Abstract
Wuhan, a city of China, is the epicenter for the pandemic outbreak of coronavirus disease‐2019 (COVID‐19). It has become a severe public health challenge to the world and established a public health emergency of international worry. This infectious disease has pulled down the economy of almost all top developed nations. The coronaviruses (CoVs) known for various epidemics caused time to time. Infectious diseases such as severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS), followed by COVID‐19, are all coronaviruses led outbreaks that scourged the history of mankind. CoVs evolved themselves to more infectious, transmissible, and more pandemic with time. To prevent the spread of the SARS‐CoV‐2, many countries have ordered the complete lockdown to combat the outbreak. This paper briefly discussed the historical background of CoVs and the evolution of human coronaviruses (HCoVs), the case studies and the development of their antiviral medications. The viral infection encountered with present‐day challenges and futuristic approaches with the help of nanotechnology to minimize the spread of infectious viruses. The antiviral drugs and their clinical advances, along with herbal medicines for viral inhibition and immunity boosters, are described. Elaboration of tables related to CoVs for the compilation of the literature has been adopted for the better understanding.
Keywords: SARS-CoV-2, Antiviral agents, Coronavirus, Herbal, Biosensors
The review article inferring nano technological aspect for infectious diseases. The quest to win the battle against COVID‐19 essentially need cumulative efforts from different scientific fields. The current pandemic situation in the world as well as future impending health crisis will greatly rely on nanotechnology based solutions in terms of detection, medication and protection.
Introduction
In last two decades, entire world faced three major outbreaks of coronaviruses like Severe Acute respiratory syndrome (SARS), middle east respiratory syndrome (MERS) and novel coronavirus disease i.e., COVID‐19. The first case of recent outbreak of COVID‐19 was recognized at Wuhan, Hubei, China five months ago. Later, it spread to the whole world and affects health as well economy globally in a very short span. Although, the world is well equipped with technological advances and well aware of the complete structural details to deal with the outbreak of coronavirus (CoVs), still all are struggling to find out its cure. Since ancient times, appearance or reappearance of several diseases have affected the human life significantly.1 From the early 1900s to date, around 250 viruses species have been evolved, and these keep on increases in the coming years (Figure 1).2
Figure 1.
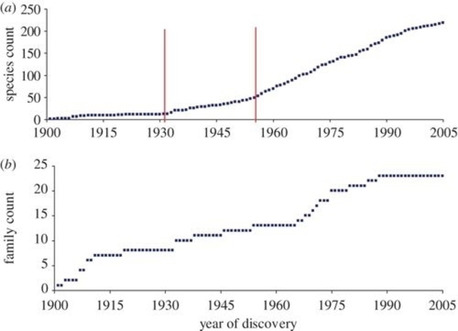
Discovery curves for human viruses. (a) Virus discovery curve by species. A cumulative number of species reported to infect humans. Statistically significant upward breakpoints are shown (vertical lines). (b) Virus discovery curve by family. A cumulative number of families containing species reported to infect humans. (Copyright permission © 2012 The Royal Society).2
Historical background
Starting from the tobacco mosaic virus (1892), foot‐mouth disease virus (1898), yellow fever virus (1901) followed by a deadly virus created influenza epidemic during World War‐I and took around 50 million lives. It is an all‐time high in the mankind history2 and this virus probably one of the influenza virus species.3
Coronavirus disease (COVID) was first reported in 1931, and the first coronavirus (HCoV‐229E) isolated from humans in 1965. These infect various birds, animals, and mammals, including humans. The isolation of the prototype murine coronavirus strain of coronavirus was reported in 1949.4 The pathogenesis and replication mechanisms of various coronaviruses have been elaborated in details since the 1970s. Human coronaviruses (HCoVs) were first identified in 1960 and caused acute upper respiratory infection (URI).5 CoVs affected the humans worldwide and are the pathogenic agents for both mammals and avian. These viruses can infect respiratory, hepatic, central nervous, and gastrointestinal systems of humans, bats, birds, mice, and some wild animals.6 These viruses are highly pathogenic to humans. Previously, CoV causes an epidemic of SARS in humans and infected thousands of people in Guangdong province of China, in 2002‐2003, followed by MERS in Saudi Arabia in 2012.
CoVs are pleiomorphic, enveloped, with an average diameter of ∼120 nm, and club‐shaped surface projections 20 nm (glycosylated spike glycoprotein) as shown in Figure 2.7 These viruses belong to family Coronaviridae, which shows crown‐like appearances under an electron microscope. The CoVs contain large, positively charged strands of RNA associated with nucleocapsid phospholipid protein and the genetic material is surrounded by viral glycoproteins. The CoVs have three types of membrane proteins, which include the spike glycoprotein, membrane protein, and small hydrophobic membrane protein. In addition to these proteins, other proteins like hemagglutinin esterase (HE) has been isolated from a different group of coronaviruses which are not present in the SARS‐CoV genome.8 The spikes of coronavirus cause infection in host cells. The structure of spike is clove shaped and has trimeric S1 head and S2 stalk, respectively (Figure 2b). During infection, S1 (head) of virus spike binds to host cell receptor for viral attachment and S2 (stalk) allow viral genome to enter into host cell through fusion of viral and host membrane surface.9
Figure 2.
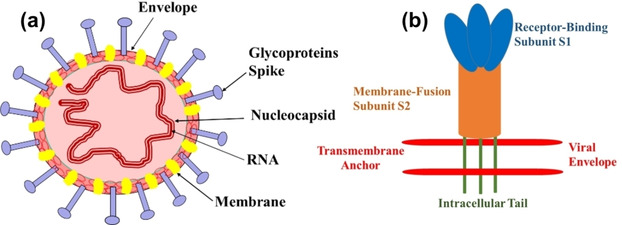
(a) General structure of CoVs and (b) Representation of the general structure of prefusion CoVs spikes.
Apart from these four principle structural proteins, various coronavirus also encodes some special structural and accessory proteins like 3a/b protein, 4a/b protein and hemagglutinin‐esterase (HE) protein.10 The subgenomic RNAs (sgRNAs) of coronaviruses encodes all the structural and accessory proteins.11 The translation of coronavirus genomic RNA is controlled by the replicase‐transcriptase protein genes. These replicase‐transcriptase proteins encoded by two open‐reading frames (ORF) namely, ORF1a and ORF1b, respectively. These two ORFs are synthesized two large polyproteins like pp1a and pp1ab. The first ORF is 2/3rd of the entire genome length from the 5′‐terminal and they encode a pp1ab polyprotein.7b The pp1ab polyprotein further divide into 16 nonstructural proteins (1‐16 nsps) except in the gamma‐coronavirus lacks nsp1. Most of the nonstructural proteins are very important and plays critical role in coronavirus viral RNA synthesis. The functions of some nonstructural proteins are not reported so far. There are 16 nsps of coronavirus form Double‐Membrane Vesicles (DMVs). Some nsps have hydrophobic transmembrane domains which acts as achor to the pp1a/pp1ab proteins with membranes during replication‐transcription complexes formation.12 Interestingly, the nsps or viral replication processes are found to be potential antiviral drug targets which might be helpful in the development of drugs against coronavirus.13 These nsps regulate genome transcription and replication processes.7b, 10 For example, nsp10 has role in viral replication/transcription complexes formation whereas the viral RNA replication and transcription are controlled by nsp12.14 It is essential to understand the function of all nsps for the development of promising drugs/vaccines against the COVID‐19 pandemic. The function of 16 nsps of coronaviruses are shown in the Table 1 that helps to interpret CoVs for effective design of drugs.
Table 1.
Functions of non‐structural proteins (nsps) of CoVs.
|
nsps |
Functions |
References |
|---|---|---|
|
nsp1 |
Promotes degradation of cellular mRNA as well as blocks translation of host cell, obstructive distinctive immunity reaction, inhibit interferon (IFN) signals |
|
|
nsp2 |
Some functions are not known, holds to prohibitin proteins |
|
|
nsp3 |
Multi‐domain large transmembrane protein, functions include: • N protein interact with Ac and Ub11 domains • Cytokine expression promote due to ADRP activity • Viral polyprotein cleaved by PLPro/Deubiquitinase domain which blocks host innate immune response • Unknown function of NAB, SUD Ubl2, G2M, and Y domains |
|
|
nsp4 |
Potential transmembrane scaffold protein, Role in (DMVs formation |
|
|
nsp5 |
chymotrypsin‐like protease (3CLpro), main protease (Mpro), cleaves viral polypeptides, inhibit interferon (IFN) signals |
|
|
nsp6 |
Restrict expansion of autophagosome, Potential transmembrane scaffold protein, double‐membrane vesicle (DMV) formation |
|
|
nsp7 |
Formation of hexadecameric complex (cofactor) with nsp8 and nsp12, role as a processivity clamp and primase for RNA polymerase |
|
|
nsp8 |
Forms hexadecameric complex (cofactor) with nsp7 and 12, may act as primase as well as processivity clamp for RNA polymerase |
|
|
nsp9 |
RNA binding protein, Dimerization |
|
|
nsp10 |
Stimulates 2‐O‐MT and ExoN activities, Scaffold protein for nsp14 and nsp16, |
|
|
nsp11 |
unknown functions |
|
|
nsp12 |
Primer and RNA‐dependent, RNA polymerase |
|
|
nsp13 |
RNA helicase, 5′ triphosphatase |
|
|
nsp14 |
Exoribonuclease activity for viral genome proofreading, N7‐Mtase activity adds 5′ cap to viral RNAs, 3′‐5′ exoribonuclease, |
|
|
nsp15 |
nsp15 endoribonuclease, evasion of dsRNA sensors |
|
|
nsp16 |
2’‐O‐Methyltransferase (2′‐O‐Mtase); avoiding MDA5 recognition, negative regulation of innate immunity |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Human coronavirus is spreading rapidly among people via viral respiratory infections like cough and sneeze. Coronavirus disease is most contagious and lethal. Various stains of coronaviruses have been reported so far (Table 2). The incubation periods and clinical symptoms of each coronaviruses are varying significantly. The symptoms and incubation periods of human coronaviruses play very important role in the identification and diagnosis of coronavirus diseases, shown in the Table 2.
