel coronavirus pneumonia (Covid‐19) patients after discharge from hospital. Chin J Tuberc Respir Dis 43: 281–284. 10.3760/cma.j.cn112147-20200229-00219. [DOI] [PubMed] [Google Scholar]
- Oh, M.D. , Park, W.B. , Choe, P.G. , Choi, S.J. , Kim, J.I. , Chae, J. , et al. (2016) Viral load kinetics of MERS coronavirus infection. N Engl J Med 375: 1303–1305. [DOI] [PubMed] [Google Scholar]
- Peng, J. , Wang, M. , Zhang, G. , and Lu, E. (2020) Seven discharged patients turning positive again for SARS‐CoV‐2 on quantitative RT‐PCR. Am J Infect Control 48: 725–726. 10.1016/j.ajic.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, J.W. , Tambyah, P.A. , and Hui, D.S. (2020) Emergence of a novel coronavirus causing respiratory illness from Wuhan, China. J Infect 80: 350–371. 10.1016/j.jinf.2020.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Cao, R. , Zhang, L. , Yang, X. , Liu, J. , Xu, M. , et al. (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res 30: 269–271. 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. , and McGoogan, J.M. (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323: 1239–1242. 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Xing, Y. , Mo, P. , Xiao, Y. , Zhao, O. , Zhang, Y. , and Wang, F. (2020) Post‐discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID‐19), China, January to February 2020. Euro Surveill 25: 2000191. 10.2807/1560-7917.ES.2020.25.10.2000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, D. , Zhang, Z. , Jin, L. , Chu, F. , Mao, Y. , Wang, H. , et al. (2005) Persistent shedding of viable SARS‐CoV in urine and stool of SARS patients during the convalescent phase. Eur J Clin Microbiol Infect Dis 24: 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanga, Y. , Peng, F. , Wang, R. , Yange, M. , Guan, K. , and Jiang, T. (2020) The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun 109: 102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, G. , Pan, Z. , Pan, Y. , Deng, Q. , Chen, L. , Li, J. , et al. (2020) Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J Infect 80: e14–e17. 10.1016/j.jinf.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, B.E. , Ong, S.W.X. , Kalimuddin, S. , Low, J.G. , Tan, S.Y. , Loh, J. , et al. (2020) Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA 23: 1488–1494. 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Du, R.‐H. , Li, B. , Zheng, X.‐S. , Yang, X.‐L. , Hu, B. , et al. (2020) Molecular and serological investigation of 2019‐nCoV infected patients:implication of multiple shedding routes. Emerging Microbes Infect 9: 386–389. 10.1080/22221751.2020.1729071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F. , Yu, T. , Du, R. , Fan, G. , Liu, Y. , Liu, Z. , et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]


