Abstract
Bruton’s tyrosine kinase (BTK) signaling is involved in innate immune responses and regulates the production of proinflammatory cytokines that can contribute to COVID-19 immunopathology. Clinical trials with BTK inhibitors in COVID-19 treatment have been proposed, and previous studies have attempted to investigate the therapeutic effects of ibrutinib and underlying mechanisms in treating viral pneumonia. These attempts, however, did not consider potential off target effect of BTK inhibitors on T cell differentiation, function, and survival, which may be beneficial in treatment for COVID-19. Here, we summarize the current knowledge of BTK/IL-2-inducible T-cell kinase (ITK) signaling in immunopathology and lymphopenia and discuss the potential of BTK/ITK dual inhibitors such as ibrutinib in modulating immunopathology and lymphopenia, for COVID-19 therapy.
Keywords: BTK, COVID-19, immunopathology, ITK, lymphopenia, therapy
Graphical Abstract
Examines BTK/ITK signaling in immunopathology and lymphopenia and describes the potential of BTK/ITK dual inhibitors such as ibrutinib in COVID-19 therapy.
Introduction
Prior to the end of 2019, severe acute respiratory syndrome (SARS) was a specific term referring to SARS-coronavirus (SARS-CoV)-induced respiratory disease. In December 2019, a cluster of SARS-like pneumonia cases emerged in Wuhan, China. The etiologic agent was later determined to be a novel beta-coronavirus and termed SARS-CoV-2, while the associated disease was named coronavirus disease of 2019 (COVID-19). SARS-CoV-2 is the third respiratory coronavirus to have caused an outbreak in the last 2 decades, along with SARS-CoV that emerged in 2002 and Middle East respiratory syndrome (MERS)-CoV that emerged in 2012. The majority of COVID-19 cases are classified as mild to moderate. However, the disease can progress to severe pneumonia, acute respiratory distress syndrome (ARDS), and multiorgan failure, most of which are fatal.1 Patients with COVID-19 display a dysregulated immune response. Elevated levels of the proinflammatory cytokines and chemokines were observed in sera of patients admitted to the intensive care unit in Wuhan, China.1 An overrepresentation of proinflammatory macrophages has been observed in the bronchoalveolar lavage (BAL) of severe cases compared with mild cases,2 and elevated IL-6 in the sera is correlated with higher mortality.3 Lymphopenia and increased number of blood neutrophils are associated with severe and fatal COVID-19.4 These observations suggest that targeting the host’s immune response including those leading to cytokine release syndrome (CRS) may be beneficial in treating immunopathology and the associated severe symptoms of the infection (Fig. 1). We write here to draw attention to lymphopenia and the potential of modulating T cells through targeting IL-2-inducible T-cell kinase (ITK) using Bruton’s tyrosine kinase (BTK)/ITK dual inhibitors being evaluated for COVID-19 therapy.
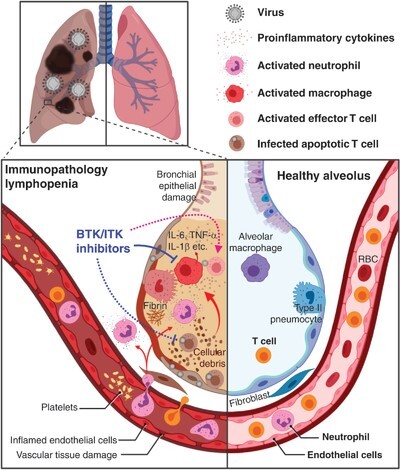
FIGURE 1.
