Key Points.
A growing body of evidence from the United Kingdom (UK), Europe, and the United States of America (USA) suggests that a number of paediatric patients could present with Kawasaki‐like symptoms such as fever, rash and shock with concomitant COVID‐19 infection which has been referred to multisystem inflammatory syndrome in children (MIS‐C).
The negative results of polymerase chain reaction (PCR) test for COVID‐19 in a patient with high levels of serum IgG could suggest that the virus had been cleared and the presence of Kawasaki‐like manifestations may be due to delayed immune‐mediated phenomenon caused by COVID‐19.
Despite the growing number of MIS‐C cases around the world, many questions remain unanswered about the underlying pathophysiology of hyperinflammatory shock related to COVID‐19 as well as treatment modalities, epidemiological and clinical characteristics associated with MIS‐C.
The novel coronavirus (SARS‐CoV‐2) that is responsible for coronavirus disease (COVID‐19) has emerged as a global crisis. 1 According to the Johns Hopkins University information site, as of 4 June 2020, over 6.29 million cases have been confirmed, and about 380 000 deaths due to COVID‐19 have been reported world‐wide. The severity of the disease has been reported to range from mild to severe and eventually death. 1 Paediatric populations seem to comprise only a small proportion of total affected individuals and are less likely than adults to be severely affected by COVID‐19. According to a recent epidemiological study, 731 cases of COVID‐19 were confirmed in children, of whom more than 90% of patients were reported as asymptomatic or with mild to moderate symptoms. 2 The clinical presentation of paediatric patients may differ from those of the adults and can range from asymptomatic to acute upper respiratory tract infection, gastrointestinal symptoms with shock or coagulation dysfunction in severe cases.
To date, the exact pathophysiology of COVID‐19 has not been fully understood. However, emerging evidence suggests that a vascular disease process may be a contributing factor in COVID‐19 pathogenesis. 3 These findings indicate that direct viral‐mediated tissue damage and endothelial dysfunction in the setting of an inflammatory state could be responsible for various adverse outcomes.
A growing body of evidence from the UK, Europe and the USA suggests that a number of paediatric patients could present with fever, rash and shock with concomitant COVID‐19 infection. Recently, the Royal College of Paediatrics and Child Health (UK) called this phenomenon paediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. 4 Similarly, the Centers for Disease Control and Prevention (USA) termed this new presentation multisystem inflammatory syndrome in children (MIS‐C). The exact underlying mechanisms for this Kawasaki disease (KD)‐like condition described in these reports are not clear; but may be due to antibody or immune‐complex mediated effects in the setting of a post‐infectious delayed inflammatory process.
Herein, we describe an Iranian paediatric case of a concurrent KD‐like inflammatory syndrome and COVID‐19 infection who presented with shock.
Case Report
The patient was a 5‐year‐old girl who presented to paediatric urgent care with 5 days of high‐grade fever, vomiting, diarrhoea and abdominal pain, which had been managed as an outpatient as a viral infection. On the third day, she developed conjunctivitis, blotchy rash and swelling of the hands. In the patient's history, she had experienced upper respiratory symptoms over the past 3 weeks that had improved with supportive care. At the initial clinical examination, the patient was ill and had generalised erythematous skin rash, non‐purulent bilateral conjunctivitis, periorbital oedema and erythema, swelling and congestion of the lips, mild swelling of the hands and moderate dehydration due to vomiting and diarrhoea (Fig. 1). There was no evidence of lymphadenopathy. Vital signs showed a temperature of 39.5°C, sinus tachycardia (165 beats/min), tachypnoea with normal breath sounds and an oxygen saturation of 98% and the blood pressure was 75/55. Laboratory tests revealed lymphopaenia, thrombocytopaenia, mildly elevated C‐reactive protein (CRP) of 23 mg/L and erythrocyte sedimentation rate of 40 mm/h. She had hyponatraemia (Na: 120 mEq/L) and elevated liver function tests (Table 1). About 4 weeks before she was admitted to our hospital, she had a positive history of exposure to her uncle who was diagnosed with COVID‐19. Owing to this history of exposure, reverse transcriptase‐polymerase chain reaction (RT‐PCR) and serology for SARS‐CoV‐2 were requested. The PCR was negative on two occasions; however, IgG for SARS‐CoV‐2 was positive (IgG: 10.3 times higher than reference standard; IgM: 0.2 times lower than reference standard; normal range was defined as lower than 1.1 ratio) suggesting previous SARS‐CoV‐2 infection.
