Abstract
The role of disease‐modifying therapies in patients with autoimmune disorders during severe acute respiratory syndrome coronavirus 2 (SARS‐CoV2) infection is controversial. Immunocompromised patients could have a more severe coronavirus disease‐2019 (COVID‐19) due to the absence of an adequate immune response against the SARS‐CoV‐2. However, therapies that act on immune response could play a protective role by dampening the cytokine‐release syndrome. Fingolimod is a drug used for immune therapy in patients with multiple sclerosis (MS) through the sequestration of activated lymphocytes in the lymph nodes. We report the case of a 57‐year‐old man with relapsing‐remitting MS treated with fingolimod that showed a reactivation of COVID‐19 with signs of hyperinflammation syndrome after fingolimod withdrawal. Our case suggests that discontinuation of fingolimod during COVID‐19 could imply a worsening of SARS‐CoV2 infection.
Keywords: coronavirus, COVID‐19, exacerbation, fingolimod, multiple sclerosis, SARS‐CoV‐2
Highlights
We report a patient with Multiple Sclerosis presenting COVID‐19 when treated with fingolimod.
Hyperinflammation syndrome was observed after fingolimod withdrawal.
Discontinuation of fingolimod during COVID‐19 could imply a worsening of SARS‐CoV‐2 infection.
1. INTRODUCTION
Multiple sclerosis (MS) is an inflammatory autoimmune disease of the central nervous system that causes demyelination and axonal damage. MS etiology involves multiple factors, with environmental factors interacting with a genetic predisposition. There is a notable interest in knowing how severe acute respiratory syndrome coronavirus 2 (SARS‐CoV2) infection can affect patients with MS, treated with disease‐modifying therapies during the COVID‐19 pandemic, which is a controversial issue. 1 Management of MS patients during COVID‐19 pandemic is also relevant considering a possible role of white matter as a virus reservoir, as observed with other coronaviruses. 2 , 3 On one side, immunocompromised patients may have a more severe COVID‐19 disease due to the absence of an adequate immune response against the SARS‐CoV‐2. But on the other, some therapies that act on immune response could play a protective role by dampening the cytokine‐release syndrome. 4 , 5 , 6 Fingolimod (FNG) is a drug used for immune therapy in patients with MS and has been associated with a rebound effect of the inflammatory activity of MS after withdrawal. 7 , 8 , 9 When the drug is withdrawn, patients may suffer an exacerbation because of the lack of therapeutic coverage, or due to clinical variability. 10 The effect of the withdrawal of this drug in patients infected with SARS‐CoV2 is not known. On one hand, it could be helpful as it increases the number of circulating lymphocytes, which should improve the defense against the viral infection; but on the other, it could also be harmful as it increases the risk of cytokine storm. We describe a case in which a significant worsening of COVID‐19 was observed in temporal association with the withdrawal of the drug.
2. CASE REPORT
We report a 57‐year‐old man with a diagnosis of relapsing‐remitting MS since 1996. He started disease‐modifying treatment with interferon β1‐a, which was switched by FNG in 2013 because of the disease activity (ie, persistent clinical and magnetic resonance imaging [MRI] inflammatory activity despite the previous treatment with interferon β1‐a). The last Expanded Disability Status Scale was 6.0, mainly due to left hemiparesis. His lymphocyte count in the previous year ranged from 700 to 900/µL, and the last MRI in 2019 showed a moderate disease burden, without any clinical and MRI sign of disease activity. The last test before admission showed 500 lymphocytes per µL.