Potential of BTK/ITK inhibitors for attenuating immunopathology and lymphopenia in COVID-19. SARS-CoV-2 infection in the lungs set off proinflammatory cytokine production by lung cells and immune cells such as macrophages and neutrophils. Cytokine release syndrome further engages pulmonary and vascular tissue damages, leukocyte recruitment, T cell activation, and other cytotoxic immune responses. T cells are possible targets of SARS-CoV-2 infection. Infected and over reactive T cells may be prompted toward apoptosis and cytolysis, resulting in infection-induced lymphopenia. BTK/ITK inhibitors may function to down-regulate proinflammatory cytokine production by innate immune populations and reduce cytotoxic T cell death while sustaining virus-specific effector T cell function, therefore exhibit therapeutic functions against immunopathology and lymphopenia. Solid-line arrows indicate known functions and dashed-line arrows indicate functions awaiting investigation
Immune Therapies Targeting CRS in Covid-19: BTK Inhibitors in the Arena
Immune therapies targeting the COVID-19-associated cytokine storm are currently being explored. Drugs that have already been approved by the United States Food and Drug Administration (US FDA) would be advantageous during this process as they would be easier to repurpose. Tocilizumab, a monoclonal antibody that blocks IL-6 signaling, is US FDA approved for treatment of rheumatoid arthritis and CRS. In early February 2020, a preliminary study in China using tocilizumab along with routine treatment, on 21 severe and critical COVID-19 patients, showed encouraging therapeutic results.5 And in the US, Roche initiated a randomized, double-blind, placebo-controlled, multicenter phase III trial of tocilizumab in severe COVID-19 patients (NCT0432061), starting on April 3, 2020. The encouraging results of the tocilizumab trial in China also motivates assessments of therapeutic strategies targeting the expression, receptor binding, and downstream signaling of proinflammatory cytokines such as IL-6, IL-1, TNF-α, type I IFN, and IL-17A. BTK is highly expressed in B cells, but is also known to be involved in signaling pathways of multiple TLRs, macrophages, and dendritic cells leading to induction of proinflammatory cytokines, including the antiviral cytokine IFN-β.6 The TLR/BTK pathway signals through the downstream NF-κB, which is up-regulated in proinflammatory macrophages that dominate the airways of severe COVID-19 patients compared with mild.2 Ex vivo analysis of macrophages from severe COVID-19 patients found higher levels of BTK phosphorylation and higher IL-6 production at resting state and when stimulated with a TLR7/8 agonist compared with the healthy controls.7 Furthermore, activation of the NLRP3 inflammasome requires BTK to convert pro-IL-1β into its active form.6 Based on the role of BTK in the production of inflammatory cytokines, clinical trials for the selective BTK inhibitors acalabrutinib and zanubrutinib on COVID-19 have been initiated by AstraZeneca (Acalabrutinib: NCT04346199) and BeiGene (Zanubrutinib: NCT04382586), respectively. Furthermore, a small off-label clinical study of severe COVID-19 patients treated with acalabrutinib for 10–14 days found the levels of IL-6 in serum decreased during this time.7 By the end of treatment, 8 out of 11 and 2 out of 8 patients that began on supplemental oxygen or mechanical ventilation, respectively, had been discharged on room air.7 In clinical practice, acalabrutinib is US FDA approved for treating chronic lymphocytic leukemia (CLL), and zanubrutinib received accelerated approval for treating mantle cell lymphoma (MCL).
