Abstract
COVID‐19 carries a high risk of severe disease course, particularly in patients with comorbidities. Therapy of severe COVID‐19 infection has relied on supportive intensive care measures. More specific approaches including drugs that limit the detrimental “cytokine storm”, such as Janus‐activated kinase (JAK) inhibitors, are being discussed. Here, we report a compelling case of a 55‐yo patient with proven COVID‐19 pneumonia, who was taking the JAK1/2 inhibitor ruxolitinib in‐label for co‐existing primary myelofibrosis for 15 months prior to coronavirus infection. The patient had significant comorbidities, including chronic kidney disease, arterial hypertension, and obesity, and our previous cohort suggested that he was thus at high risk for acute respiratory distress syndrome (ARDS) and death from COVID‐19. Since abrupt discontinuation of ruxolitinib may cause fatal cytokine storm and ARDS, ruxolitinib treatment was continued and was well tolerated, and the patient´s condition remained stable, without the need for mechanical ventilation or vasopressors. The patient became negative for SARS‐CoV‐2 and was discharged home after 15 days. In conclusion, our report provides clinical evidence that ruxolitinib treatment is feasible and can be beneficial in patients with COVID‐19 pneumonia, preventing cytokine storm and ARDS.
Keywords: COVID‐19, cytokine storm, mild course, primary myelofibrosis, ruxolitinib
Key points.
JAK inhibition by ruxolitinib may facilitate a mild COVID‐19 course
Ruxolitinib treatment was continued in a patient with COVID‐19‐related pneumonia and pre‐existing myeloproliferative neoplasm (MPN), who, despite significant comorbidities and risk factors, experienced a favorable clinical course of the infection
This observation clearly supports the rationale of investigating ruxolitinib in order to avoid a “cytokine storm” in non‐MPN patients with COVID‐19 infection
1. INTRODUCTION
Advanced age and comorbidities are risk factors for severe SARS‐CoV‐2 infection and development of ARDS. 1 , 2 Elevated inflammatory parameters such as an increased white blood cell (WBC) count, C‐reactive protein (CRP), interleukin‐6 (IL‐6), and soluble IL‐2 receptor correlate with ARDS and multiorgan failure (MOF) in COVID‐19 patients, 2 comparable to the so‐called “cytokine storm” syndrome. Frontline treatment of COVID‐19 infection consists of supportive care. In addition, off‐label and experimental treatment options against SARS‐CoV‐2 comprising direct antiviral agents and control of overwhelming inflammation reaction are currently being evaluated.
Outside of COVID‐19, treatment of overwhelming inflammatory mechanisms responsible for ARDS and MOF includes anti‐IL‐6R antagonists and inhibitors of Janus kinase 1/2 (JAK1/2) 3 such as baricitinib or ruxolitinib (which is approved for the treatment of patients with myelofibrosis and polycythemia vera). JAK inhibitors have been proposed for patients with severe COVID‐19, and the first clinical trials have recently been initiated using ruxolitinib (NCT04414098, NCT04361903, NCT04348071, NCT04331665, NCT04355793, NCT04377620, NCT04362137, NCT04334044, NCT04337359, NCT04338958, NCT04359290) or pacritinib (NCT04404361). However, to the best of our knowledge, no clinical reports for ruxolitinib use in MPN pts with COVID‐19 have been published so far. Here, we describe the clinical course of a patient with COVID‐19 and concomitant ruxolitinib treatment for myelofibrosis.
2. CASE PRESENTATION
A 55‐year‐old male patient with known calreticulin (CALR del52)‐mutant primary myelofibrosis, diagnosed 12 years ago and treated with ruxolitinib, was admitted to our academic center with SARS‐CoV2‐positive pneumonia. Ruxolitinib treatment at 10mg BID had been initiated 15 months ago for constitutional symptoms and splenic pain. The current dynamic international prognostic scoring system (DIPSS) group was intermediate‐2 (anemia and constitutional symptoms).
The patient also had stage III chronic kidney disease (CKD) and associated secondary hyperparathyroidism, and tinnitus. Cardiovascular risk factors included arterial hypertension, obesity (BMI 30 kg/m2), and hyperuricemia. His medications included candesartan, low‐dose acetylsalicylic acid, torasemide, and colecalciferol.
