Abstract
Background
Although type 2 diabetes mellitus (T2DM) patients with coronavirus disease 2019 (COVID‐19) develop a more severe condition compared to those without diabetes, the mechanisms for this are unknown. Moreover, the impact of treatment with antihyperglycemic drugs and glucocorticoids is unclear.
Methods
From 1584 COVID‐19 patients, 364 severe/critical COVID‐19 patients with clinical outcome were enrolled for the final analysis, and patients without preexisting T2DM but elevated glucose levels were excluded. Epidemiological data were obtained and clinical status evaluation carried out to assess the impact of T2DM and its management on clinical outcomes.
Results
Of 364 enrolled severe COVID‐19 inpatients, 114 (31.3%) had a history of T2DM. Twenty‐seven (23.7%) T2DM patients died, who had more severe inflammation, coagulation activation, myocardia injury, hepatic injury, and kidney injury compared with non‐DM patients. In severe COVID‐19 patients with T2DM, we demonstrated a higher risk of all‐cause fatality with glucocorticoid treatment (adjusted hazard ratio [HR], 3.61; 95% CI, 1.14‐11.46; P = .029) and severe hyperglycemia (fasting plasma glucose ≥11.1 mmol/L; adjusted HR, 11.86; 95% CI, 1.21‐116.44; P = .034).
Conclusions
T2DM status aggravated the clinical condition of COVID‐19 patients and increased their critical illness risk. Poor fasting blood glucose (≥ 11.1 mmol/L) and glucocorticoid treatment are associated with poor prognosis for T2DM patients with severe COVID‐19.
Keywords: antihyperglycemic drugs, clinical status, coronavirus disease 2019 (COVID‐19), glucocorticoid, type 2 diabetes
Highlights
Type 2 diabetes mellitus (T2DM) patients with severe acute respiratory syndrome coronavirus 2 infection had more severe inflammation, coagulation activation, myocardia injury, hepatic injury, and kidney injury. T2DM aggravated the clinical status of coronavirus disease 2019 (COVID‐19) patients and increased their critical illness rate and mortality.
Glucocorticoid treatment and poor fasting blood glucose (≥11.1 mmol/L) control were found to be risk factors of fatality in T2DM patients with severe COVID‐19.
摘要
背景
虽然2019冠状病毒病(COVID‐19)合并2型糖尿病(T2DM)患者比未患糖尿病的患者病情更加严重, 但其发病机制尚不清楚。此外, 降糖药和糖皮质激素治疗对COVID‐19的影响尚不清楚。
方法
对我院1584例COVID‐19住院患者的临床资料进行回顾性分析。我们排除了既往无T2DM但血糖升高的患者, 得到364例重型/危重型COVID‐19患者作为最终分析对象, 收集其流行病学资料和临床转归, 以评估T2DM及其治疗对COVID‐19临床预后的影响。
结果
在364例重型/危重型COVID‐19住院患者中, 114例(31.3%)有T2DM病史。27例(23.7%)T2DM患者死亡, 这些患者比非糖尿病患者具有更严重的炎症反应、凝血激活、心肌损伤、肝损伤和肾损伤。在T2DM合并COVID‐19重症患者中, 糖皮质激素治疗(校正HR, 3.61;95%CI, 1.14‐11.46;P=0.029)和严重高血糖(空腹血糖≥11.1 mmol/L;校正HR, 11.86;95%CI, 1.21‐116.44;P=0.034)的全因死亡风险更高。
结论
T2DM加重COVID‐19病情并增加其危重症风险。空腹血糖不良(≥11.1mmol/L)和糖皮质激素治疗与重症COVID‐19患者预后不良相关。
Keywords: 降糖药, 临床状况, 2019冠状病毒病, 糖皮质激素, 2型糖尿病。
1. INTRODUCTION
The pandemic of coronavirus disease 2019 (COVID‐19) has now infected over 14 million people worldwide. It has had a catastrophic impact on human lives, particularly the elderly and those with comorbidities. 2 , 3 People with type 2 diabetes mellitus (T2DM) appear to have a higher risk for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection with a prevalence of 5% to 20% 2 , 3 , 4 , 5 , 6 , 7 because they are generally older and often have other comorbidities. Previous studies have also shown that diabetes is a risk factor for severe cases of viral infections, including SARS, Middle East respiratory syndrome (MERS), and novel influenza A (H1N1). 8 , 9 , 10 , 11 Moreover, most diabetes patients with COVID‐19 end up with a severe form of the disease. 3 In a series of 174 inpatients with COVID‐19, 24 of whom had diabetes, there was a rapid progression of the chest infection necessitating a chest CT scan test within 24 to 48 hours, 12 a worse prognosis, and higher risk/percentage to develop composite endpoints 3 in the diabetes patients. More importantly, diabetes patients appear to have a higher fatality rate. 3 , 13