Dysregulated T Lymphocytes in Severe Covid-19
A robust and balanced adaptive immune response is important for both viral clearance and limiting immunopathology. SARS-CoV-2 infection however triggers significant lymphocyte apoptosis, as revealed by postmortem examinations of patients succumbed to fatal COVID-19.8 In addition to the lymphopenia observed in severe COVID-19 cases, there is also evidence suggesting T cell dysfunction positively associated with the severity of COVID-19.9,10 Moreover, delayed development of an adaptive immune response was associated with mortality during SARS-CoV outbreak during 2002–2004.11 An increased frequency of exhausted CD8+ T cells and reduced frequency of functional cytotoxic CD8+ T cells in the peripheral blood of COVID-19 patients have been reported.9 In addition, a reduced proportion of IFN-γ+ and TNF-α+ CD4+ T cells, coinciding with CD8+ T cells expressing increased levels HLA-DR and TIGIT, has been reported in severe cases.9 These activation and exhaustion phenotypes of T cells were mainly observed in PBMCs from COVID-19 patients. Single cell RNA sequencing of cells in the BAL suggests reduced frequency of CD8+ T cells among the total immune cell population in severe COVID-19 patients compared with the mild cases.2 Mild cases had a highly expanded effector CD8+ T cells in the airway, with a more diverse T cell repertoire. By contrast, there is far less expansion and diversity of CD8+ T cells in the BAL of severe patients.2 It is not yet clear whether this represents delayed kinetics of the adaptive response or reduced survival of the highly activated effector CD8+ T cells. The functional evaluation of immune cells in the respiratory system, in particular, lymphocyte activation and function as identified by biomarkers at protein level and functional assays awaits further investigation. Regardless, current data suggest that T cell dysfunction may be a contributing factor in COVID-19. Clinical trials targeting lymphocytes are ongoing or have been proposed, including IL-7 treatment in lymphopenic COVID-19 patients (http://NCT04379076, http://NCT04426201) and low-dose IL-2 treatment to expand Treg cells and limit immunopathology (http://NCT04357444).
SARS-CoV-2 Infection in T Lymphocytes
This speculation is further supported by recent findings that SARS-CoV-2 is able to enter human T cells, although replication inside T cells was not observed (as determined via RT-qPCR with probes targeting the viral Nucleocapsid, 5′ UTR, or Envelope genes).12,13 Despite the apparent lack of viral genome replication, the SARS-CoV-2 nucleocapsid protein can persist within T cells up to 24 h postinfection13; and viral particles could be observed via electron microscopy in SARS-CoV-2-infected primary PBMCs (from healthy donors) in CD4+ T cells but not CD8+ T cells, at 48 h post in vitro infection.12 Given that this was observed with PBMCs infected in vitro, further studies are required to determine whether this represents a true preference in COVID-19. Although the consequences of T cell infection by SARS-CoV-2 have not been investigated at this time, MERS-CoV has been reported to be able to infect and induce apoptosis of human T cells, with a preference for CD4+ over CD8+ T cells.14 Lymphopenia is positively associated with severity and fatality of COVID-19 cases,4 and there is a possibility that virus-induced apoptosis may be a potential explanation. It should be stressed that at the time of writing this article, the consequences of viral entry into human T cells is unknown. However, although potential SARS-CoV-2 infection of T cells is a fascinating finding, much more investigation is required to get a better understanding of the mechanism of its entry, consequences on T cell function, and its physiologic impact in COVID-19 patients.
Potential of BTK/ITK Dual Inhibitors for Targeting CRS and Promoting T Cell Function
Given this backdrop, targeting molecules that play critical signaling functions in T lymphocytes, ideally those that preferentially regulate T cell apoptosis and exhaustion over activation, may be a potential strategy for treating patients with severe COVID-19. ITK is highly expressed in T cells, and regulates the activation and function of both CD4+ and CD8+ T cells, including cytokine production and cytotoxic function.15 In addition to acalabrutinib and zanubrutinib, ibrutinib is also a US FDA-approved BTK inhibitor, but is less specific as it also inhibits ITK and TEC kinases, both of which are highly expressed in T cells. Ibrutinib has been approved for treating CLL, MCL, chronic graft-versus-host disease, and others.