Table 2.
HCoVs and their clinical symptoms30
|
HCoVs |
Clinical Symptoms |
Incubation Period |
References |
|---|---|---|---|
|
HKU1 |
Fever, Cough, Respiratory Tract Illness (RTI), Sneezing, Dyspnea, Pneumonia |
2–4 days |
|
|
229E |
Fever, Chills, Cough, Acute Rhinorrhea, Malaise, Nasal Congestion and Discharge, Headache, Throat Sore |
2–5 days |
|
|
OC43 |
Fever, Cold, Cough, Sputum, Dyspnea, Headache, Nasal Congestion and Discharge, Sneezing, Throat Sore |
2–5 days |
|
|
NL63 |
Fever, Cold, Cough, Respiratory Distress, Wheeze, Rales, Rhinorrhea, Tachypnea, Hypoxia |
2–4 days |
|
|
MERS‐CoV |
Fever, Cough, Cold, Shortness of Breath, Gastrointestinal Symptoms, Sore Throat, Arthralgia, Diarrhea and Vomiting, Pneumonia, Acute Renal Impairment, Multiple Organ Failures, Rapid Kidney Failure |
2–13 days |
|
|
SARS‐CoV |
Fever, Cough, Cold, Rigor, Shortness Of Breath, Gastrointestinal Symptoms, Myalgias, Headache, Malaise, Dyspnea, Respiratory Distress, Diarrhea, Pneumonia |
2–11 days |
|
|
SARS‐CoV‐2 (COVID‐19) |
Fever, Coughing, Cold, Sore Throat, Nasal Congestion and Rhinorrhea, Diarrhea, Asymptomatic, Organ Function Damage, Acute Kidney And Cardiac Infection, Liver Dysfunction, Pneumothorax |
2–14 days but in some cases extended to 24 days |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The viral spike glycoprotein is dimer or trimer and has two functions: attaching the virus to receptor sites located on the host surface and activate the virion membrane to fuse with host membrane so that RNA genome released inside the host cell.38 It may be noted that coronaviruses attack inside the cell membrane rather than acting on the surface of host cells. CoVs genome is non‐segmental, and the two‐third portion consists of overlapping ORFs responsible for translating the polyproteins. Remaining ORFs of genomes contained for structural proteins. Depending upon the protein sequences CoVs are subcategorised into four genera (α‐, β‐, γ‐, and δ‐CoVs).39 The two genera α‐ and β‐ were primarily associated with rodents and bats for their genes source, although birds are the key reservoir for γ‐ and δ‐CoVs. So far, there are seven human coronaviruses (HCoVs) reported in literature where two of the coronaviruses (229E and NL63) are α‐CoVs, while remaining five coronaviruses namely, SARS, MERS, OC43, HKU1, and SARS‐CoV‐2 are β‐CoVs.40 However, past few years, studies are underway to explore these viruses and develop potential drugs to cure the people from the infection.7b, 30, 32c, 33a, 41
There are various natural hosts of coronaviruses have been reported so far. Natural hosts are mainly mammals, birds, dog, man, pig, cat, mouse, bat, mouse, tree sparrow, chicken, etc. (Table 3). These hosts are very important in the identification of specific coronavirus strains. Several coronaviruses are classified as species in the Coronaviridae.7a, 42 A total of 37 coronaviruses and their natural hosts are shown in Table 3, which provides information about specific genera like α, β, δ and γ and acronym of each CoVs. Woo et al. analyzed 3306 birds species in which only 1.1% of species found positive for CoV as mentioned in Table 3.42b
Table 3.
|
S.No. |
Natural Host |
Virus |
Acronym |
Genere |
|---|---|---|---|---|
|
1 |
Dog |
Canine coronavirus |
CCV |
α |
|
2 |
Dog |
Canine enteric coronavirus |
CECV |
α |
|
3 |
Man |
Human coronavirus 229E |
HCoV‐229E |
α |
|
4 |
Man |
Human coronavirus NL63 |
HCoV‐NL63 |
α |
|
5 |
Cat |
Feline infectious peritonitis virus |
FIPV |
α |
|
6 |
Pig, Swine |
Porcine epidemic diarrhea coronavirus |
PEDV |
α |
|
7 |
Pig, Swine |
Porcine hemagglutinating encephalomyelitis virus |
HEV |
α |
|
8 |
Pig |
Porcine transmissible gastroenteritis virus |
TGEV |
α |
|
9 |
Pig |
Porcine coronavirus |
PorCoV‐HKU15 |
α |
|
10 |
Bat |
Rhinolophus bat coronavirus |
HKU2 |
α |
|
11 |
Bat |
Myotis bat coronavirus |
HKU6 |
α |
|
12 |
Bat |
Miniopterus bat coronavirus |
HKU7 |
α |
|
13 |
Bat |
Miniopterus bat coronavirus |
HKU8 |
α |
|
14 |
Bat |
Hipposideros and Rousettus bat coronavirus |
HKU10 |
α |
|
15 |
Cattle |
Bovine coronavirus |
BCV |
β |
|
16 |
Man |
Human coronavirus OC43 |
HCV OC43 |
β |
|
17 |
Man |
Human coronavirus HKU1 |
HCoV‐HKU1 |
β |
|
18 |
Man |
Middle East respiratory syndrome coronavirus |
MERS‐CoV |
β |
|
19 |
Mouse |
Murine hepatisis virus |
MHV |
β |
|
20 |
Puffin |
Puffin coronavirus |
PuCoV |
β |
|
21 |
Bat |
SARS‐related Rhinolophus bat coronaviruse |
SARSr−Rh‐Bat HKU3 |
β |
|
22 |
Bat |
Tylonycteris bat coronavirus |
HKU4 |
β |
|
23 |
Bat |
Pipistrellus bat coronavirus |
HKU5 |
β |
|
24 |
Bat |
Rousettus bat coronavirus |
HKU9 |
β |
|
25 |
Man |
Severe acute respiratory syndrome coronavirus |
SARS‐CoV |
β |
|
26 |
Chinese bulbul, Red‐whiskered bulbul, Sooty‐headed bulbul |
Bulbul coronavirus |
BuCoV HKU11 |
δ |
|
27 |
Grey‐backed thrush |
Thrush coronavirus |
ThCoV HKU12 |
δ |
|
28 |
White‐rumped munia, Chestnut munia |
Munia coronavirus |
MunCoV HKU13 |
δ |
|
29 |
Japanese white eye |
White‐eye coronavirus |
WECoV HKU16 |
δ |
|
30 |
Tree sparrow |
Sparrow coronavirus |
SpCoV HKU17 |
δ |
|
31 |
Oriental magpie robin |
Magpie robin coronavirus |
MrCoV HKU18 |
δ |
|
32 |
Black‐crowned night heron |
Night heron coronavirus |
NHCoV HKU19 |
δ |
|
33 |
Eurasian wigeon |
Wigeon coronavirus |
WiCoV HKU20 |
δ |
|
34 |
Common moorhen |
Common moorhen coronavirus |
CMCoV HKU21 |
δ |
|
35 |
Chicken, Fowl |
Infectious bronchitis virus |
IBV |
γ |
|
36 |
Pheasant |
Pheasant coronavirus |
PhCoV |
γ |
|
37 |
Turkey |
Turkey coronavirus |
TCV |
γ |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Bats and birds are reservoirs of distinct types of CoVs. The evolution model of HCoVs is shown in Figure 3.30 According to the evolutionary model (Figure 3), the origin of first CoVs might be in the bat and transfer to birds or vice‐versa. Based on the Figure 3, the bat coronavirus family transfer the virus to other bat species and then, further transfers to mammals, including humans. Similarly, the bird CoVs transfer to another bird species and further transfer in birds and mammals like pigs and whale.15d
Figure 3.
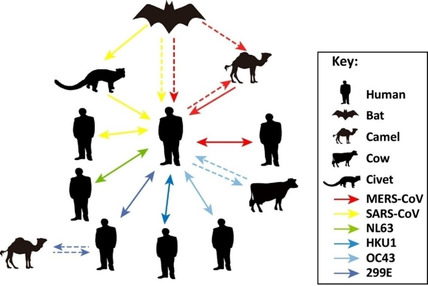
Intra‐ and Inter‐Species Transmission of Human Coronaviruses. Red, yellow, green, blue, brown, and purple arrows represent the transmission of MERS‐CoV, SARS‐CoV, NL63, HKU1, OC43, and 229E, respectively, between bats, camels, cows, humans, and masked palm civets (shown in a legend on the side of the figure). Unbroken arrows represent confirmed transmission between the two species in question, and broken arrows represent suspected transmission (Copyright permission @ 2016 Elsevier Ltd.30).