Patients with diabetes may be susceptible to more severe SARS‐CoV‐2 infection due to immune system dysfunction. 14 Viral infections could also induce a diabetes state or worsen hyperglycemia in people with diabetes, which may adversely influence prognosis. 15 , 16 , 17 , 18 Moreover, glucocorticoid use may further aggravate the situation. Antihyperglycemic treatments may be limited because some oral drugs (ie, metformin) are potentially harmful to COVID‐19 patients with hypoxia. In this observational study, we characterized risk factors for severe COVID‐19 with and without T2DM and described the effects of commonly prescribed antihyperglycemic drugs and glucocorticoid therapy on clinical outcomes in hospitalized T2DM patients with severe COVID‐19, which still remain unclear. 19 , 20 , 21 , 22
2. METHODS
2.1. Study design and participants
The diagnosis and clinical classification (mild, moderate, severe, and critical) of COVID‐19 patients were carried out by two independent doctors based on the Guideline of Novel Coronavirus Pneumonia (seventh Edition) issued by the Chinese National Health Commission. 23 Real‐time reverse transcription polymerase chain reaction (RT‐PCR) assay for SARS‐CoV‐2 was performed based on the recommendation by the National Institute for Viral Disease Control and Prevention (China). 24 All enrolled patients were confirmed COVID‐19 cases with RT‐PCR and admitted to Renmin Hospital of Wuhan University from 30 January 2020, when the first COVID‐19 patient was admitted, to 26 April 2020, when the last COVID‐19 patient was discharged. The clinical outcomes (cured or died) and laboratory parameters on admission and endpoint were recorded. This case series study was approved by the institutional ethics board of Renmin Hospital of Wuhan University (No. WDRY2020‐K081). Written, informed consent was waived in light of the urgent need to collect the data for this study.
2.2. Data collection
The Chinese guideline classified the patients into five categories: asymptomatic (positive virology test without symptoms), mild (symptoms), moderate (CT scan test for viral pneumonia), severe (oxygen saturation ≤ 93% or oxygenation index <300), and critical (require intensive care unit admission or invasive oxygen treatment). 23
We encountered many difficulties in justifying requests for some tests during the outbreak of COVID‐19. Oral glucose tolerance tests to diagnose T2DM and glycosylated hemoglobin were not routinely requested since they were considered low priorities for COVID‐19 patients, making the diagnosis of new onset T2DM screening impossible. We therefore included patients with known T2DM history in the diabetes group and excluded patients with elevated glucose (fasting blood glucose ≥6.1 mmol/L or random glucose ≥11.1 mmol/L) from the nondiabetic group to make the analysis more coherent.
Epidemiological, clinical characteristics, laboratory parameters, clinical status, and outcomes were obtained from the electronic medical records of Renmin Hospital of Wuhan University. The data were entered and cross‐reviewed by at least two independent team members. Information recorded included demographic data, medical history, underlying comorbidities, symptoms, signs, laboratory findings (eg, random blood glucose on admission, cellular immunity, metabolic enzymes, and other biochemical parameters), treatment measures (eg, oxygen therapy and ventilator use), and drugs (eg, insulin, antihyperglycemic agents, and glucocorticoids use). Fasting plasma glucose (FPG) was measured for all patients during hospitalization.