The patient had coughing and dyspnea, accompanied by fever and chills, eleven days before admission. One day before admission, he was tested at a regional COVID‐19 testing site with a positive test result on the same day of testing. Upon the advice of his general practitioner, the patient presented himself to our emergency outpatient clinic on the following day with clinical suspicion of COVID‐19 pneumonia.
Upon admission, oxygen saturation was 88% on room air, and he was given supplemental oxygen per nasal cannula (4L/min). The physical examination showed tachypnea (breathing frequency at rest 23/min) but was otherwise unremarkable. Capillary blood gas analysis in the presence of 4 L O2/min showed hypoxemia (PaO2 of 59.7 mmHg) and hypocapnia (PaCO2 26.2 mmHg). EKG showed tachycardia (103/min). Computed tomography (CT) showed bilateral infiltrates (Figure 1A) and moderate enlargement of the spleen (14 × 6.5 cm). Laboratory analysis and vital signs during the 15‐day hospital stay are displayed in Figure 1B.
FIGURE 1.
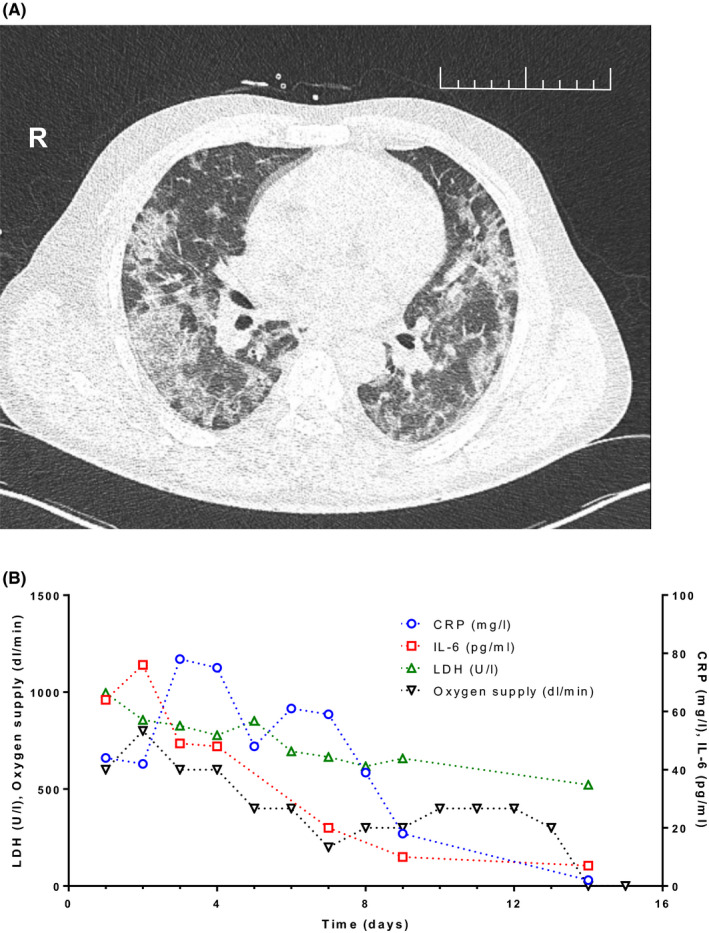
Chest CT scan at admission and clinical and laboratory parameters during the course of treatment. A, The low‐dose computed tomography (CT) of the chest on the day of admission showed bilateral, predominantly peripheral “ground glass” opacities, focal consolidations, and so‐called “crazy paving,” that is, superimposed intra‐ and interlobular interstitial thickening. No pleural or pericardial effusion or coronary artery sclerosis was observed. No thoracic lymphadenopathy. In summary, the pattern of findings was categorized to be highly suggestive of COVID‐19 associated pneumonia (COV‐RADS 5). B, Laboratory parameters lactate dehydrogenase, C‐reactive protein (CRP), interleukin‐6 (IL‐6), and administered oxygen supply of the patient during the hospital stay (days after admission)
The patient was admitted to the intensive care unit (ICU) with hypotension (BP 95/59 mm Hg), tachycardia (heart rate 116/min), tachypnea (respiratory rate 23/min), and hypoxia (SaO2:88%) despite oxygen supplementation (4‐8 L/min). However, the patient neither required vasopressor treatment nor mechanical ventilation. No antibiotics were administered. Despite a potential immunosuppressive effect of ruxolitinib, treatment with ruxolitinib was continued at 10 mg BID, given that abrupt discontinuation of the drug had been described to be associated with cases of severe cytokine storm and sometimes fatal ARDS. 4 Five days after admission, the patient was tested negative for SARS‐CoV‐2 by PCR, and seven days after admission, he was transferred from the ICU to the regular pneumology ward. He still needed 4 L/min of supplemental oxygen but was afebrile and able to ambulate. 15 days after admission and after two additional negative SARS‐CoV‐2 PCRs, the patient was discharged in good health condition and without the need for supplemental oxygen. IL‐6 levels had peaked on the third day after admission and gradually declined afterward to essentially normal levels on the day of discharge, while soluble IL‐2R levels remained to be elevated by two‐ to threefold. CRP was normalized on the day prior to discharge from hospital. In total, he had been oxygen‐dependent for 12 days (Table 1) (Supplementary Tables S1 and S2).