Fig 1.
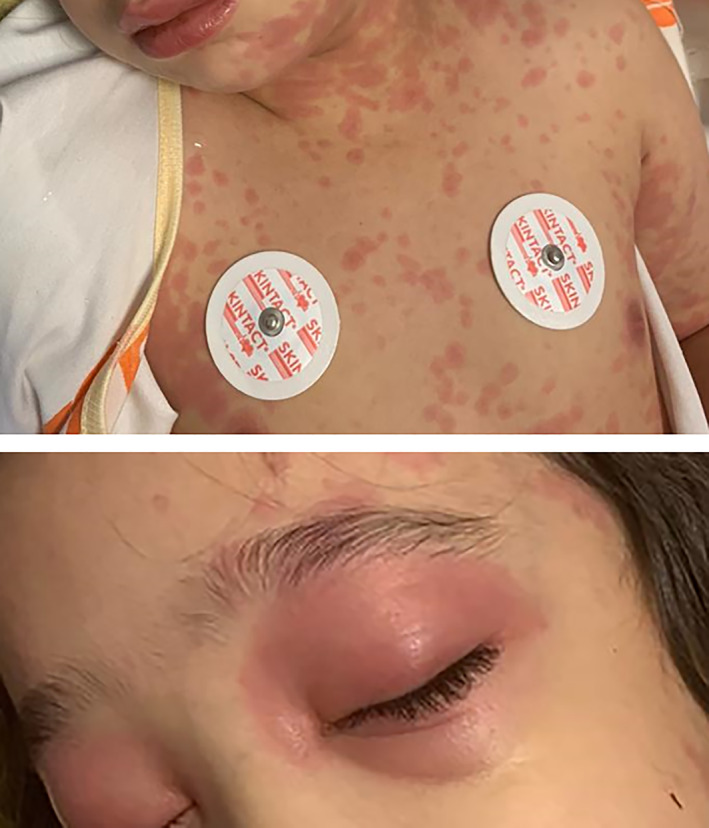
Skin rash, congestion of the lips and periorbital edema and erythema. Image shared with parental permission.
Table 1.
The laboratory data of the patient at baseline, during and after treatment
| Laboratory tests | Baseline† | During hospitalisation‡ | After treatment§ |
|---|---|---|---|
| WBC, ×103/mm3 | 5.2 (Neu: 73%, Lym: 22%, Band: 3%, EO: 2%) | 7.3 | 6.8 |
| Hb, g/dL | 11.5 | 9.7 | 8 |
| Platelet count, mg/dL | 93 | 187 | 294 |
| ESR, mm/h | 40 | 89 | 3 |
| CRP, mg/L | 23 | 103–220 | 6 |
| d‐Dimer, mg/L | 2.5 | 1.2 | 0.3 |
| Procalcitonine, ng/mL | NA | >100 | 1.4 |
| AST, U/L | 94 | 114 | NA |
| ALT, U/L | 111 | 76 | NA |
| BUN, mg/dL | 15 | 10.2 | NA |
| Cr, mg/dL | 0.8 | 0.21 | NA |
| Na, mEq/L | 120 | 127 | NA |
| K, mEq/L | 4.2 | 4.9 | NA |
| Ca, mg/dL | 8.6 | NA | NA |
Baseline laboratory values referred to outpatient results prior to admission.
Referred to 1 week after admission.
Referred to 1 week post‐discharge.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; Hb, haemoglobin; NA, not available; WBC, white blood cell.
Prompt treatment with fluid resuscitation was initiated due to the patient's dehydration and low blood pressure. After vital signs stabilised, the patient was evaluated for the possible diagnosis of KD. After establishing the diagnosis, standard treatment for KD was started according to guidelines. She was treated with a single dose of 2 g/kg intravenous immunoglobulin (IVIG) and high‐dose acetylsalicylic acid. The patient's fever subsided after the initiation of IVIG and she was without fever for about 24 h. However, she developed high‐grade fever again as well as hypotension the next day. In serial laboratory tests, a markedly elevated CRP level along with high procalcitonin and d‐dimer were observed (Table 1). There was evidence of shock and IVIG‐resistant KD. Inotropic management of the shock with epinephrine was started for the patient. According to advice from the infectious diseases team, broad‐spectrum antibiotics including meropenem, vancomycin and ciprofloxacin was also administered. After the shock resolved, a second dose of the IVIG was initiated.