Comorbidities, including diabetes, cerebral diseases, cardiovascular diseases, chronic renal diseases, digestive diseases, pulmonary diseases, and surgical history were defined as documented history in the admission notes. Cerebral diseases refer to cerebral infarction, epilepsy, Alzheimer disease, and Parkinson disease. Cardiovascular diseases refer to hypertension, coronary heart disease, arrhythmia, cardiomyopathy, and heart failure. Chronic renal diseases refer to chronic renal insufficiency, chronic renal failure, chronic nephritis, and nephrotic syndrome. Digestive diseases refer to gastritis, gastric and duodenal ulcer, enteritis, cholecystitis, and pancreatitis. Pulmonary diseases refer to asthma, chronic bronchitis, emphysema, chronic obstructive pulmonary disease, tuberculosis, pulmonary embolism, and interstitial pneumonia. Surgical history refers to major abdominal surgery, brain surgery, and cardiothoracic surgery including cardiac bypass, lung surgery, etc.
Clinical Status Evaluation. A five‐category ordinal scale of clinical status was used for ventilation status evaluation which ranged from 1 to 5 25 : 1‐not requiring supplemental oxygen, 2‐requiring low‐flow oxygen therapy, 3‐requiring high‐flow nasal cannula oxygen therapy, 4‐requiring noninvasive mechanical ventilation, and 5‐requiring extracorporeal membrane oxygenation (ECMO)/invasive mechanical ventilation (IMV). The National Early Warning Score 2 (NEWS2) is an aggregated weighted score of 0 to 20 based on measurements of heart rate, systolic blood pressure, arterial oxygen saturation, respiratory rate, level of consciousness, temperature, and supplemental oxygen. 26
2.3. Statistical analysis
Categorical variables were described as frequency and percentages (%), and continuous variables were described with median and interquartile range (IQR) values. Means for continuous variables were compared using independent group t tests when the data were normally distributed; otherwise, the Mann‐Whitney test was used. Proportions for categorical variables were compared using Fisher exact test or the χ 2 test. Logistic regression analysis was used to analyze independent risk factors for mortality of COVID‐19 patients. The risk for composite endpoints and the corresponding hazard ratio (HR) were analyzed using the Cox proportional hazards model. The cumulative rates of death were plotted by applying the Kaplan‐Meier method. All statistical analyses were performed using SPSS 22.0 (IBM Software, Armonk, New York) and GraphPad Prism 8 (GraphPad, San Diego, California), and P < .05 was considered statistically significant.