TABLE 1.
Patient's clinical characteristics
| Characteristics | |
| Age (ys) | 55 |
| Sex | male |
| Duration from first symptom to (days) | |
| Hospitalization | 10 |
| Intensive care | 10 |
| Duration of (days) | |
| Fever | 17 |
| Hospitalization | 15 |
| Therapy | |
| Intensive care | 7 |
| Mechanical ventilation | NA |
| ECMO | NA |
| Oxygen supplementation | 12 |
| Dialysis | NA |
Abbreviations: ECMO, Extracorporal membrane oxygenation; NA, Not applicable.
3. RESULTS AND DISCUSSION
Drugs such as ruxolitinib targeting the “cytokine storm” in severe COVID‐19 infection are currently under clinical investigation (see introduction). By now, the first results on ruxolitinib in the non‐MPN patient population with severe COVID‐19 have been published: a single‐blind randomized trial of 43 patients receiving ruxolitinib vs. placebo, showing numerically faster clinical improvement, as measured by mortality, chest CT and recovery from lymphocytopenia, as well as a favorable side‐effect profile of ruxolitinib. 5 In a second trial, 14 out of 105 patients with a newly developed “COVID‐19 inflammation score (CIS)” of ≥ 10 out of 16 received ruxolitinib, with 11 of these pts showing sustained clinical improvement, as detected by a ≥ 25% CIS improvement and “clinical control,” while grade 3 toxicity was only seen in three patients (anemia, liver toxicity). 6 79% of the 14 pts received glucocorticoids. 6 Skin toxicity was observed upon ruxolitinib treatment in two hydrochloroquine/lopinavor/ritonavir‐pretreated pts with COVID‐19. 7 In a fourth report, two young pts in their 20´s with alopecia areata continued their treatment with ruxolitinib when they developed proven COVID‐19 and showed a mild COVID‐19 course. 8
However, no clinical experience is currently available on ruxolitinib treatment for COVID‐19 in MPN patients. Ruxolitinib has been associated with an increased frequency of infections, including zoster, urinary tract infection, pneumonia, sepsis, and tuberculosis, 9 but also reactivation of hepatitis B, which mandates testing for hepatitis B and tuberculosis before initiation of ruxolitinib treatment. Therefore, both a worsening of the COVID‐19 course due to the immunosuppressive effect of the drug and an improvement due to a positive impact on the cytokine storm are conceivable. However, the patient reported here experienced a rather mild clinical course of COVID‐19 despite significant comorbidities. Such comorbidities are associated with a more severe course of COVID‐19 in cohorts of patients requiring mechanical ventilation. 2 Tachycardia, dyspnea, hypoxia, hypocapnia, and marked bilateral infiltrates were present in our patient upon transfer to the ICU. In addition, the patient showed elevated LDH, creatine kinase, and substantially elevated inflammatory markers, all parameters associated with a more severe disease course in our previous cohort. 2 Surprisingly, the patient did not require mechanical ventilation during the course of infection, cleared the virus within four days, and recovered unusually rapidly from pneumonia. One potential explanation for this rather moderate COVID‐19 course could be the continuing treatment with ruxolitinib for his underlying myelofibrosis. Ruxolitinib had been shown to improve disease‐related splenomegaly and blood count alterations, but also MPN‐related symptoms, fever, and pro‐inflammatory cytokines. 10 Interestingly, the IL‐6 level had been normal (5.4 pg/mL) approximately a year ago in our patient, suggesting that there was an approximately 10‐fold increase during the COVID‐19 infection. However, both CRP and IL‐6 levels remained in the lower range of ARDS‐bearing patients requiring mechanical ventilation in our recently reported COVID‐19 cohort. 2
Excessive levels of pro‐inflammatory cytokines as well as macrophage activation syndrome have been observed during severe courses of COVID‐19, and ruxolitinib has recently shown activity in patients with secondary hemophagocytic lymphohistiocytosis, 11 which is similarly characterized by both cytokine storm and macrophage overactivation.