High expression of Fas coinciding with increased lymphocyte apoptosis has been observed in the lymph nodes and spleens of patients that died of COVID-19 compared with the healthy controls.8 Along with alterations in B cells, CLL patients treated with ibrutinib displayed an increase in total number of T cells, which is not seen in patients treated with the more specific acalabrutinib.16 The observed accumulation of T cells may be due to a decrease in activation-induced cell death, which has been shown to be a result of the ITK signaling-mediated up-regulation of FasL, which promotes activation-induced T cell death. Indeed, human T cells stimulated in vitro in the presence of ibrutinib express lower levels of FasL and undergo apoptosis at a lower rate.16 This is consistent with findings of the mouse models of OVA-induced allergic asthma where inhibition of ITK resulted in an increase in total CD4+ T cells coinciding with a decreased T cell apoptosis in the spleen and draining lymph node.17
Several lines of evidence suggest a Th1 response is critical in controlling respiratory coronavirus infection. Convalescent COVID-19 patients display predominately a Th1-polarized SARS-CoV-2-specific memory T cell response.18–21 Mice vaccinated with virus replicon particles expressing SARS-CoV or MERS-CoV N protein resulted in IFN-γ+ CD4+ T cell-dependent protection from challenge,22 whereas mouse models of SARS-CoV infection found the recruitment of IFN-γ+ CD4+ cells to be an important event in controlling virus replication and lung immunopathology during primary infection.23 Ibrutinib can irreversibly bind to ITK, but does not affect Th1 and CD8+ T cell effector function, likely because of the expression of redundant Tec family kinases, such as RLK, which is not targeted by ibrutinib.24 Furthermore, ibrutinib can functionally restore antigen-specific Th1 and CD8+ effector T cells in a mouse model of leukemia/listeriosis.24
In a mouse model of ARDS induced by lethal dose infection with influenza A H1N1 virus, ibrutinib exhibited therapeutic effects in reducing the production of inflammatory cytokines, lung tissue damage, and mortality.25 In patients who were on ibrutinib treatment for Waldenstrom’s Macroglobulinemia and recently diagnosed with COVID-19 (6 patients reported), 5 out of 6 continued with regular dosage of ibrutinib and developed very mild COVID-19 symptoms, whereas 1 out of 6 held ibrutinib after contracting COVID-19 needed to be hospitalized because of severe symptoms and had to restart ibrutinib treatment; eventually, all recovered from the infection.26 Similarly, in patients who were on BTK inhibitor treatment for CLL, 2 (out of 8) who continued with regular dosage of ibrutinib after contracting COVID-19 developed milder symptoms and required significantly shorter time of hospitalization than 6 (out of 8) who held BTK inhibitor treatment.27 It is unclear whether ibrutinib treatment changed viral growth in the hosts, altered T cell activation, survival and function, and so provided benefits in disease controls against viral pneumonia. All current studies focused the discussion of the effects of ibrutinib on macrophage and cytokine-mediated lung inflammation, without mentioning the potential roles of T cell immunity.
Concluding Remarks
The potential of modulating T cells through BTK/ITK dual inhibitors such as ibrutinib for COVID-19 therapy has not been considered to date. A clinical trial using ibrutinib to treat COVID-19 (NCT04375397) began in early May 2020. When interpreting results of clinical trials of BTK inhibitors in COVID-19, along with anti-inflammatory therapeutic effects and the potential adverse effects/toxicity of these drug candidates,28 the effects of such inhibitors on T cell activation, survival, and function will need to be fully considered and carefully evaluated (Fig. 1). We have provided a brief summary here of the rapidly evolving understanding of the lymphocyte response during COVID-19, and how our current knowledge of the Tec family kinases (BTK and ITK) can be utilized to improve outcomes. Further exploration of these kinase pathways may help contribute to the broadening arsenal of approaches for treating COVID-19.
Authorship
M.C.M., A.A., and W.H. wrote the manuscript.
Acknowledgments
We thank Dr. Lu Lu at Fudan University, Dr. Wanli Liu at Tsinghua University, and Dr. Mobin Karimi at SUNY Upstate Medical University for helpful discussions. Research related to Tec family kinases, T cell functions in viral infection and lung inflammation in the authors’ laboratories is supported in part by grants from the National Institutes of Health (R01AI120701, R01AI138570, R21AI129422, R56AI146226, R21AI137822, and P20GM130555-6610).