3. RESULTS
3.1. Study sample
From 30 January 2020 to 26 April 2020, 1584 patients diagnosed with COVID‐19 were admitted to Renmin Hospital of Wuhan University, 1093 of whom were admitted in the first month from 30 January to 29 February 2020. After excluding patients with pregnancy (n = 30) and those transferred to other facilities (n = 243), 274 mild/moderate cases and 546 severe/critical cases were included in the study sample. Patients with fasting glucose ≥6.1 mmol/L or random glucose ≥11.1 mmol/L in those without a previous diagnosis of T2DM (n = 182) were excluded. This left 114 patients with T2DM history and 250 patients without T2DM history (Figure 1).
FIGURE 1.
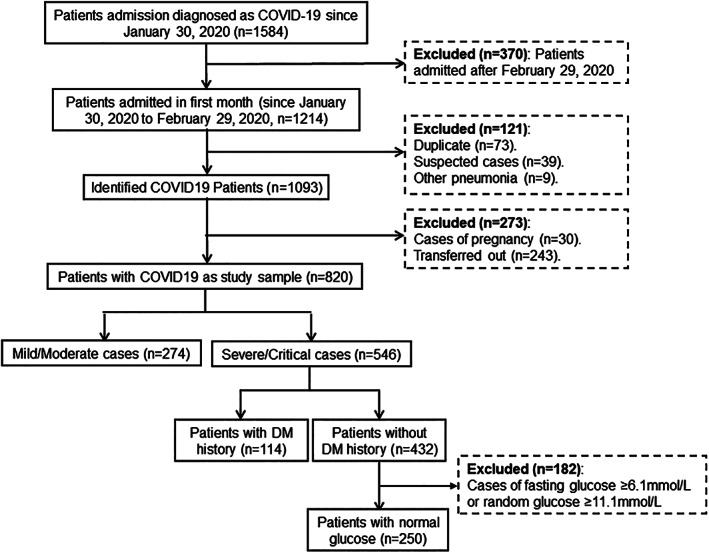
Flow diagram showing enrollment of COVID‐19 inpatients and the recruitment of severe/critical cases with and without a history of type 2 diabetes
3.2. Presenting characteristics
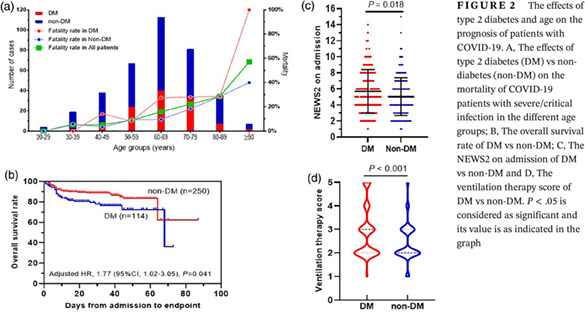
Of the 364 confirmed severe/critical COVID‐19 patients, 305 (83.8%) were discharged and 59 (16.2%) died. A total of 66.5% (242) had one or more coexisting comorbid medical condition. The five most common coexisting conditions were: cardiovascular diseases (42.3%, n = 154), DM (31.3%, n = 114), surgical history (12.4%, n = 45), pulmonary disease (10.7%, n = 39), and digestive disease (10.2%, n = 37). The patients aged 60 to 69 years had the highest percentage (31.0%, n = 113) of COVID‐19 compared to other age groups. The fatality rate increased with increasing age (Figure 2A). There was a higher fatality rate (27 [23.7%] vs 32 [12.7%]; P = .014] and older age (median [IQR], 66 [57‐73] vs 64 [52‐73]; P = .044] in T2DM patients compared with non‐DM patients (Table 1). Days from symptom onset to admission or gender did not differ between T2DM and non‐DM patients (Table 1).
FIGURE 2.
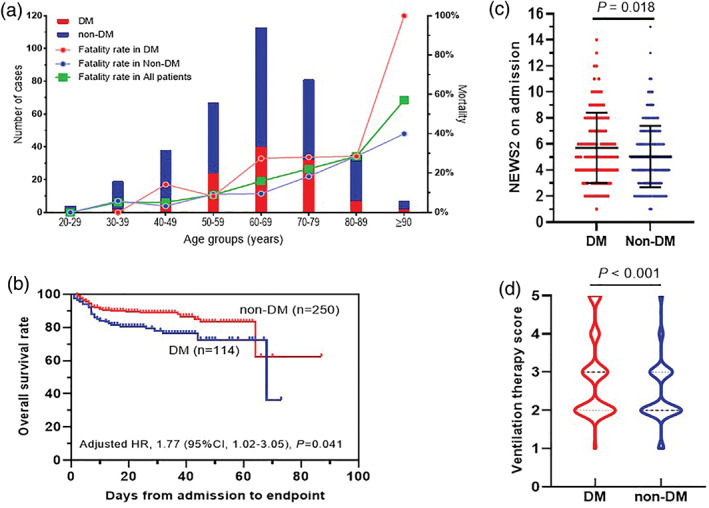
The effects of type 2 diabetes and age on the prognosis of patients with COVID‐19. A, The effects of type 2 diabetes (DM) vs non‐diabetes (non‐DM) on the mortality of COVID‐19 patients with severe/critical infection in the different age groups; B, The overall survival rate of DM vs non‐DM; C, The NEWS2 on admission of DM vs non‐DM and D, The ventilation therapy score of DM vs non‐DM. P < .05 is considered as significant and its value is as indicated in the graph
TABLE 1.