There were no significant findings on chest X‐ray and the echocardiogram was normal with no evidence of coronary artery dilation. Our patient suffered from severe generalised abdominal pain. On abdominal ultrasound scan, a fluid accumulation measuring 33 × 34 × 14 mm was localised in the right lower quadrant and a volume of 9 mL was reported. After 48 h, the abdominal ultrasound scan was repeated in which a mild free fluid collection was noted in the right paracolic area and pelvis. During this time, the patient had orthostatic hypotension and lower limb mottling while standing which was due to the presence of hyperinflammatory shock (Fig. 2). The symptoms and fever were present for another 48 h. All of our patient's signs and symptoms began to improve on the third day of hospitalisation after starting the second dose of IVIG and antibiotics. Antibiotic treatment was continued for 1 week. The patient's blood culture and adenovirus‐specific PCR tests were negative. At the time of discharge, evidence of desquamation was observed on the patient's fingers. The patient was discharged with instructions to take low dose aspirin (3 mg/kg daily) and plans for repeating her follow‐up echocardiogram 1 week after discharge.
Fig 2.
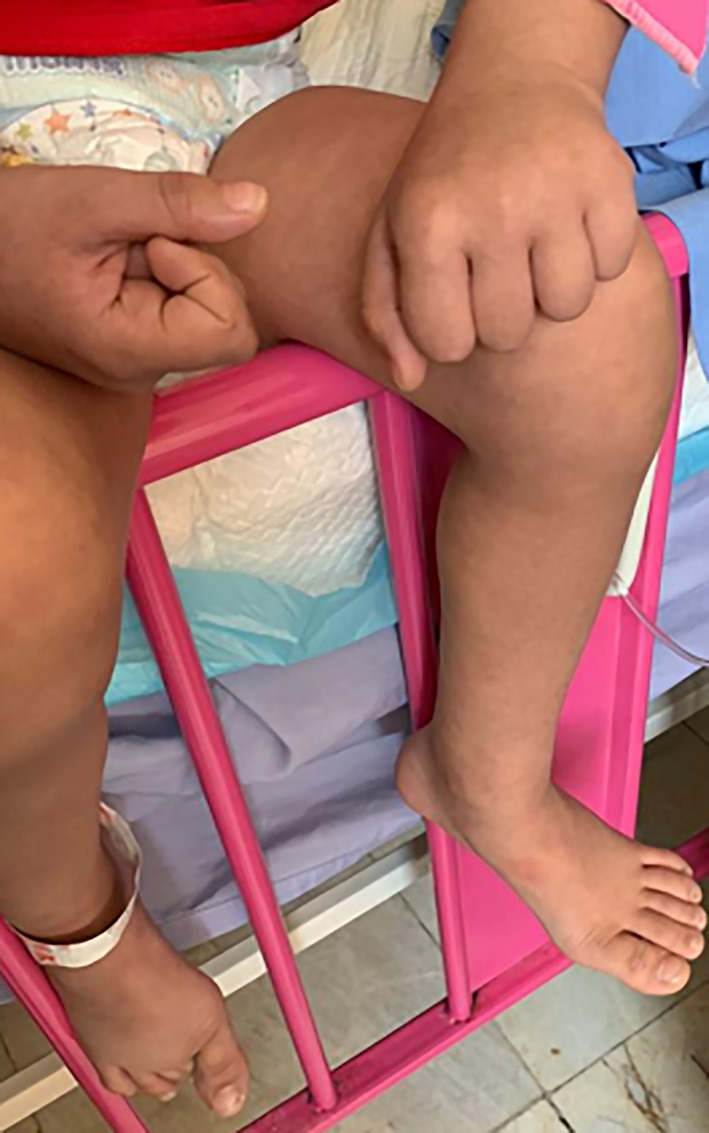
Lower limb mottling.