Human coronaviruses
The human coronavirus first emerged in 1965 and was obtained from human embryonic tracheal culture from adults suffering from the common cold by Tyrell and Bynoe.43 Similar findings were carried out by Hamre and Procknow44 on common cold specimens and isolated a new category of virus known as 229E from WI‐38 lung cell line culture. These two human respiratory viruses were sensitive to ethers and get multiplied in the presence of inhibitors of DNA synthesis.45 Later, McIntosh and his group at the NIH, Bethesda isolated six morphologically related viruses grown in organ culture, OC43 and OC38. The term “coronaviruses” described in 1968 based on crown‐like projections on the surface for the new generation of viruses.46
Human and animal coronaviruses were classified under three main categories, category‐I has 229E and similar type of other viruses, category‐II has OC43 and similar viruses, while in category‐III, avian infectious bronchitis viruses (IBVs) were included.47 Both 229E and OC43 coronaviruses are impacting globally in the winter season, and incubation time is less than one weak.48 Another epidemic was caused in 2002 emerged from Guangdong Province, China caused by SARS‐HCoV. The virus spread in almost 30 countries, and more than 7900 patients were reported to suffer from this viral infection. The SARS‐HCoV was isolated from the Himalayan palm civets and was believed to originated from a live animal market in Guangdong Province, China.49 The worldwide spread of the virus was accompanied by the incubation period of 4–7 days and the highest viral load on the 10th day.50 Patients infected with this virus show myalgia, headache, fever, and respiratory difficulties. Severe conditions present with lymphopenia, liver disfunction, diffused alveolar damage, an increased number of macrophages.42e, 51 The virus was re‐emerged in late 2003 because of favourable condition available for amplification of virus and it was isolated from horseshoe bats.50
The fourth human coronavirus strain, HCoV‐NL63 was identified by Hoek and co‐workers52 in Netherland and was isolated from seven months old baby in 2004. It was observed that about 4.7% of common respiratory problems in children due to HCoV‐NL63.30 In 2004 again, fifth human coronavirus strain, HCoV‐HKU1 was isolated by Patrick and co‐workers53 from 71 years old man suffering from pneumonia and bronchiolitis in Hong Kong. The old person was returned from Shenzhen, China. The virus causes acute asthmatic exacerbation and it was different from common pneumonia.22
In Saudi Arabia 2012, MERS‐HCoV (6th HCoV) reported in an old aged patient who suffered from acute pneumonia and renal failure. MERS‐HCoV sequenced and resemble with SARS‐CoV group and placed in the same category with a new lineage. MERS‐HCoV, unlike SARS‐CoV, observed with severe symptoms along with more epidemic and sporadic nature.54 Recently, emergence of 7th HCoV reported in late December 2019 from Wuhan of China. The pandemic outbreak of the novel coronavirus is most devastated to date resulting in 6.27 million infected and 379 thousands deaths so far until this paper was written up to 3rd June 2020. SARS‐CoV‐2, which shares 82% nucleotides similarities with SARS‐HcoV..55 The novel coronavirus shows an incubation period of 2–14 days but in some cases extended to 24 days and spread rapidly through inanimate surfaces (metal, plastic, and glass) where it persists up to 9 days. The pandemic outspread can only be controlled through surface disinfestation procedures by applying ethanol (62‐71%), 1‐propanol (70%), 2‐propanol (70%), H2O2 (0.5%), and sodium hypochlorite (0.1%).56
Genome replication and transcription
Coronavirus enters into host cells through viral spike (S) glycoproteins.9b, 57 Spike (S) contains two subunits, namely, S1 and S2. The S1 subunit contains bind to host cell receptors via the receptor‐binding domain. Receptor binding is a crucial step to determine the host range for a coronavirus. Some human coronavirus has receptors like angiotensin‐converting enzyme 2 (ACE2) for SARS‐CoV and HCoV‐NL63, aminopeptidase N (APN) for HCoV‐229E, 9‐O‐acetylated sialic acid HCoV‐HKU1, and HCoV‐OC43, and dipeptidyl peptidase 4 (DPP4) for MERS‐CoV.58 In the case of novel COVID‐19, the receptor‐binding domain (RBD) of S protein has a strong affinity to angiotensin‐converting enzyme 2 (ACE2) receptors of the bat and human.59 While S2 subunit comprises specialized fusion machinery that mediates fusion of viral and host cell membranes.9a, 9b, 57, 60 Later, S is cleaved at S2 of fusion peptide by proteases activities of the host in all coronaviruses.61 After entry, viruses get uncoated and start cap‐dependent translation of ORF1a from viral genomic RNA to produce polyprotein pp1a, and further translation of ORF1b encodes longer polyprotein pp1ab.62 The pp1a and pp1ab produce 15‐16 nonstructural proteins (nsps) through autoproteolytic cleavage. Significantly, nsp12 encodes the RNA‐dependent RNA polymerase (RdRP) activity,25 whereas nsp3 and nsp5 encode papain‐like protease (PLPro) and main protease (Mpro) activities, respectively.63 The nsp3, nsp4, and nsp6 from DMVs or spherules via also induce the rearrangement of the cellular membrane.20a, 64 Further, the coronavirus replication transcription complex (RTC) is anchored and assembled at DMVs. The entire life cycle, replication and pathogenicity of SARS‐CoV‐2 is shown in Figure 4.58a, 65
Figure 4.
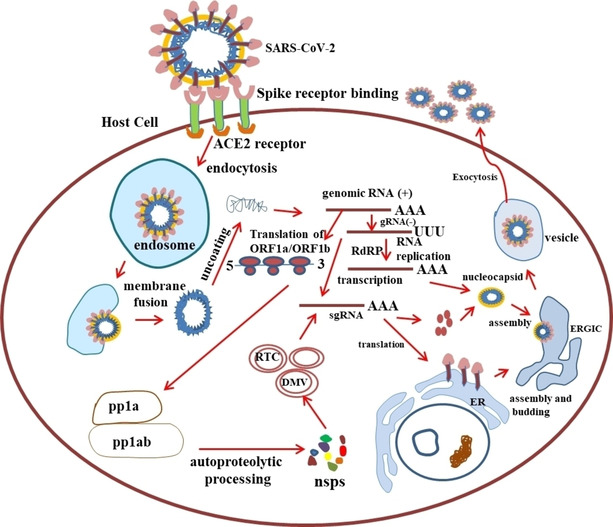
Modified schematic overview of SARS‐CoV‐2 life cycle in host cells. gRNA‐genomic RNA; ERGIC‐ER‐Golgi intermediate complex; sgRNA‐ subgenomic RNA; RTC‐replication transcription complexes; RdRP‐ RNA‐dependent RNA polymerase; nsps‐non‐structural proteins.
Case studies
HCoVs have different symptoms for various host, and accordingly dissimilar disease caused as mentioned in Table 2. Dromedary camels (Saudi Arabia) were responsible for the spread of different HCoVs in the middle east, including South Korea (2015).30 Several countries such as the United States of America, the United Kingdom, Saudi Arabia, Kenya, France, China, and other countries are profoundly affected by different types of HCoVs before Wuhan 2019 outbreak (Table 4). In 2012, a novel β‐CoV MERS was found in an old aged patient in Jeddah, Saudi Arabia suffered from pneumonia, renal failure, and respiratory infection who linked to Jordon. Later on, doctors and nurses were examined for the CoV, but they found negative, which proposed that it was not scattered easily. The MERS‐CoV caused lower respiratory tract illness (RTI) in almost all over the globe, predominantly in the Middle East, and took 356 lives by February 2015.42d, 66 Sipilwa et al. have carried out the study to find and describe HCoV strains spreading all regions of Kenya and explored the epidemiological significance of human coronaviruses.41a
Table 4.
Case study of HCoVs in several countries.
|
S.No. |
Country |
Coronaviruses strains |
Year |
Effect |
Ref. |
|---|---|---|---|---|---|
|
1 |
Chicago, USA |
HCoV 229E |
1962 |
Respiratory infections among medical students at the University of Chicago |
|
|
2 |
Netherlands |
HCoV‐NL63 |
2002‐2003 |
Replicated faster in monkey kidney cells as compared to HCoV 229E and OC43, matched with porcine epidemic diarrhea virus and HCoV 229E |
|
|
3 |
Frankfurt, Germany |
SARS‐CoV |
2003 |
The patient traveled Singapore, Hong Kong, New York and During a stopover in Frankfurt, Germany on day 7 |
|
|
4 |
Hong‐Kong |
MERS‐CoV, SARC‐CoV |
2003 |
SARS‐CoV behind the outbreak of severe respiratory infection in China in this period |
|
|
5 |
Shenzhen, China |
SARS‐CoV, NL63, HKU1 |
2003‐2004 |
Isolation of SARS‐CoV from civet cats, the study revealed that it might be originated from animals |
|
|
6 |
Kenya |
HCoV 229E, NL63, OC43, HKU1 |
2009–2012 |
Out of 417 samples, 2.4% HCoV‐NL63, ∼3% OC43, ∼2% HKU1, 1% 29E. CoVs are spreaded globally |
|
|
7 |
Jordan, Saudi Arabia |
MERS‐CoV |
2012 |
Relatives are bat coronaviruses HKU4 and HKU5, 60 years old man died due to renal failure and respiratory infection linked to Jordon and SA |
|
|
8 |
UK |
The origin of this novel coronavirus is unknown |
2012 |
Severe respiratory infection, the patient traveled from Qatar and Saudi Arabia |
|
|
9 |
France |
HCoV‐OC43 |
2001‐2013 |
Genomic studies revealed to understand the dynamics of evolution of CoVs |
|
|
10 |
USA |
HCoVs OC43, NL63, 229E, HKU1 |
2014‐2017 |
Out of 854575 cases; 2.2% OC43, 1.0% NL63, 0.8% 229E, 0.6% HKU1. Cases inceased in between December and March |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Drugs and vaccines in the current scenario
Presently, the entire world is fighting againt COVID‐19 pandemic which is caused by SARS‐CoV‐2. This disease was first reported in the Hubai, China, in December 2019 and spreaded throughout the globe in a very short time.1, 37a According to “WHO” as of 3rd June 2020, the total number of confirmed COVID‐19 cases is approximately 6.27 million and 379 thousand deaths throughout the 216 countries in the world. At present, there is no effective drug available in the market to control the spread of COVID‐19. The researchers are working for the development of therapeutic drugs to treat infections.