He experienced a fall at home in the first week of March 2020. Since then, he started complaining of back pain at the dorsal‐lumbar region that worsened with trunk movements. On March 25, he developed fever and malaise. He was admitted to the hospital, where a temperature of 38°C (100.4°F) was registered. Neurological examination showed no changes in comparison to the last assessment in the MS clinic. Blood tests showed elevated C‐reactive protein (12.83 mg/dL [normal range < 0.50]) and lymphopenia (500/µL). Chest X‐ray was unremarkable (Figure 1A), but a fracture of the sixth thoracic vertebra was confirmed. The real‐time polymerase chain reaction (PCR) for SARS‐CoV‐2 from a nasopharyngeal swab was positive, and the patient was started on hydroxychloroquine for 7 days. FNG was maintained, and during the following 3 weeks, he persisted with intermittent fever, but no respiratory complications (pO2 82 mm Hg, pCO2 35 mm Hg) and normal chest radiographs (Figure 1B,C).
Figure 1.
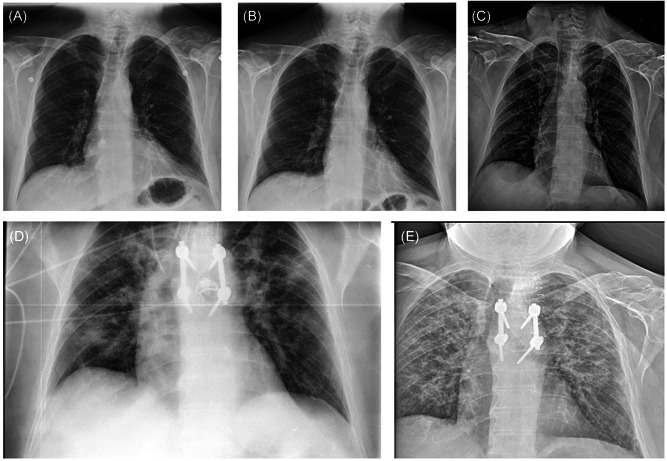
Radiological evolution of coronavirus disease‐2019 (COVID‐19) disease in the patient. Chest X‐ray on days 4 (A), 7 (B), and 14 (C) from the onset of COVID‐19 symptoms, showing no abnormalities. After the suspension of fingolimod (day 25), a radiological worsening is noted on the radiographs of day 30 (D) and day 42 (E)
He underwent back surgery on 18 April due to the risk of spinal instability, but without complications. Two days later, FNG was discontinued due to low lymphocyte count (100/µL). After a week (day 30 since symptom onset), lymphocytes increased (800/µL), but the patient progressively developed fever (38.5°C) and dyspnea, requiring oxygen via nasal cannula. A new chest X‐ray was performed, showing new bilateral infiltrates (Figure 1D). C‐reactive protein persist elevated (6.93 mg/dL). At this moment, pO2 was 70 mm Hg and pCO2 was 36 mm Hg, respectively. Other infectious etiologies were ruled out, and the PCR for SARS‐CoV‐2 remained positive. A delayed clinical exacerbation of COVID‐19 was the main possibility, and thus, methylprednisolone (80 mg/day) was prescribed for a week. 11 The patient progressively improved during the following week, he did not require oxygen supplementation anymore but persisted with an abnormal chest radiograph (Figure 1E) and a positive nasopharyngeal PCR. FNG was restarted after lowering of methylprednisolone, without further complications.