Demographic data, laboratory indices, clinical course, and outcomes of diabetes versus nondiabetes patients with COVID‐19
| Characteristics | DM (n = 114) | non‐DM (n = 250) | Total (n = 364) | P |
|---|---|---|---|---|
| Age, years | 66 (57‐73) | 64 (52‐73) | 65 (55‐73) | .044 |
| Gender (men) | 62 (54.4%) | 144 (57.6%) | 206 (56.6%) | .571 |
| Days from onset to admission | 10 (7‐14) | 10 (7‐14) | 10 (7‐14) | .568 |
| Died patients | 27 (23.7%) | 32 (12.7%) | 59 (16.2%) | .014 |
| Laboratory parameters (at admission) | ||||
| WBC (×109/L) | 6.77 (5.30‐8.61) | 5.27 (3.99‐7.28) | 5.64 (4.26‐7.86) | <.001 |
| LYM (×109/L) | 0.87 (0.55‐1.27) | 1.0 (0.75‐1.41) | 0.97 (0.71‐1.38) | .134 |
| CRP (mg/L) | 56.6 (17.2‐111.9) | 28.6 (6.6‐70.5) | 37.8(9.4‐81.3) | <.001 |
| IL‐6 (pg/mL) | 17.34 (6.59‐38.39) | 6.56(3.34‐23.13) | 8.99(4.28‐28.81) | .009 |
| ALT (U/L) | 24.5(17.8‐57.0) | 23.0(16.0‐36.0) | 24.0(16.0‐40.0) | .873 |
| AST (U/L) | 28.5(20.0‐47.3) | 28.0(20.0‐40.0) | 28.0(20.0‐41.0) | .032 |
| ALB (g/L) | 34.5(31.8‐37.4) | 36.0(32.8‐39.3) | 35.5(32.4‐38.6) | .012 |
| CR (μmol/L) | 65.0(52.0‐74.8) | 64.0(51.0‐76.5) | 64.0(51.0‐76.0) | .729 |
| eGFR (mL/min·1.73 m2) | 94.4 (74.0‐101.8) | 94.2(85.1‐106.0) | 94.3(83.1‐104.6) | .007 |
| LDH (U/L) | 326(238‐449) | 281(216‐360) | 295(222‐377) | <.001 |
| NT‐proBNP (pg/mL) | 289.8(133.1‐986.0) | 122.4 (46.3‐429.4) | 171.2(56.1‐544.8) | .014 |
| CK (U/L) | 55(36‐119) | 63(40‐111) | 61(38‐113) | .835 |
| CK‐MB (ng/mL) | 1.31(0.85‐2.62) | 1.05(0.66‐1.59) | 1.14 (0.70‐1.90) | <.001 |
| Myoglobin (μg/L) | 60.59(34.13‐122.80) | 45.59(29.38‐82.35) | 49.99(31.46‐92.01) | .008 |
| cTnI (ng/mL) | 0.008(0.005‐0.029) | 0.005(0.005‐0.018) | 0.005(0.005‐0.022) | <.001 |
| D‐dimer (mg/L) | 1.31(0.62‐5.79) | 0.89(0.42‐3.31) | 1.03(0.49‐3.91) | .02 |
| LA (mmol/L) | 2.1(1.7‐2.9) | 2.0(1.6‐2.8) | 2.1(1.6‐2.9) | .488 |
| CD3 (/μl) | 505(284‐850) | 604 (399‐847) | 561(364‐846) | .131 |
| CD4 (/μl) | 321(167‐491) | 380(239‐531) | 357(217‐514) | .215 |
| CD8 (/μl) | 136(75‐289) | 209(129‐327) | 194 (110‐305) | .147 |
| CD4/CD8 | 1.94 (1.34‐3.16) | 1.77(1.23‐2.69) | 1.83(1.24‐2.78) | .109 |
| CD19 (/μl) | 136(80‐192) | 127(76‐196) | 129(77‐193) | .5 |
| CD16 + 56 (/μl) | 111(55‐191) | 116(75‐188) | 112(70‐190) | .755 |
Note: Data are shown as median (interquartile range) and no. of patients (%). P < .05 for DM vs non‐DM was considered statistically significant.
Abbreviations: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CK‐MB, creatine kinase myocardial band isoenzyme; COVID‐19, coronavirus disease 2019; CR, creatinine; CRP, C‐reactive protein; cTnI, cardiac troponin I; DM, diabetes; eGFR, estimated glomerular filtration rate; IL‐6, interleukin‐6; LA, lactic acid; LDH, lactate dehydrogenase; LYM, lymphocyte; NT‐proBNP, N‐terminal prohormone brain natriuretic peptide; WBC, white blood cell.
In multiple logistic regression analysis for fatality cohorts including age, gender, and comorbidities (diabetes, cerebral diseases, cardiovascular diseases, chronic renal diseases, digestive disease, pulmonary disease, and surgical history), age (OR, 1.04; 95% CI, 1.02‐1.07; P < .001), T2DM (OR, 1.81; 95% CI, 1.06‐3.07; P = .029), and surgical history (OR, 2.37; 95% CI, 1.20‐4.71; P = .014) were found to be risk factors for fatality of severe/critical inpatients with COVID‐19.