In silico methods offer a way to methodically and rapidly yield additional repurposing candidates. Three‐dimensional structure for the main protease of SARS‐CoV‐2 in complex with N3 (PDB ID: 6LU7) available on the RCSB protein databank is being use in insilico study. Bioinformatics analysis is used to virtually screen the molecules to get the promising candidate against the main protease of SARS‐CoV‐2. A recent study published, relied on this approach, using the predicted structure of all SARS‐CoV‐2 proteins based on their homology with other known coronavirus protein structures, and identified several compounds with potential antiviral activity.41d, 72 Since the outbreak of SARS‐CoV in 2003 and MERS‐CoV during 2005, evoked scientists to explore and elaborate the HCoVs.7b, 40b Complete information at molecular level of any disease causing agents (virus/bacteria) may helpful in the development of the medicine. There are many approved drugs to cure the patients suffering from different diseases. These durgs are approved based on the efficacy and safety (pharmacology, formulation and toxicity) of medicines to humans. Subsequently, then many therapeutic and defensive agents have been discovered. More than 500 patents have been filed to combat these viruses in various authorities worldwide, so far.73 The treatment regimen of this epidemic appearance hard as the world is eyeing for the finding of effective drugs and vaccines. The pioneer of the pathophysiology of COVID‐19 sacked the world to discover the drug/antiviral.37a, 41d, 74 Many of the broad‐spectrum antiviral drugs are in the trial stage at different levels to combat the effect of COVID‐19 by blocking the RNA polymerase, RdRp.73, 75 Some of the drugs are in trial stages such as quinine, chloroquine, lopinavir, ritonavir, alferon LDO, poly‐ICLC, streptokinase, arbidol and glucocorticoid alone or in a combination of hydroxychloroquine, ritonavir/ribavirin, ASC09/ritonavir, daunavir/lopinavir, interferon α‐2b/ribavirin, camrelizumab/thymosin, etc.73, 75a, 76 Till date, none of the compounds is approved drugs for curing from COVID‐19 infection in satisfactory way. In most of the countries on the trial basis, hydroxychlroquine with azithromycin have been used to cure the infected people. Further, it is reported that hydroxychlroquine is less effective when administered alone than in combination with the azithromycin. Although, hydroxychlroquine and azithromycin have toxicity to heart and hydroxychloroquine showed toxic effect on eye.39, 77
A biological preparation provides active acquired immunity against particular infectious disease like COVID‐19. It contains an agent that is similar to virus or microorganism caused disease. There are many vaccines are under preparation and at developmental stages throughout the world. Many vaccines are at first stage and few are waiting for human trial very soon. Till date, eight vaccines are under clinical trials throughout the world. These are Adenovirus Type 5 Vector, ChAdOx1, DNA plasmid vaccine with electropolation,78 Inactivated, Inactivated + alum, mRNA and LNP‐encapsulated mRNA and these are developed by CanSino Biological Inc./Beijing Institute of Biotechnology, University of Oxford, Inovio Pharmaceuticals, Wuhan Institute of Biological Products/Sinopharm, Beijing Institute of Biological Products/Sinopharm, Sinovac, BioNTech/Fosun Pharma/Pfizer and Moderna/NIAID, respectively.79
Nanotechnology: viral inhibition and recognition with potent nanomedicines
Still, we are strugling to find out rapid detection kit, effective antiviral and drug to make potent theranostics against SARS‐CoV‐2. The peripheral protein sequence adaptation and genome modification of virus lead to the resistance toward conventional therapies. With the advent of nanotechnology in recent years, nanomedicine have emerged to be an alternative for conventional therapeutics.80 Professor Chan emphasis on the development of nanotechnological tools as best weapon to deal with COVID‐19 and focussed on rapid point‐of‐care diagnostics, surveillance and monitoring, therapeutics and vaccine development. Identification, treatment and prevention using nanotechnology tools would escalate the development to fight against COVID‐19 as frontline tools.81 Kerry et al. explored the nano‐based technique to combat NIPAH virus and a similar method may be explored to combat HCoVs where nanosystems could be developed and used to treat viral diseases.80, 82 In the last few years, nanomaterials have also been investigated against various viral strains, and promising results were attained. A prominent approach, development of iron‐based magnetic nanoparticles (IMNPs) as nanomedicine would help immensely to deal with COVID‐19.80 The movement of IMNPs can be controlled by the external magnetic field and targeted to affected organs invaded by COVID‐19, i. e., lung, throat, etc. without going to other organelles, as shown in Figure 5.80 IMNPs alone could interact with the nanovirus, COVID‐1983 as of similar size regime and destroy or inhibit it. Additionally, in combination with photodynamic therapy, it deforms the structure of nanovirus and inhibit the growth.83 Except magnetic NPs, other nanomedicine can be utilized as antiviral agents such as Ti‐based NPs known for influenza virus,84 carbon‐based NPs elaborated for ebola, respiratory syncytial virus, etc.85 Inhibition of norovirus and pseudorabies virus through viral replications or by attacking histo‐blood group antigens using Carbon dots,86 graphene oxides based nanomaterials preventing viral spread (coronavirus, respiratory syncytial virus) on the damaging virus by interacting with negative charge present over it87 and similarly other NPs such as Se NPs, solid lipid NPs, Ag NPs, Au NPs, etc. are also active in fighting against viruses.88 Huy et al. reported the electrochemical synthesis of AgNPs and explored their activity against non‐enveloped viruses. The concentration of AgNPs up to 100 ppm or less was not cytotoxic, while antiviral activities were found in 3.13 ppm for 30 minutes of incubation with poliovirus.89 Viruses inactivation were carried out with different nanoparticles such as TiO2 NPs,84b Au NPs,88d and Silica NPs.90 The composite of various nanomaterials also exhibits promising results that may comprise the delivery of target species through nanocarrier. The nucleic acid‐based antiviral drugs face problems of cell penetration. The fragments of nucleic acid, therefore, introduced with the help of nanomaterials. Such an attempt was made by Levina et al., where they prepared titanium oxide nanoparticles and polylysine containing oligonucleotides (TiO2‐PL‐DNA) and used to target the influenza A virus (IAV).84c The authors reported (‐) RNA strands as more sensitive for IAV segment 5. However, IMNPs have great advantageous here over other nanomedicine and drug because of less toxicity.
Figure 5.
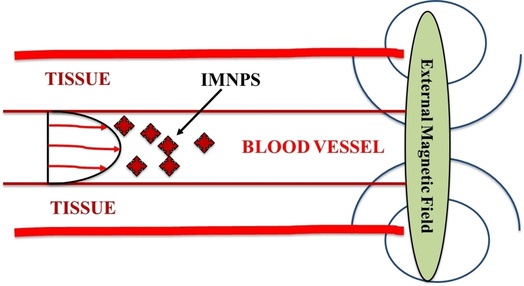
Diagrammatic illustration of IMNPs based drug delivery system.
Another strategy could be the development of nano‐biosensor to recognize and neutralize the epidemic of virus based on earlier development and detection of coronavirus. Nanosensor would open new possibilities to stop the outbreak of HCoVs by identifying the molecular species in real‐time. These sensors, based on semiconductors or hybrid nanomaterials, show rapid identification of the virus even at ultra‐low concentration.82, 91 CdTe quantum dots (CdTe QDs) mixed with sandwiched complexes, NH2‐reporter, and biotin receptor and target DNA accelerating the research for developing a sensor for human T‐lymphotropic virus‐1 (HTLV‐1) identification.92 Au NPs based materials are well known for the detection of various viruses such as dengue virus, influenza virus, bovine viral diarrhea coronavirus, etc.93 Recently, Seo et al.94 reported the field‐effect transistor (FET) based biosensor for SARS‐CoV‐2. The novel biosensor was fabricated by immobilizing the SARS‐CoV‐2 spike antibody on the graphene surface through 1‐pyrenebutyric acid N‐hydroxysuccinimide ester (PBASE) as probe linker. The FET based biosensor exhibited a good response for clinical samples obtained from nasopharyngeal swabs and cultured SARS‐CoV‐2 with a limit of detection of 1 fg/mL. Further, thiol modified antisense oligonucleotides capped AuNPs showed better response and sensitivity against RNA sequence of SARS‐CoV‐2 (0.18 ng/μL virus load) by modification in surface plasmon resonance behavior.95
Role of medicinal plants and herbs in the treatment of COVID‐19
Till today, there is no single vaccine developed for effective treatment of deadly coronavirus disease COVID‐19. On exposure to the deadly virus, the recovery rate of the patient mainly depends upon the strength of the immune system. According to WHO, 80% population of the developing country make use of herbal plants for successful treatment of various disease as well as for boosting up the immunity of individuals. The best preventive measures under practice to minimize the rate of infection includes primarily controlling the source of infection along with maintaining proper hygiene, avoiding crowded places, isolation, early diagnosis, and supportive treatments (boosting the immune system using herbal medicine). The herbal drugs are designed using specific parts of plants (roots, stem, bark, flowers, seeds, and fruits) to increase the resistance against the emerging and re‐emerging viruses and bacteria (used in Ayurveda, as mentioned in Charaka Samhita and Susruta Samhita). Various plants produce beneficial ingredients such as oregano oil, fennel, garlic, peppermint, echinacea, sambucus, licorice, astragalus, ginger, tulsi (ocimum tenuiflorum), turmeric, etc. which contain different flavones, alkaloids, polyphenols, etc. These substances release interferons and antibodies which play an essential role to fight against various viral disease. It also increases the quantity of phagocytosis that protects the body from harmful particles. One of the herbal drugs ‘fifatrol’, a natural antibiotic, is a combination of vital phytoconstituents, immunomodulatory and antioxidants. It is advantagenous against viruses, allergens, and bacteria, thereby providing multi symptoms relief.
The essential oil of garlic is a source of organosulfur compounds and allicin is a reactive sulfur species found in it. The organosulfur in the essential garlic oil inhibit the ACE2 (host‐receptor site of the virus) and main protease of the virus as well as to treat the infection due to SARS‐CoV‐2. Docking simulation and synergistic interactions show that the essential garlic oil has the ability to inhibit ACE2 and resist CoV‐19.96 This oil is also beneficial in treatment of antithrombotic, hypoglycemia, hypotension, and immunomodulation.