3. DISCUSSION
The consequences of MS therapy on the immune response during SARS‐CoV2 infection appear to be complex. 12 The most relevant mechanism of action of FNG is the sequestration of activated lymphocytes in the lymph nodes. In this regard, Giovannoni et al 13 defined the rebound as the MS exacerbation caused by lymphocyte retrafficking, and there is experimental evidence supporting this mechanism. 14
In this study, we report a patient treated with FNG who developed a mild and long‐lasting course of COVID‐19 disease without respiratory involvement. After discontinuation of FNG due to severe lymphopenia, he progressed with respiratory insufficiency and bilateral pneumonia with signs of hyperinflammation syndrome. Other alternative diagnoses were ruled out, and PCR for SARS‐CoV‐2 remained positive. Overall, this is consistent with a delayed clinical exacerbation of COVID‐19 disease, 1 month after disease onset and coinciding with FNG discontinuation. We hypothesize that FNG may have played a protective role, leading to a milder course of the disease, but discontinuation of FNG could suppose that sequestered infected lymphocytes are released into the bloodstream, and as the circulating viral load increases, the disease reactivates, similar to what happens with relapse rebound. Fortunately, the patient responded to corticosteroid therapy. Three previous cases have recently been published of COVID‐19 in patients taking FNG. 15 , 16 , 17 In the first report, 14 the patient suffered the COVID‐19 under FNG treatment. The drug was not withdrawn and the patient recovered from COVID‐19. The authors suggest the potential beneficial effect of FNG by enhancing lung endothelial cell integrity and reducing cytokine storm. In the second patient, FNG was discontinued due to a low number of lymphocytes, but did not show clinical changes. 14 In the third case, the patient worsened after fingolimod withdrawal, but was controlled with an interleukin‐6 inhibitor drug. 16 It is interesting to compare the number of circulating lymphocytes at the time of FNG withdrawal. It shows how the worsening occurs in those cases where the number was lower, suggesting a greater influx of lymphocytes sequestered into the bloodstream. Hypothetically, it could be explained as an increase in circulating viral load or a greater immune activity (Table 1).
Table 1.
Patients reported with fingolimod (FNG) treatment and concomitant coronavirus disease‐2019 (COVID‐19) infection
Since some immunosuppressive drugs have been suggested as a potential treatment in COVID‐19 to prevent or treat cytokine storm, 18 our case may support the idea of maintaining FNG in patients with MS presenting mild forms of COVID‐19. In addition, our case may suggest that FNG discontinuation during the active infection may worsen COVID‐19. Our report suggests that FNG withdrawal could trigger worsening of the clinical situation of COVID‐19, which could be transient 19 and probably suggests the withdrawal of this drug should be done though gradual dose reduction (eg alternating days) while monitoring lymphocyte counts to avoid a rapid increase. Additionally, this case seems to suggest a potential beneficial effect of FNG through lymphocyte sequestration, which may be of interest in other situations that affect COVID‐19. However, further research is necessary to confirm these preliminary findings and clarify the best management in patients with MS developing COVID‐19 infection.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
The study about the effects of COVID‐19 in MS was approved by the Clinical Research Ethics Committee of Hospital Clínico San Carlos [ref. 20/242‐E]). Oral consent was obtained from the patient described in this article. Data were handled in observance of Spanish legislation on data protection (Organic Law 15/1999 of 13 December). The study complies with the principles of the Declaration of Helsinki (“Recommendations guiding doctors in biomedical research involving human subjects,” Helsinki 1964, modified in October 2013).
Gomez‐Mayordomo V, Montero‐Escribano P, Matías‐Guiu JA, González‐García N, Porta‐Etessam J, Matías‐Guiu J. Clinical exacerbation of SARS‐CoV2 infection after fingolimod withdrawal. J Med Virol. 2021;93:546–549. 10.1002/jmv.26279
REFERENCES