Abbreviations
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar lavage
- BTK
Bruton’s tyrosine kinase
- CLL
chronic lymphocytic leukemia
- COVID-19
coronavirus disease of 2019
- CRS
cytokine release syndrome
- US FDA
United States Food and Drug Administration
- ITK
IL-2-Inducible T-cell kinase
- MCL
mantle cell lymphoma
- MERS
Middle East respiratory syndrome
- SARS
severe acute respiratory syndrome
- SARS-CoV
SARS-coronavirus
- UTR
untranslated region
Contributor Information
Michael C McGee, Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, Louisiana, USA.
Avery August, Department of Microbiology & Immunology, College of Veterinary Medicine, Cornell University, Ithaca, New York, USA.
Weishan Huang, Department of Pathobiological Sciences, School of Veterinary Medicine, Louisiana State University, Baton Rouge, Louisiana, USA; Department of Microbiology & Immunology, College of Veterinary Medicine, Cornell University, Ithaca, New York, USA.
Disclosures
W.H. receives research support from MegaRobo Technologies Corporation. A.A. receives research funding from 3M Company. M.C.M. declares no competing financial interests.
References
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26:842-844. [DOI] [PubMed] [Google Scholar]
- Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intens Care Med. 2020;46:846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber ANR, Bittner Z, Liu X, Dang TM, Radsak MP, Brunner C. Bruton’s tyrosine kinase: an emerging key player in innate immunity. Front Immunol. 2017;8:1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschewski M, Lionakis MS, Sharman JP, et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci Immunol. 2020;5:eabd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Diao B, Wang R, et al. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv. 2020, 10.1101/2020.03.27.20045427. [DOI] [Google Scholar]
- Zheng HY, Zhang M, Yang CX, et al. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol Immunol. 2020;17:541-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MJ, Ran L, Xu L, et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol. 2007;81:8692-8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Nasir JA, Budylowski P, et al. Isolation, sequence, infectivity and replication kinetics of SARS-CoV-2. bioRxiv. 2020, 10.1101/2020.04.11.037382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xu W, Hu G, et al. SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Zhou J, Wong BH, et al. Middle east respiratory syndrome coronavirus efficiently infects human primary T lymphocytes and activates the extrinsic and intrinsic apoptosis pathways. J Infect Dis. 2016;213:904-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solouki S, August A, Huang W. Non-receptor tyrosine kinase signaling in autoimmunity and therapeutic implications. Pharmacol Ther. 2019;201:39-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127:3052-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Peng I, Webster JD, et al. Inhibition of the kinase ITK in a mouse model of asthma reduces cell death and fails to inhibit the inflammatory response. Sci Signal. 2015;8:ra122. [DOI] [PubMed] [Google Scholar]
- Peng Y, Mentzer AJ, Liu G, et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent COVID-19 patients. bioRxiv. 2020, 10.1101/2020.06.05.134551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020, 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D, Schmitz KS, Raadsen MP, et al. Phenotype of SARS-CoV-2-specific T-cells in COVID-19 patients with acute respiratory distress syndrome. medRxiv. 2020, 10.1101/2020.04.11.20062349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidleman J, Luo X, Frouard J, et al. SARS-CoV-2-specific T cells exhibit unique features characterized by robust helper function, lack of terminal differentiation, and high proliferative potential. bioRxiv. 2020, 10.1101/2020.06.08.138826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhao J, Mangalam AK, et al. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lau YF, Lamirande EW, et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: cD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84:1289-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence JM, Krupa A, Booshehri LM, Davis SA, Matthay MA, Kurdowska AK. Inhibiting Bruton’s tyrosine kinase rescues mice from lethal influenza-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2018;315:L52-L58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treon SP, Castillo JJ, Skarbnik AP, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135:1912-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaud S, Tremblay D, Bhalla S, Zimmerman B, Sigel K, Gabrilove J. Protective role of Bruton tyrosine kinase inhibitors in patients with chronic lymphocytic leukaemia and COVID-19. Br J Haematol. 2020, 10.1111/bjh.16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paydas S. Management of adverse effects/toxicity of ibrutinib. Crit Rev Oncol Hematol. 2019;136:56-63. [DOI] [PubMed] [Google Scholar]