Natural products present in common vegetables are explored due to its tendency to effectively bind the active sites of COVID‐19 protease and hinder the viral replication. Turmeric (Curcuma longa), coriandrin, apple peels (ursolic acid), cucurbit vegetables (hederagenin), olive oil (oleanolic acid), rosemary, mint family plants (sageone), red pepper (apigenin), glycyrrhiza glabra (glabridin) are some of the natural compounds that have better insight into the mode of inhibition of viruses and have the potential for further research.97 Further, Anthocyanins represent a class of glycosylated polyphenol, that can act as a potential therapeutic antiviral compounds. It produces anthocyanidins whose nanoformulation are used in drug delivery systems and can reveal antiviral effects through a number of ways, i. e., by binding to host cells, inhibiting viral life cycle, or stimulating host immunity.98 Arundina gramnifolia belongs to Orchid family and produces gramniphenol (orange gum) and others phenolic compounds that can unveil anti‐tobacco mosaic virus activity, in addition to anti‐HIV‐1 activity.99 Codiaeum peltatum bark extract is used as an anti viral, attributed to its structural novelty and antiviral potential against zika, chikungunya and dengue.100 Similarly, zingiber officinale (ginger) was used as an antiviral for the treatment of chikungunya virus.101
The anti‐infectives is highly dependent on natural products and their structures. Combinatorial chemistry techniques have emerged as a successful approach for optimizing structures and have been used effectively in the optimization of various drugs.102 Few natural products that can act as starting materials for host‐targeting antiviral drugs show a broad spectrum in treating antiviral problems. Tannins that are normally found in plants acts as antimicrobial secondary metabolites. Hydrolysable tannins i. e. chebulagic acid and punicalagin possess inhibitory mechanism against bacteria, viruses, and eukaryotic microorganisms.103 Mycalamide is used for treatment of polio, HSV‐1 Influenza and CoV−A59 (Mice survival 14 days after A59 CoV infection under 0.1 mg/kg of 2% mycalamide A) and Griffithsin exhibits inhibitory effects against SARS‐CoV virus.103 Finally, there is a urgent need to develop natural antiviral medicines which can possess high chemical diversity and biochemical specificity to control coronavirus pandemics in future.
Future challenges and perspectives
Controlling and development of vaccines to prevent the outbreak of infectious diseases such as COVID‐19, needs fast action. Till date, no efficient vaccine/drug for its treatment is developed. Clinically patients treatment is mainly supported by emphasizing on the strength of their immune systems. In case of respiratory failure, patients are kept in the intensive care unit. Various nonspecific drugs such as hydroxychloroquine, ribavirin, lopinavir etc. are used for treating COVID‐19; however, their actual effectiveness is still not apparent.
Since nanotechnology has improvised therapeutic advancements in recent years and is an advanced combating tool in drug designing as well as drug targeting. Scientists could be encouraged towards the use of nanomaterials for targeting viral structures and depriving the impact of such novel viral infections.
The traditional diagnostic methods for viral disease sometimes are bulky, expensive and require skilled staff to operate, as it requires cell culture and sample preparation and thus, they are limited to centralized laboratories. But, the application of nanosensors, new tools offers massive advantages in the diagnosis of viral diseases; it requires only suitable nanoparticles. The development of nanosensors will be beneficial due to low cost, rapid detection tool and can help in the epidemics such as COVID‐19. Moreover, the existing toxicity evaluations of the NPs, though, cannot eliminate health risks.
To encounter the pandemic situation in the current scenario is herculean. However, it could be possible by undergoing strong preventive major like social distancing, avoiding crowded places, wearing a good quality mask, maintaining personal hygiene like washing hands regularly, and early diagnosis of infection is of utmost crucial if symptoms of disease appear.
The herbal drugs designed using specific parts of plants (roots, stem, bark, flowers, seeds, and fruits) increase the resistance against the emerging and re‐emerging viruses and bacteria (used in Ayurveda, as mentioned in Charaka Samhita and Susruta Samhita). Oregano oil, fennel, garlic, peppermint, echinacea, sambucus, licorice, astragalus, ginger, tulsi, turmeric, etc. have flavinoids, alkaloids, polyphenol's plays an essential role against various viral infections. These herbs release interferons and antibodies against viruses. It also increases the quantity of phagocytosis that protects the body by harmful particles. One of the herbal drugs ‘fifatrol’ natural antibiotic combination of vital phytoconstituents, immunomodulatory and antioxidants is helpful against viruses, allergens, and bacteria, thereby providing multi symptoms relief.104
Since COVID‐19 outbreak is a global concern, therefore, confronting similar future situations needs uncompromising regulations. Zoonotic origin and genetically crossed species barriers need to be seriously encountered through the scientific database. Since 2019‐nCoV probably originated from bats and there are many future chances to get more viruses like this, which may prove more deadly and transmissible. Therefore, scientists must take fast action to recognize the origin of this deadly virus, discover active and safe drug and therapeutics to prevent its escalation and to combat any upcoming pandemic.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Dr. Akanksha Gupta did Ph.D. in Materials Chemistry from Department of Chemistry, University of Delhi, Delhi, India. She has vast experience in synthesis, crystallography, and characterization of several mixed metal oxides viable for potential applications in cathode materials for lithium‐ion batteries, magnetism and photocatalysis. Dr. Gupta has published several research articles, reviews and book chapters in journals of international repute. Currently, she is an Assistant Professor in Sri Venkateswara College, University of Delhi, INDIA.

Biographical Information
Sanjay Kumar is an Assistant Professor in the department of Chemistry, Deshbandhu College, University of Delhi. He is perusing his PhD from Department of Chemistry, University of Delhi. He completed his MSc. and M.Phil. in Chemistry from Himachal Pradesh University, Shimla. His area of interest entails material chemistry and supramolecular nano assemblies and their exploitation in environmental science.

Biographical Information
Dr. Ravinder Kumar is an Assistant Professor in the Department of Chemistry, Gurukula Kangri Vishwavidyalaya, Haridwar, Uttarakhand, India. He completed his Ph.D. in Coordination Chemistry from Department of Chemistry, University of Delhi, Delhi and completed M.Sc in Inorganic Chemistry from Kirori Mal College, University of Delhi and B.Sc. from Kurukshetra University, Kurukshetra, India. He worked on the synthesis, structural and catalytic application of metal complexes for organic transformation reactions as well as activation and his current work is focused on the development of heterogeneous catalytic systems for sustainable and efficient catalysis.

Biographical Information
Dr. Ashish Kumar Choudhary did Ph.D. in Plant Unusual Lipid Metabolism from the Department of Botany, University of Delhi, Delhi, India. He did his M.Sc. in Biotechnology from Lalit Narayan Mithila University, Darbhanga, India. He worked as an Assistant Professor at Swami Shraddhanand College, University of Delhi, India. His research interests focus on plant molecular biology and genetic engineering, lipid biochemistry, proteomics, metabolomics and nanotechnology.

Biographical Information
Dr. Kamlesh Kumari studied Zoology at Dayalbagh Educational Institute, Agra, Uttar Pradesh, India and did M.Sc. in 2007. She joined the research group of Dr. Samim, Jamia Hamdard, Delhi, India and worked in the area of nanoscience. Then, she worked with Prof. Gopal K. Mehrotra, Motilal Nehru National Institute of Technology, Allahabad, Uttar Pradesh, India. She received her Ph.D. in 2015. She is working as Assistant Professor of Zoology at Deen Dayal Upadhyaya College, University of Delhi since 2016. Her research interest is bioinformatics and nanotechnology. She has published about 50 research articles/ reviews/ chapters in journals of international repute.

Biographical Information
Dr. Prashant Singh studied Chemistry at Indian Institute of Technology, Delhi, India and did M.Sc. in 2005. He then joined the research group of late Dr. N. N. Ghosh, Prof. Ramesh Chandra and Dr. Anju Katyal at Dr. B. R. Ambedkar Centre for Biomedical Research, University of Delhi, Delhi, India. He received his Ph.D. in January, 2010. He obtained the position of Assistant Professor at Atma Ram Sanatan Dharma (ARSD) College, University of Delhi in 2006 and published more than 75 research articles/ reviews/ chapters in journals of international repute. Citation of his work is more than 1100. He is awarded with UGC postdoctoral research fellowship and also offered for post‐doctoral fellowship from North‐West University, South‐Africa.

Biographical Information
Dr. Vinod Kumar is an Assistant Professor in the Special Centre for Nanosciences, Jawaharlal Nehru University, Delhi, India. He completed his Ph.D. in Materials Chemistry from Department of Chemistry, University of Delhi, Delhi, India. He studied M.Sc. and B.Sc. (Hons) in Chemistry from Kirori Mal College, University of Delhi, India. His present research interests include synthesis of functionalized binary and mixed metal oxide nanomaterials as well as their applications in energy and environmental related areas, magnetism, water treatment and molecular docking. Dr. Kumar has published several research articles, reviews and book chapters in journals of international repute.

A. Gupta, S. Kumar, R. Kumar, A. K. Choudhary, K. Kumari, P. Singh, V. Kumar, ChemistrySelect 2020, 5, 7521.
Contributor Information
Dr. Ashish Kumar Choudhary, Email: ash4biotech@gmail.com.
Dr. Vinod Kumar, Email: kumarv@mail.jnu.ac.in, Email: vinod7674@gmail.com.
References
- 1. Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R., Science 2020, 368, 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woolhouse M., Scott F., Hudson Z., Howey R., Chase-Topping M., Philos. Trans. R. Soc. London Ser. B 2012, 367, 2864–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.
- 3a. Billings M., Web page, June 1997; [Google Scholar]
- 3b. Riley S., Proc. Natl. Acad. Sci. USA 2014, 111, 7892–7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.
- 4a. Cheever F. S., Daniels J. B., Pappenheimer A. M., Bailey O. T., J. Exp. Med. 1949, 90, 181; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4b. Bailey O. T., Pappenheimer A. M., Cheever F. S., Daniels J. B., J. Exp. Med. 1949, 90, 195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.
- 5a. Hamre D., Procknow J. J., Proc. Soc. Exp. Biol. Med. 1966, 121, 190–193; [DOI] [PubMed] [Google Scholar]
- 5b. Kendall E., Bynoe M., Tyrrell D., Br. Med. J. 1962, 2, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.
- 6a. Wang L. F., Shi Z., Zhang S., Field H., Daszak P., Eaton B. T., Emerging Infect. Dis. 2006, 12, 1834; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6b. Ge X. Y., Li J. L., Yang X. L., Chmura A. A., Zhu G., Epstein J. H., Mazet J. K., Hu B., Zhang W., Peng C., Nature 2013, 503, 535–538; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6c. Chen Y., Guo D., Virol. Sin. 2016, 31, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.
- 7a. Cavanagh D., Coronaviruses with Special Emphasis on First Insights Concerning SARS, Springer, 2005, pp. 1–54; [Google Scholar]
- 7b. Cui J., Li F., Shi Z. L., Nat. Rev. Microbiol. 2019, 17, 181–192; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7c. Li F., Annu. Rev. Virol. 2016, 3, 237–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D. A. Brian, B. G. Hogue, T. E. Kienzle, The Coronaviridae, Springer, 1995, pp. 165–179.