3.3. Laboratory parameters in COVID‐19 patients with and without diabetes
There were several differences in laboratory findings between T2DM and non‐DM patients, including lower levels of serum albumin and estimated glomerular filtration rate (eGFR), as well as higher levels of white blood cells (WBC), C‐reactive protein (CRP), interleukin‐6 (IL‐6), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), N‐terminal prohormone brain natriuretic peptide (NT‐proBNP), creatine kinase myocardial band isoenzyme (CK‐MB), myoglobin, cardiac troponin I (cTnI), and D‐dimer in T2DM (P < .05 or P < .001). The levels of lymphocytes, alanine aminotransferase, creatinine, creatine kinase, lactic acid, and cellular immune responses (CD3+, CD4+, CD8+, CD19+, CD16 + 56 + cell counts, and CD4+/CD8+) did not differ between T2DM and non‐DM (Table 1). Taken together, our results suggest that T2DM patients with COVID‐19 had more severe inflammation, coagulation activation, myocardia injury, hepatic injury, and kidney injury.
3.4. The impact of T2DM status on the prognosis of COVID‐19 patients
On admission, the NEWS2 score of T2DM patients was higher than that of non‐DM patients (median [IQR], 5 [4‐8] vs 5 [3‐6]; P = .018) (Figure 2C). It indicated that T2DM patients were more severely ill than non‐DM patients on admission. During hospitalization, their ventilation therapy was defined using a five‐category ordinal scale of clinical status; the score in T2DM was higher than in non‐DM (median [IQR]: 3 [2, 3] vs 2 [2, 3]; P < .001) (Figure 2D). More T2DM patients required IMV/ECMO therapy than non‐DM patients (15 [13.2%] vs 8 [3.2%]; P < .001). After adjusting for age, gender, comorbidities, and NEWS2 on admission, the survival rate of T2DM patients (adjusted HR, 1.77; 95% CI, 1.02‐3.05; P = .041) was lower than that of non‐DM patients with COVID‐19 (Figure 2B).
3.5. Glucocorticoid therapy and the prognosis of COVID‐19 patients with T2DM
In this study, 74 (64.9%) of T2DM patients and 134 (53.6%) of non‐DM patients had glucocorticoid (GC) therapy (dose from 20 mg/day to 160 mg/day, duration range of 1‐28 days). Admission NEWS2 scores did not differ significantly between GC and no GC treatment in both T2DM and non‐DM patients (P > .05; Figure 3D). After adjusting for age, gender, comorbidities, and NEWS2 on admission, the results significantly demonstrated a lower overall survival rate in the patients treated with GC compared with no GC treatment (adjusted HR, 3.61; 95% CI, 1.14‐11.46; P = .029) (Figure 3A). This contrasted with non‐DM patients in whom GC treatment was not a risk factor for fatality (adjusted HR, 1.41; 95% CI, 0.67‐2.96; P = .362) (Figure 3B). The fatality rate for DM patients treated with GC was 31.1%, which was about three times higher than that of patients with no GC treatment in both the T2DM (10.0%, P = .012) and non‐DM group (10.3%, P < .001) and about twice that of non‐DM patients with GC treatment (14.9%, P = .007) (Figure 3C). During hospitalization, more patients treated with GC required invasive ventilation than patients without GC treatment both in T2DM and non‐DM patients (P < .001; Figure 3E). The level of IL‐6, a marker of inflammation, increased significantly after GC treatment in T2DM patients (P = .049), but not in non‐DM patients (P = .36) (Figure 3F).
FIGURE 3.