In conclusion, this report suggests that ruxolitinib treatment is feasible (most likely due to its beneficial effect on the cytokine milieu observed) and not detrimental (due to a potential immunosuppressive effect) in patients with COVID‐19, strongly supporting current recommendations 12 not to stop ruxolitinib treatment if patients with MPN develop COVID‐19. In addition, our results seem to support the rationale underlying ongoing clinical trials with ruxolitinib treatment to prevent cytokine storm in COVID‐19 pneumonia even in the absence of MPN.
CONFLICT OF INTEREST
SK reports honoraria, advisory board honoraria, and travel support by Novartis, Incyte, Celgene, Bristol‐Myers Squibb (BMS), CTI Biopharma, as well as, additionally, research funding by Novartis and BMS. THB reports consultancy for Novartis, Pfizer, Ariad, Janssen, and Merck, research funding from Novartis and Pfizer and honoraria from Pfizer. MD reports consultancy and fees for speaking for Novartis. GM reports honoraria, research funding, advisory board, and travel support by BBraun, Adrenomed, and 4teen4.
AUTHORSHIP CONTRIBUTIONS
SK, EJ, THB, and MD wrote the paper. CC, TM, MS‐H, JB, GM, NM, and MD were involved in primary care of the patient in the ICU and/or on the ward and in the collection of the data. SK, EJ, and THB were hematology consultants. MK performed, analyzed, and interpreted SARS‐CoV2 PCR and serological tests. All authors read and approved the final manuscript.
Supporting information
Table S1‐S2
Koschmieder S, Jost E, Cornelissen C, et al. Favorable COVID‐19 course despite significant comorbidities in a ruxolitinib‐treated patient with primary myelofibrosis. Eur J Haematol. 2020;105:655–658. 10.1111/ejh.13480
Steffen Koschmieder, Edgar Jost, Tim H. Brümmendorf and Michael Dreher equal contribution.
Contributor Information
Tim H. Brümmendorf, Email: tbruemmendorf@ukaachen.de.
Michael Dreher, Email: mdreher@ukaachen.de.
REFERENCES
- 1. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dreher M, Kersten A, Bickenbach J, et al. The characteristics of 50 hospitalized COVID‐19 patients with and without ARDS. Deutsches Ärzteblatt. 2020;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stebbing J, Phelan A, Griffin I, et al. COVID‐19: combining antiviral and anti‐inflammatory treatments. Lancet Infect Dis. 2020;20(4):400‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tefferi A, Pardanani A. Serious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosis. Mayo Clin Proc. 2011;86(12):1188‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID‐19): A multicenter, single‐blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146(1):136‐146. 10.1016/j.jaci.2020.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. La Rosee F, Bremer HC, Gehrke I, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID‐19 with severe systemic hyperinflammation. Leukemia. 2020;34(7):1805‐1815. 10.1038/s41375-020-0891-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaspari V, Zengarini C, Greco S, Vangeli V, Mastroianni A. Side effects of ruxolitinib in patients with SARS‐CoV‐2 infection: Two case reports. International Journal of Antimicrobial Agents. 2020;106023. 10.1016/j.ijantimicag.2020.106023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peterson D, Damsky W, King B. The use of Janus kinase inhibitors in the time of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). J Am Acad Dermatol. 2020;82(6):e223‐e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long‐term findings from COMFORT‐II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30(8):1701‐1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahmed A, Merrill SA, Alsawah F, et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open‐label, single‐centre, pilot trial. Lancet Haematol. 2019;6(12):e630‐e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. COVID‐19‐Empfehlungen aktualisiert; 2020. https://www.onkopedia.com/de/news/covid-19-empfehlungen-aktualisiert. Accessed April 25, 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2