- 9.
- 9a. Kirchdoerfer R. N., Cottrell C. A., Wang N., Pallesen J., Yassine H. M., Turner H. L., Corbett K. S., Graham B. S., McLellan J. S., Ward A. B., Nature 2016, 531, 118–121; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9b. Walls A. C., Tortorici M. A., Bosch B.-J., Frenz B., Rottier P. J., DiMaio F., Rey F. A., Veesler D., Nature 2016, 531, 114–117; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9c. Beniac D. R., Andonov A., Grudeski E., Booth T. F., Nat. Struct. Mol. Biol. 2006, 13, 751–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., The Lancet 2020, 395, 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hussain S., Chen Y., Yang Y., Xu J., Peng Y., Wu Y., Li Z., Zhu Y., Tien P., Guo D., J. Virol. 2005, 79, 5288–5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sawicki S. G., Sawicki D. L., Siddell S. G., J. Virol. 2007, 81, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zumla A., Chan J. F., Azhar E. I., Hui D. S., Yuen K.-Y., Nat. Rev. Drug Discovery 2016, 15, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang S. G., Shen H., Wang J., Tay F. P., Liu D. X., Virology 2008, 379, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.
- 15a. Huang C., Lokugamage K. G., Rozovics J. M., Narayanan K., Semler B. L., Makino S., J. Virol. 2011, 85, 638–643; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15b. Kamitani W., Huang C., Narayanan K., Lokugamage K. G., Makino S., Nat. Struct. Mol. Biol. 2009, 16, 1134; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15c. Tanaka T., Kamitani W., DeDiego M. L., Enjuanes L., Matsuura Y., J. Virol. 2012, 86, 11128–11137; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15d.A. R. Fehr, S. Perlman, Coronaviruses, Springer, 2015, pp. 1–23.
- 16.
- 16a. Graham R. L., Sims A. C., Brockway S. M., Baric R. S., Denison M. R., J. Virol. 2005, 79, 13399–13411; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16b. Cornillez-Ty C. T., Liao L., Yates J. R., Kuhn P., Buchmeier M. J., J. Virol. 2009, 83, 10314–10318; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16c. Gadlage M. J., Graham R. L., Denison M. R., J. Virol. 2008, 82, 11964–11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.
- 17a. Lei J., Kusov Y., Hilgenfeld R., Antiviral Res. 2018, 149, 58–74; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17b. Serrano P., Johnson M. A., Chatterjee A., Neuman B. W., Joseph J. S., Buchmeier M. J., Kuhn P., Wüthrich K., J. Virol. 2009, 83, 12998–13008; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17c. Neuman B. W., Joseph J. S., Saikatendu K. S., Serrano P., Chatterjee A., Johnson M. A., Liao L., Klaus J. P., Yates J. R., Wüthrich K., J. Virol. 2008, 82, 5279–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.
- 18a. Clementz M. A., Kanjanahaluethai A., O′Brien T. E., Baker S. C., Virology 2008, 375, 118–129; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18b. Gadlage M. J., Sparks J. S., Beachboard D. C., Cox R. G., Doyle J. D., Stobart C. C., Denison M. R., J. Virol. 2010, 84, 280–290; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18c. Beachboard D. C., Anderson-Daniels J. M., Denison M. R., J. Virol. 2015, 89, 2080–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.
- 19a. Lu Y., Lu X., Denison M. R., J. Virol. 1995, 69, 3554–3559; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19b. Stobart C. C., Sexton N. R., Munjal H., Lu X., Molland K. L., Tomar S., Mesecar A. D., Denison M. R., J. Virol. 2013, 87, 12611–12618; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19c. Zhu X., Fang L., Wang D., Yang Y., Chen J., Ye X., Foda M. F., Xiao S., Virology 2017, 502, 33–38; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19d. Zhu X., Wang D., Zhou J., Pan T., Chen J., Yang Y., Lv M., Ye X., Peng G., Fang L., J. Virol. 2017, 91, e00003–00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.
- 20a. Angelini M. M., Akhlaghpour M., Neuman B. W., Buchmeier M. J., mBio 2013, 4, e00524–00513; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20b. Oostra M., Hagemeijer M. C., van Gent M., Bekker C. P., te Lintelo E. G., Rottier P. J., de Haan C. A., J. Virol. 2008, 82, 12392–12405; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20c. Cottam E. M., Whelband M. C., Wileman T., Autophagy 2014, 10, 1426–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.
- 21a. Zhai Y., Sun F., Li X., Pang H., Xu X., Bartlam M., Rao Z., Nat. Struct. Mol. Biol. 2005, 12, 980–986; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21b. Kirchdoerfer R. N., Ward A. B., Nat. Commun. 2019, 10, 1–9; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21c. Te Velthuis A. J., van den Worm S. H., Snijder E. J., Nucleic Acids Res. 2012, 40, 1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imbert I., Guillemot J. C., Bourhis J. M., Bussetta C., Coutard B., Egloff M. P., Ferron F., Gorbalenya A. E., Canard B., EMBO J. 2006, 25, 4933–4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.
- 23a. Egloff M. P., Ferron F., Campanacci V., Longhi S., Rancurel C., Dutartre H., Snijder E. J., Gorbalenya A. E., Cambillau C., Canard B., Proc. Natl. Acad. Sci. USA 2004, 101, 3792–3796; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23b. Zeng Z., Deng F., Shi K., Ye G., Wang G., Fang L., Xiao S., Fu Z., Peng G., J. Virol. 2018, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.
- 24a. Decroly E., Debarnot C., Ferron F., Bouvet M., Coutard B., Imbert I., Gluais L., Papageorgiou N., Sharff A., Bricogne G., PLoS Pathog. 2011, 7; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24b. Ma Y., Wu L., Shaw N., Gao Y., Wang J., Sun Y., Lou Z., Yan L., Zhang R., Rao Z., Proc. Natl. Acad. Sci. USA 2015, 112, 9436–9441; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24c. Chen Y., Su C., Ke M., Jin X., Xu L., Zhang Z., Wu A., Sun Y., Yang Z., Tien P., PLoS Pathog. 2011, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu X., Liu Y., Weiss S., Arnold E., Sarafianos S. G., Ding J., Nucleic Acids Res. 2003, 31, 7117–7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.
- 26a. Ivanov K. A., Thiel V., Dobbe J. C., van der Meer Y., Snijder E. J., Ziebuhr J., J. Virol. 2004, 78, 5619–5632; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26b. Ivanov K. A., Ziebuhr J., J. Virol. 2004, 78, 7833–7838; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26c. Hao W., Wojdyla J. A., Zhao R., Han R., Das R., Zlatev I., Manoharan M., Wang M., Cui S., PLoS Pathog. 2017, 13, e1006474; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26d. Adedeji A. O., Lazarus H., mSphere 2016, 1, e00235–00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.
- 27a. Eckerle L. D., Becker M. M., Halpin R. A., Li K., Venter E., Lu X., Scherbakova S., Graham R. L., Baric R. S., Stockwell T. B., PLoS Pathog. 2010, 6; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27b. Chen Y., Cai H., Xiang N., Tien P., Ahola T., Guo D., Proc. Natl. Acad. Sci. USA 2009, 106, 3484–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.
- 28a. Bhardwaj K., Sun J., Holzenburg A., Guarino L. A., Kao C. C., J. Mol. Biol. 2006, 361, 243–256; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28b. Deng X., Hackbart M., Mettelman R. C., O'Brien A., Mielech A. M., Yi G., Kao C. C., Baker S. C., Proc. Natl. Acad. Sci. USA 2017, 114, e4251-E4260; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28c. Zhang L., Li L., Yan L., Ming Z., Jia Z., Lou Z., Rao Z., J. Virol. 2018, 92, e00893–00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi P., Su Y., Li R., Liang Z., Dong S., Huang J., Virus Res. 2019, 265, 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Su S., Wong G., Shi W., Liu J., Lai A. C., Zhou J., Liu W., Bi Y., Gao G. F., Trends Microbiol. 2016, 24, 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.
- 31a. Sloots T. P., McErlean P., Speicher D. J., Arden K. E., Nissen M. D., Mackay I. M., J. Clin. Virol. 2006, 35, 99–102; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31b. Esper F., Weibel C., Ferguson D., Landry M. L., Kahn J. S., Emerging Infect. Dis. 2006, 12, 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.
- 32a. Hendley J. O., Fishburne H. B., J. M. Gwaltney Jr , Am. Rev. Respir. Dis. 1972, 105, 805–811; [DOI] [PubMed] [Google Scholar]
- 32b. Bradburne A., Bynoe M., Tyrrell D., Br. Med. J. 1967, 3, 767; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32c. Mäkelä M. J., Puhakka T., Ruuskanen O., Leinonen M., Saikku P., Kimpimäki M., Blomqvist S., Hyypiä T., Arstila P., J. Clin. Microbiol. 1998, 36, 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.
- 33a. Gaunt E. R., Hardie A., Claas E. C., Simmonds P., Templeton K. E., J. Clin. Microbiol. 2010, 48, 2940–2947; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33b. f Zhang S., l Tuo J., b Huang X., Zhu X., Zhang D.-m., Zhou K., Yuan L., Luo H.-j., Zheng B.-j., Yuen K. Y., PLoS One 2018, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.
- 34a. Arden K. E., Nissen M. D., Sloots T. P., Mackay I. M., J. Med. Virol. 2005, 75, 455–462; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34b. Hofmann H., Pyrc K., van der Hoek L., Geier M., Berkhout B., Pöhlmann S., Proc. Natl. Acad. Sci. USA 2005, 102, 7988–7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.
- 35a. Momattin H., Mohammed K., Zumla A., Memish Z. A., Al-Tawfiq J. A., Int. J. Infect. Dis. 2013, 17, e792–798; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35b. Goh G. K. M., Dunker A. K., Uversky V., PLoS Curr. 2013, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.