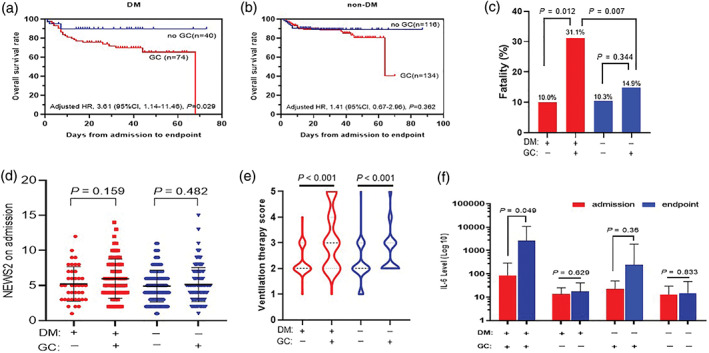
The effects of glucocorticoid (GC) therapy on the prognosis of type 2 diabetes (DM) and non‐diabetes (non‐DM) patients with severe/critical COVID‐19. A, The overall survival rate of GC therapy in DM patients; B, The overall survival rate of GC therapy in non‐DM patients; C, The fatality of GC treatment (+) vs no GC treatment (−) in DM (+) and non‐DM (−) patients; D, The NEWS2 of GC treatment (+) vs no GC treatment (−) in DM (+) and non‐DM (−) patients; E, Ventilation therapy scores of GC treatment (+) vs no GC treatment (−) in DM (+) and non‐DM (−) patients; F) The effects of GC on IL‐6. P < .05 is considered as significant and its value is as indicated in the graph
3.6. Antihyperglycemic drug use and prognosis of diabetes patients with COVID‐19
Among the 114 T2DM patients, 83.3% (n = 95) were treated with one or more antihyperglycemic drugs including: basal insulin (24.6% [n = 28]), premixed insulin (14.0% [n = 16]), Aspart/Lispro/Human insulin (42.1% [n = 48]), acarbose (46.5% [n = 53]), metformin (26.3% [n = 30]), sulfonylureas (14.9% [n = 17]), dipeptidyl peptidase IV inhibitors (DDP‐IVi) (6.1% [n = 7]), and sodium glucose cotransporter‐2 inhibitors (SGLT2i) (0.9% [n = 1]). All these drugs were prescribed in the conventional dosages according to the instructions. A total of 16.7% (n = 19) of T2DM patients did not use any antihyperglycemic drugs and were on diet treatment alone. The fatality rates of patients treated with basal insulin (14.3%, P = .045), premix insulin (6.3%, P = .022), metformin (6.7%, P = .008), acarbose (7.5%, P = .002), sulfonylureas (5.9%, P = .02), insulin (including basal insulin, premixed insulin, and Aspart/Lispro/Human insulin) and oral antihyperglycemic drugs (OAH, including acarbose, metformin, sulfonylureas, DDP‐IVi, and SGLT2i; 7.3%; P = .003), and OAH alone (7.4%, P = .009) were lower than that of diet‐alone‐treated T2DM patients (42.1%) (Table 2). NEWS2 scores on admission did not differ among these patients (P > .05). Patients treated with DDP‐IVi or OAH alone had lower ventilation scores than those with diet treatment alone (P < .05). Premix insulin, acarbose, metformin, DDP‐IVi, insulin and OAH, and OAH alone improved fasting blood glucose metabolism (P < .05 or P < .001) (Table 2).
TABLE 2.
Antihyperglycemic drug use and prognosis of type 2 diabetes patients with COVID‐19
| Antihyperglycemic drugs | Died patients | n | NEWS2 on admission | Ventilation score | FPG | ||
|---|---|---|---|---|---|---|---|
| Admission | Endpoint | P ** | |||||
| Insulin alone | 14 (51.9%) | 27 | 6.0(4.0‐8.0) | 3.0(2.0‐5.0) | 9.22(6.49‐15.42) | 9.20(5.74‐12.89) | .822 |
| OAH alone | 2(7.4%)* | 27 | 5.0(4.0‐7.0) | 2.0(2.0‐3.0)* | 9.17(6.59‐10.39) | 6.23(5.51‐8.35) | .015 |