Discussion
Since the first reported KD‐like case of a 6‐month‐old female infant who was concurrently diagnosed with COVID‐19 in Stanford Children's Hospital in USA, a growing number of MIS‐C cases have been reported world‐wide. 5 Recently, an Italian cohort of KD‐like cases in Bergamo province, which experienced the highest rate of COVID‐19 infection and death in Italy, reported a sudden surge of new cases of KD with an increased incidence of nearly 30‐fold over the past 5 years in that region. 6 Although it has been hypothesised that a delayed immune response to the virus might be contributing to the clinical feature of MIS‐C, the exact link between COVID‐19 and MIS‐C remains unclear.
The role of respiratory viruses in the pathogenesis of KD is not fully understood. According to previous studies, the most common viruses detected in KD patients were rhinovirus and enterovirus. 7 Although most of the reported COVID‐19 cases among children experienced mild symptoms, the potential to progress to a more severe course such as with shock still exist and could lead to permanent organ damage and mortality. The observed negative results of PCR in our patient along with high levels of serum IgG could suggest that the virus had been cleared and the manifestations displayed in this patient may be due to delayed immune‐mediated phenomenon caused by COVID‐19. 8 However, limited data exist regarding the exact immunological mechanism of COVID‐19 in predisposing individuals to hyperinflammatory shock.
The patient presented in this study had severe symptoms consistent with MIS‐C and developed hyperinflammatory shock during the admission but had a good response to standard treatments. The high procalcitonin and CRP levels along with symptoms of shock in this patient suggest a serious and life‐threatening medical condition. With respect to her history of contact with COVID‐19, preceding upper respiratory tract symptoms, positive IgG for SARS‐CoV‐2 and negative blood culture; our patient's symptoms appear to be related to COVID‐19. As more cases of concurrent COVID‐19 and MIS‐C have been increasingly reported world‐wide, whether this relationship is causation or association is not yet fully elucidated. On the epidemiological aspect of the current pandemic, the timing between positive cases of COVID‐19 and identification of new cases of MIS‐C in various locations, suggest that this phenomenon may be partly contributed to by a delayed immune‐mediated process that is triggered by the SARS‐CoV‐2 infection. This observation is supported by the fact that the majority of reported cases were PCR negative for SARS‐CoV‐2, while serology testing has been found to be positive in many cases. 6 Nevertheless, it is worth noting that although vasculitis is known to be caused by coronavirus, it is too early to conclude that KD is due to coronavirus.
Despite the growing number of reported cases, many questions remain unanswered about the new coronavirus such as the treatment modalities, epidemiological, and clinical features associated with COVID‐19, especially in the paediatric population. Early recognition and management of this novel phenomenon is crucial in order to minimise potential complications of this disease in the paediatric population. Further studies are needed to increase our knowledge regarding MIS‐C related to COVID‐19 pathogenesis.
Written informed consent for publication of this patient's clinical details and clinical images was obtained from the parents.
Acknowledgement
We would like to thank both our patient and her family for letting us share their experience. Also, we thank all doctors and nurses who fought bravely during this pandemic.
Conflict of interest: None declared.
References
- 1. Wu F, Zhao S, Yu B et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579: 265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Tong S. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics 2020; e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 3. Leisman DE, Deutschman CS, Legrand M. Facing COVID‐19 in the ICU: Vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020; 28: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Royal College of Paediatrics and Child Health . Guidance—Paediatric Multisystem Inflammatory Syndrome Temporally Associated with COVID‐19. UK: Royal College of Paediatrics and Child Health (RCPCH); 2020. Available from: https://www.rcpch.ac.uk/resources/guidance‐paediatric‐multisystem‐inflammatory‐syndrome‐temporally‐associated‐covid‐19 [accessed 25 May 2020].
- 5. Jones VG, Mills M, Suarez D et al. COVID‐19 and Kawasaki disease: Novel virus and novel case. Hosp. Pediatr. 2020; 10: 537–40. [DOI] [PubMed] [Google Scholar]
- 6. Verdoni L, Mazza A, Gervasoni A et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: An observational cohort study. Lancet 2020; 395: 1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turnier JL, Anderson MS, Heizer HR, Jone PN, Glode MP, Dominguez SR. Concurrent respiratory viruses and Kawasaki disease. Pediatrics 2015; 136: e609–6. [DOI] [PubMed] [Google Scholar]
- 8. Giamarellos‐Bourboulis EJ, Netea MG, Rovina N et al. Complex immune dysregulation in COVID‐19 patients with severe respiratory failure. Cell Host Microbe 2020; 27: 992–1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]