- 36a. Christian M. D., Loutfy M., McDonald L. C., Martinez K. F., Ofner M., Wong T., Wallington T., Gold W. L., Mederski B., Green K., Emerging Infect. Dis. 2004, 10, 287; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36b. Cameron M. J., Bermejo-Martin J. F., Danesh A., Muller M. P., Kelvin D. J., Virus Res. 2008, 133, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.
- 37a. Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Lancet Respir. Med. 2020; [Google Scholar]
- 37b. Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Nat. Med. 2020, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simmons G., Reeves J. D., Rennekamp A. J., Amberg S. M., Piefer A. J., Bates P., Proc. Natl. Acad. Sci. USA 2004, 101, 4240–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nguyen L. S., Dolladille C., Drici M. D., Fenioux C., Alexandre J., Mira J. P., Moslehi J. J., Roden D. M., Funck-Brentano C., Salem J. E., Circulation 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.
- 40a. Killerby M. E., Biggs H. M., Haynes A., Dahl R. M., Mustaquim D., Gerber S. I., Watson J. T., J. Clin. Virol. 2018, 101, 52–56; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40b.M. E. Killerby, H. M. Biggs, A. Haynes, 2018.
- 41.
- 41a. Sipulwa L. A., Ongus J. R., Coldren R. L., Bulimo W. D., Virology journal 2016, 13, 18; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41b. Dominguez S. R., Robinson C. C., Holmes K. V., J. Med. Virol. 2009, 81, 1597–1604; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41c. Talbot H. K., Shepherd B. E., J. E. Crowe Jr , Griffin M. R., Edwards K. M., Podsiad A. B., Tollefson S. J., Wright P. F., Williams J. V., Pediatr. Infect. Dis. J. 2009, 28, 682; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41d. Dong L., Hu S., Gao J., Drug Discoveries Ther. 2020, 14, 58–60. [DOI] [PubMed] [Google Scholar]
- 42.
- 42a.D. Cavanagh, D. Brian, M. Brinton, L. Enjuanes, K. Holmes, M. Horzinek, M. Lai, H. Laude, P. Plagemann, S. Siddell, in Coronaviruses, Springer, 1994, pp. 255–257; [DOI] [PubMed]
- 42b. Woo P. C., Lau S. K., Lam C. S., Lau C. C., Tsang A. K., Lau J. H., Bai R., Teng J. L., Tsang C. C., Wang M., J. Virol. 2012, 86, 3995–4008; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42c. Fouchier R. A., Hartwig N. G., Bestebroer T. M., Niemeyer B., de Jong J. C., Simon J. H., Osterhaus A. D., Proc. Natl. Acad. Sci. USA 2004, 101, 6212–6216; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42d. Zaki A. M., Van Boheemen S., Bestebroer T. M., Osterhaus A. D., Fouchier R. A., N. Engl. J. Med. 2012, 367, 1814–1820; [DOI] [PubMed] [Google Scholar]
- 42e. To K. K., Hung I. F., Chan J. F., Yuen K. Y., J. Thorac. Dis. 2013, 5, S103; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42f. Lau S. K., Woo P. C., Li K. S., Huang Y., Wang M., Lam C. S., Xu H., Guo R., h Chan K., j Zheng B., Virology 2007, 367, 428–439; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42g. Woo P. C., Lau S. K., Li K. S., Poon R. W., Wong B. H., w Tsoi H., Yip B. C., Huang Y., Chan K.-h., y Yuen K., Virology 2006, 351, 180–187; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42h. Woo P. C., Huang Y., Lau S. K., Yuen K.-Y., Viruses 2010, 2, 1804–1820; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42i. Lau S. K., Poon R. W., Wong B. H., Wang M., Huang Y., Xu H., Guo R., Li K. S., Gao K., Chan K.-H., J. Virol. 2010, 84, 11385–11394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Tyrrell D. A., Bynoe M. L., Lancet 1966, 1, 76–77. [DOI] [PubMed] [Google Scholar]
- 44. Hamre D., Procknow J. J., Proc. Soc. Exp. Biol. Med.. 1966, 121, 190–193. [DOI] [PubMed] [Google Scholar]
- 45. Almeida J. D., Tyrrell D. A., J. Gen. Virol. 1967, 1, 175–178. [DOI] [PubMed] [Google Scholar]
- 46. Tyrrell D. A., Almeida J. D., Cunningham C. H., Dowdle W. R., Hofstad M. S., McIntosh K., Tajima M., Zakstelskaya L. Y., Easterday B. C., Kapikian A., Bingham R. W., Intervirology 1975, 5, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Holmes K. V., Lai M. M. C., Fields Virol. 2001, 3, 1075–1093. [Google Scholar]
- 48. McIntosh K., Dees J. H., Becker W. B., Kapikian A. Z., Chanock R. M., Proc. Natl. Acad. Sci. USA 1967, 57, 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guan Y., Zheng B., He Y., Liu X., Zhuang Z., Cheung C., Luo S., Li P., Zhang L., Guan Y., Science 2003, 302, 276–278. [DOI] [PubMed] [Google Scholar]
- 50. Cheng V. C. C., Lau S. K. P., Woo P. C. Y., Yuen K. Y., Clin. Microbiol. Rev. 2007, 20, 660–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peiris J., Lai S., Poon L., Guan Y., Yam L., Lim W., Nicholls J., Yee W., Yan W., Cheung M., Lancet 2003, 361, 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van der Hoek L., Pyrc K., Jebbink M. F., Vermeulen-Oost W., Berkhout R. J., Wolthers K. C., Wertheim-van Dillen P. M., Kaandorp J., Spaargaren J., Berkhout B., Nat. Med. 2004, 10, 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Woo P. C. Y., Lau S. K. P., Chu C.-M., Chan K.-h., Tsoi H.-W., Huang Y., Wong B. H. L., Poon R. W. S., Cai J. J., Luk W.-k., Poon L. L. M., Wong S. S. Y., Guan Y., Peiris J. S. M., y Yuen K., J. Virol. 2005, 79, 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee S. S., Wong N. S., Int. J. Infect. Dis. 2015, 38, 65–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.
- 55a. Chan J. F. W., Kok K. H., Zhu Z., Chu H., To K. K. W., Yuan S., Yuen K. Y., Emerg. Microbes Infect. 2020, 9, 221–236; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55b. Surveillances V., China CDC Weekly 2020, 2, 113–122. [Google Scholar]
- 56. Kampf G., Todt D., Pfaender S., Steinmann E., J. Hosp. Infect. 2020, 104, 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Walls A. C., Tortorici M. A., Snijder J., Xiong X., Bosch B. J., Rey F. A., Veesler D., Proc. Natl. Acad. Sci. USA 2017, 114, 11157–11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.
- 58a. Fung T. S., Liu D. X., Annu. Rev. Microbiol. 2019, 73, 529–557; [DOI] [PubMed] [Google Scholar]
- 58b. Lim Y. X., Ng Y. L., Tam J. P., Liu D. X., Diseases 2016, 4, 26 (1-28). [Google Scholar]
- 59. Tai W., He L., Zhang X., Pu J., Voronin D., Jiang S., Zhou Y., Du L., Cell. Mol. Immunol. 2020, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Song W., Gui M., Wang X., Xiang Y., PLoS Pathog. 2018, 14, e1007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.
- 61a. Millet J. K., Whittaker G. R., Virus Res. 2015, 202, 120–134; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61b. Madu I. G., Roth S. L., Belouzard S., Whittaker G. R., J. Virol. 2009, 83, 7411–7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Masters P. S., Adv. Virus Res. 2006, 66, 193–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ziebuhr J., Snijder E. J., Gorbalenya A. E., J. Gen. Virol. 2000, 81, 853–879. [DOI] [PubMed] [Google Scholar]
- 64. Maier H. J., Hawes P. C., Cottam E. M., Mantell J., Verkade P., Monaghan P., Wileman T., Britton P., mBio 2013, 4, e00801–00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shereen M. A., Khan S., Kazmi A., Bashir N., Siddique R., J. Adv. Res. 2020, 24, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hijawi B., Abdallat M., Sayaydeh A., Alqasrawi S., Haddadin A., Jaarour N., El Sheikh S., Alsanouri T., East Mediterr Health J. 2013, 1 9, S12-S18. [PubMed] [Google Scholar]
- 67. Drosten C., Günther S., Preiser W., Van Der Werf S., Brodt H.-R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R. A., N. Engl. J. Med. 2003, 348, 1967–1976. [DOI] [PubMed] [Google Scholar]
- 68. Chiu S. S., Hung Chan K., Wing Chu K., Kwan S. W., Guan Y., Man Poon L. L., Peiris J., Clin. Infect. Dis. 2005, 40, 1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Woo P. C., Lau S. K., Chu C. M., Chan K. H., Tsoi H. W., Huang Y., Wong B. H., Poon R. W., Cai J. J., Luk W. K., J. Virol. 2005, 79, 884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bermingham A., Chand M., Brown C., Aarons E., Tong C., Langrish C., Hoschler K., Brown K., Galiano M., Myers R., Eurosurveillance 2012, 17, 20290. [PubMed] [Google Scholar]
- 71. Kin N., Miszczak F., Lin W., Gouilh M. A., Vabret A., Viruses 2015, 7, 2358–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.
- 72a. Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F., Cell Discovery 2020, 6, 1–18; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72b.C. Harrison, Nat. Biotechnol 2020.
- 73. Liu C., Zhou Q., Li Y., Garner L. V., Watkins S. P., Carter L. J., Smoot J., Gregg A. C., Daniels A. D., Jervey S., ACS Cent. Sci. 2020, 6, 315–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kumar D., Kumari K., Jayaraj A., Kumar V., Kumar R. V., Dass S. K., Chandra R., Singh P., J. Biomol. Struct. Dyn. 2020, 1–14 (https://doi.org/10.1080/07391102.2020.1752310); b) D. Kumar, K. Kumari, V. Kumar, Vishvakarma, A. Jayaraj, D. Kumar, V. K. Ramappa, R. Patel, V. Kumar, S. K. Dass, R. Chandra, P. Singh, J. Biomol. Struct. Dyn 2020 (https://doi.org/10.1080/07391102.2020.1779131). [Google Scholar]
- 75.