- 1. Giovannoni G, Hawkes C, Lechner‐Scott J, Levy M, Waubant E, Gold J. The COVID‐19 pandemic and the use of MS disease‐modifying therapies. Mult Scler Relat Disord. 2020;39:102073. 10.1016/j.msard.2020.102073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burks JS, DeVald BL, Jankovsky LD, Gerdes JC. Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science. 1980;209:933‐934. 10.1126/science.7403860 [DOI] [PubMed] [Google Scholar]
- 3. Matías‐Guiu J, Gomez‐Pinedo U, Montero‐Escribano P, Gomez‐Iglesias P, Porta‐Etessam J, Matias‐Guiu JA. Should we expect neurological symptoms in the SARS‐CoV‐2 epidemic? Neurologia. 2020;35:170‐175. 10.1016/j.nrl.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Giovannoni G. Anti‐CD20 immunosuppressive disease‐modifying therapies and COVID‐19. Mult Scler Relat Disord. 2020;41:102135. 10.1016/j.msard.2020.102135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montero‐Escribano P, Matías‐Guiu J, Gómez‐Iglesias P, Porta‐Etessam J, Pytel V, Matias‐Guiu JA. Anti‐CD20 and COVID‐19 in multiple sclerosis and related disorders: a case series of 60 patients from Madrid, Spain. Mult Scler Relat Disord. 2020;42:102185. 10.1016/j.msard.2020.102185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matias‐Guiu J, Montero‐Escribano P, Pytel V, Porta‐Etessam J, Matias‐Guiu JA. Potential COVID‐19 infection in patients with severe multiple sclerosis treated with alemtuzumab. Mult Scler Rel Disord. 2020;44:102297. 10.1016/j.msard.2020.102297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatcher SE, Waubant E, Nourbakhsh B, Crabtree‐Hartman E, Graves JS. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol. 2016;73:790‐794. 10.1001/jamaneurol.2016.0826 [DOI] [PubMed] [Google Scholar]
- 8. Andrade C. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol. 2016;73:1375‐1376. 10.1001/jamaneurol.2016.3507 [DOI] [PubMed] [Google Scholar]
- 9. Merschhemke M, Tomic D, Putzki N. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol. 2016;73:1375. 10.1001/jamaneurol.2016.3199 [DOI] [PubMed] [Google Scholar]
- 10. Voskuhl R. Rebound relapses after ceasing another disease‐modifying treatment in patients with multiple sclerosis: are there lessons to be learned? JAMA Neurol. 2016;73:775‐776. 10.1001/jamaneurol.2016.0934 [DOI] [PubMed] [Google Scholar]
- 11. Johnson RM, Vinetz JM. Dexamethasone in the management of COVID‐19. BMJ. 2020;340:m2648. [DOI] [PubMed] [Google Scholar]
- 12. Baker D, Amor S, Kang AS1, Schmierer K1, Giovannoni G. The underpinning biology relating to multiple sclerosis disease modifying treatments during the COVID‐19 pandemic. Mult Scler Relat Disord. 2020;43:102174. 10.1016/j.msard.2020.102174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giovannoni G, Hawkes C, Waubant E, Lublin F. The 'Field Hypothesis': rebound activity after stopping disease‐modifying therapies. Mult Scler Relat Disord. 2017;15:A1‐A2. 10.1016/j.msard.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 14. Cavone L, Felici R, Lapucci A, et al. Dysregulation of sphingosine 1 phosphate receptor‐1 (S1P1) signaling and regulatory lymphocyte‐dependent immunosuppression in a model of post‐fingolimod MS rebound. Brain Behav Immun. 2015;50:78‐86. 10.1016/j.bbi.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 15. Barzegar M, Mirmosayyeb O, Nehzat N, et al. COVID‐19 infection in a patient with multiple sclerosis treated with fingolimod. Neurol Neuroimmunol Neuroinflamm. 2020;7:e753. 10.1212/NXI.0000000000000753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foerch C, Friedauer L, Bauer B, Wolf T, Adam EH. Severe COVID‐19 infection in a patient with multiple sclerosis treated with fingolimod. Mult Scler Relat Disord. 2020;42:102180. 10.1016/j.msard.2020.102180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valencia‐Sanchez C, Wingerchuk DM. A fine balance: immunosuppression and immunotherapy in a patient with multiple sclerosis and COVID‐19. Mult Scler Relat Disord. 2020;42:102182. 10.1016/j.msard.2020.102182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chakraborty C, Sharma AR, Bhattacharya M, Sharma G, Lee SS, Agoramoorthy G. COVID‐19: consider IL‐6 receptor antagonist for the therapy of cytokine storm syndrome in SARS‐CoV‐2 infected patients. J Med Virol. 2020. 10.1002/jmv.26078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiarini M, Paghera S, Moratto D, et al. Immunologic characterization of a immunosuppressed multiple sclerosis patient that recovered from SARS‐CoV‐2 infection. Neuroimmunology. 2020;345:577282. 10.1016/j.jneuroim.2020.577282 [DOI] [PMC free article] [PubMed] [Google Scholar]