- 75a. Li G., De Clercq E., Nat. Rev. Drug Discovery 2020; [DOI] [PubMed] [Google Scholar]
- 75b. Qiu R., Wei X., Zhao M., Zhong C., Zhao C., Hu J., Li M., Huang Y., Han S., He T., MedRxiv 2020, https://doi.org/10.1101/2020.03. 04. 20031401. [Google Scholar]
- 76.
- 76a. Wu S. C., Biotechnol. J. 2020, 15, 2000147; [Google Scholar]
- 76b. Caddy S., British Medical Journal Publishing Group, 2020; [Google Scholar]
- 76c. Le T. T., Andreadakis Z., Kumar A., Roman R. G., Tollefsen S., Saville M., Mayhew S., Nat. Rev. Drug Discovery 2020, 19, 305–306; [DOI] [PubMed] [Google Scholar]
- 76d. Peeples L., Proc. Natl. Acad. Sci. USA 2020, 117, 8218–8221; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76e. Yamey G., Schäferhoff M., Hatchett R., Pate M., Zhao F., McDade K. K., Lancet 2020, 395, 1405–1406; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76f. Lythgoe M. P., Middleton P., Trends Pharmacol. Sci. 2020, 41, 363–382; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76g.W. H. Organization, World 2020 (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines);
- 76h. Talevi A., Bellera C. L., Expert Opin. Drug Discovery 2020, 15, 397–401. [DOI] [PubMed] [Google Scholar]
- 77. Kamp T. J., Hamdan M. H., January C. T., J. Am. Heart Assoc. 2020, 9, e016887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.
- 78a. Kim Y. C., Dema B., Reyes-Sandoval A., NPJ Vaccines 2020, 5, 1–3; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78b. Diamond M. S., Pierson T. C., Cell Host Microbe 2020, 27, 699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.
- 79a. Espeseth A. S., Cejas P. J., Citron M. P., Wang D., DiStefano D. J., Callahan C., O'Donnell G., Galli J. D., Swoyer R., Touch S., NPJ vaccines 2020, 5, 1–14; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79b. Ella K. M., Mohan V. K., Indian Pediatr. 2020, 57, 407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kumar V., Choudhary A. K., Kumar P., Sharma S., Nanoscience & Nanotechnology-Asia 2019, 9, 64–78. [Google Scholar]
- 81.
- 81a. Chan W. C., ACS Nano 2020, 14, 4, 3719–3720; [DOI] [PubMed] [Google Scholar]
- 81b. Fadeel B., Kostarelos K., Nat. Nanotechnol. 2020, 15, 164; [DOI] [PubMed] [Google Scholar]
- 81c.A. Gupta, S. Kumar, V. Kumar, Challenges for Assessing Toxicity of Nanomaterials in Biochemical Toxicology-Heavy Metals and Nanomaterials, IntechOpen, 2019.
- 82. Kerry R. G., Malik S., Redda Y. T., Sahoo S., Patra J. K., Majhi S., Nanomedicine: Nanotechnology, Biology and Medicine 2019, 18, 196–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hu T. Y., Frieman M., Wolfram J., Nat. Nanotechnol. 2020, 15, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.
- 84a. Mazurkova N., Spitsyna Y. E., Shikina N., Ismagilov Z., Zagrebel'nyi S., Ryabchikova E., Nanotechnol. Russ. 2010, 5, 417–420; [Google Scholar]
- 84b. Syngouna V. I., Chrysikopoulos C. V., J. Colloid Interface Sci. 2017, 497, 117–125; [DOI] [PubMed] [Google Scholar]
- 84c. Levina A. S., Repkova M. N., Bessudnova E. V., Filippova E. I., Mazurkova N. A., Zarytova V. F., Beilstein J. Nanotechnol. 2016, 7, 1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.
- 85a. Luczkowiak J., Muñoz A., Sánchez-Navarro M., Ribeiro-Viana R., Ginieis A., Illescas B. M., Martín N., Delgado R., Rojo J., Biomacromolecules 2013, 14, 431–437; [DOI] [PubMed] [Google Scholar]
- 85b. Dostalova S., Moulick A., Milosavljevic V., Guran R., Kominkova M., Cihalova K., Heger Z., Blazkova L., Kopel P., Hynek D., Monatsh. Chem. 2016, 147, 905–918. [Google Scholar]
- 86.
- 86a. Du T., Liang J., Dong N., Liu L., Fang L., Xiao S., Han H., Carbon 2016, 110, 278–285; [Google Scholar]
- 86b. Dong X., Moyer M. M., Yang F., Sun Y. P., Yang L., Sci. Rep. 2017, 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.
- 87a. Ye S., Shao K., Li Z., Guo N., Zuo Y., Li Q., Lu Z., Chen L., He Q., Han H., ACS Appl. Mater. Interfaces 2015, 7, 21571–21579; [DOI] [PubMed] [Google Scholar]
- 87b. Yang X. X., Li C. M., Li Y. F., Wang J., Huang C. Z., Nanoscale 2017, 9, 16086–16092; [DOI] [PubMed] [Google Scholar]
- 87c. Chen Y. N., Hsueh Y. H., Hsieh C. T., Tzou D. Y., Chang P. L., Int. J. Environ. Res. Public Health 2016, 13, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.
- 88a. Li Y., Lin Z., Guo M., Xia Y., Zhao M., Wang C., Xu T., Chen T., Zhu B., Int. J. Nanomed. 2017, 12, 5733; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88b. Torrecilla J., Rodríguez A. D. P., Solinís M. Y., Apaolaza P. S., Herranz B. B., López C. R., Herranz A. B., Gascón A. R., Colloids Surf B Biointerfaces 2016, 146, 808–817; [DOI] [PubMed] [Google Scholar]
- 88c. Gaikwad S., Ingle A., Gade A., Rai M., Falanga A., Incoronato N., Russo L., Galdiero S., Galdiero M., Int. J. Nanomed. 2013, 8, 4303; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88d. Paul A. M., Shi Y., Acharya D., Douglas J. R., Cooley A., Anderson J. F., Huang F., Bai F., J. Gen. Virol. 2014, 95, 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Huy T. Q., Hien Thanh N. T., Thuy N. T., Chung P. V., Hung P. N., Le A.-T., Hong Hanh N. T., J. Virol. Methods 2017, 241, 52–57. [DOI] [PubMed] [Google Scholar]
- 90. Lee E. C., Davis-Poynter N., Nguyen C. T., Peters A. A., Monteith G. R., Strounina E., Popat A., Ross B. P., Nanoscale 2016, 8, 16192–16196. [DOI] [PubMed] [Google Scholar]
- 91.
- 91a. Zhu H., Fohlerová Z., Pekárek J., Basova E., Neužil P., Biosens. Bioelectron. 2020, 112041; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91b. Mokhtarzadeh A., Eivazzadeh-Keihan R., Pashazadeh P., Hejazi M., Gharaatifar N., Hasanzadeh M., Baradaran B., de la Guardia M., TrAC Trends Anal. Chem. 2017, 97, 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Norouzi M., Zarei Ghobadi M., Golmimi M., Mozhgani S.-H., Ghourchian H., Rezaee S. A., Anal. Lett. 2017, 50, 2402–2411. [Google Scholar]
- 93.
- 93a. Carter J. R., Balaraman V., Kucharski C. A., Fraser T. S., Fraser M. J., Virol. J. 2013, 10, 201; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93b. Gopinath S. C., Awazu K., Fujimaki M., Shimizu K., Shima T., PLoS One 2013, 8, e69121; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93c. Askaravi M., Rezatofighi S. E., Rastegarzadeh S., Shapouri M. R. S. A., AMB Express 2017, 7, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Seo G., Lee G., Kim M. J., Baek S. H., Choi M., Ku K. B., Lee C. S., Jun S., Park D., Kim H. G., Kim S. J., Lee J. O., Kim B. T., Park E. C., Kim S. I., ACS Nano 2020, 14(4), 5135–5142. [DOI] [PubMed] [Google Scholar]
- 95. Moitra P., Alafeef M., Dighe K., Frieman M., Pan D., ACS Nano 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Thuy B. T. P., My T. T. A., Hai N. T. T., Hieu L. T., Hoa T. T., Thi Phuong Loan H., Triet N. T., Anh T. T. V., Quy P. T., Tat P. V., ACS Omega 2020, 5(14), 8312–8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.
- 97a. Khaerunnisa S., Kurniawan H., Awaluddin R., Suhartati S., Soetjipto S., Prepr. doi10. 20944/preprints202003. 0226. v1 2020, 1–14; [Google Scholar]
- 97b. Sampangi-Ramaiah M. H., Vishwakarma R., Shaanker R. U., Curr. Sci. 2020, 118, 1087. [Google Scholar]
- 98. Pour P. M., Fakhri S., Asgary S., Farzaei M. H., Echeverria J., Front. Pharmacol. 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hu Q. F., Zhou B., Huang J. M., Gao X. M., Shu L. D., Yang G. Y., Che C. T., J. Nat. Prod. 2013, 76, 292–296. [DOI] [PubMed] [Google Scholar]
- 100. Olivon F., Remy S., Grelier G., Apel C. C., Eydoux C. C., Guillemot J. C., Neyts J., Delang L., Touboul D., Roussi F., J. Nat. Prod. 2019, 82, 330–340. [DOI] [PubMed] [Google Scholar]
- 101.S. Kaushik, G. Jangra, V. Kundu, J. P. Yadav, S. Kaushik, VirusDis 2020 (https://doi.org/10.1007/s13337-020-00584-0).
- 102. Newman D. J., Cragg G. M., J. Nat. Prod. 2020, 83, 770–803. [DOI] [PubMed] [Google Scholar]
- 103. Martinez J., Sasse F., Brönstrup M., Diez J., Meyerhans A., Nat. Prod. Rep. 2015, 32, 29–48. [DOI] [PubMed] [Google Scholar]
- 104. Ganjhu R. K., Mudgal P. P., Maity H., Dowarha D., Devadiga S., Nag S., Arunkumar G., VirusDis. 2015, 26, 225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]