| Insulin + OAH | 3(7.3%)* | 41 | 5.0(3.0‐7.0) | 2.0(2.0‐3.0) | 11.05(7.39‐16.77) | 6.83(5.80‐9.16) | <.001 |
| Insulin | |||||||
| Basal | 4 (14.3%)* | 28 | 5.0(4.0‐7.8) | 3.0(2.0‐3.0) | 12.45(8.79‐19.99) | 7.41(5.80‐10.14) | .145 |
| Premix | 1(6.3%)* | 16 | 5.0(4.3‐8.0) | 2.5(2.0‐3.0) | 12.70(8.57‐18.26) | 6.47(5.39‐9.60) | .002 |
| Aspart/Lispro/Human | 16(33.3%) | 48 | 5.0(4.0‐8.0) | 3.0(2.0‐4.0) | 11.20(7.14‐15.97) | 8.28(5.80‐10.95) | .144 |
| OAH | |||||||
| Metformin | 2(6.7%)* | 30 | 4.0(3.0‐6.0) | 2.0(2.0‐3.0) | 9.86(6.82‐13.86) | 6.36(5.87‐8.36) | .021 |
| Acarbose | 4 (7.5%)* | 53 | 5.0(4.0‐8.0) | 2.0(2.0‐3.0) | 10.15(7.17‐14.79) | 6.83(5.56‐9.16) | <.001 |
| Sulfonylureas | 1 (5.9%)* | 17 | 4.0(2.5‐7.0) | 2.0(2.0‐3.0) | 7.59(5.33‐9.95) | 5.80(4.91‐8.51) | .134 |
| DDP‐IVi | 0(0) | 7 | 6.0(5.0‐8.0) | 2.0(2.0‐2.0)* | 10.28(7.13‐11.93) | 6.10(4.92‐9.20) | .028 |
| SGLT2i | 0(0) | 1 | 9.0 | 3.0 | 17.22 | 6.3 | / |
| Diet treatment alone | 8(42.1%) | 19 | 6.0(4.0‐8.0) | 3.0(2.0‐4.0) | 9.53(6.84‐15.21) | 6.07(5.84‐9.60) | .595 |
Note: Data are shown as numbers (percentages, %), and median (interquartile range).
Abbreviations: COVID‐19, coronavirus disease 2019; DDP‐IVi, dipeptidyl peptidase IV inhibitor; FPG, fasting plasma glucose; NEWS2, National Early Warning Score 2; OAH, oral antihyperglycemic drugs; SGLT2i, sodium glucose cotransporter 2 inhibitor.
P < .05 vs diet treatment alone.
P, FPG on admission vs FPG at endpoint.
The average FPG was analyzed and divided into three groups including: euglycemia (3.9‐6.0 mmol/L), moderate hyperglycemia (6.1‐11.0 mmol/L), and severe hyperglycemia (≥11.1 mmol/L). No hypoglycemia readings (<3.9 mmol/L) were recorded. By applying a mixed‐effect Cox model adjusting for age, gender, comorbidities, NEWS2 on admission, GC treatment, and antihyperglycemic treatment compared with euglycemia and severe hyperglycemia (adjusted HR1, 11.86; 95% CI, 1.21‐116.44; P = .034) showed higher risk of all‐cause fatality in T2DM patients; however, moderate hyperglycemia (adjusted HR2, 4.51; 95% CI, 0.40‐51.30; P = .225) showed no difference (Figure 4).
FIGURE 4.
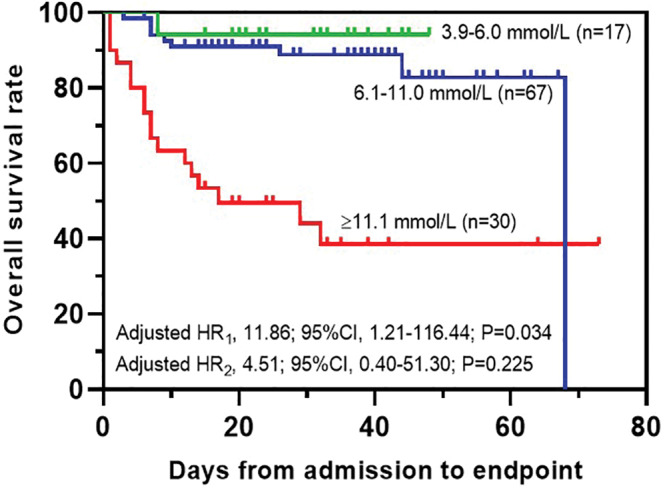
The overall survival rates of average fasting plasma glucose (FPG). P < .05 is considered as significant and its value is as indicated in the graph
4. DISCUSSION
It has been suggested that diabetes confers worse prognosis in COVID‐19 patients. 3 , 13 However, the impact of T2DM and its management, especially fasting glucose control, on the prognosis of patients with COVID‐19 have not been fully evaluated. Until now, no specific treatment has been validated for its effectiveness, and no antiviral agent has been found to provide benefit in reducing mortality of COVID‐19 patients, except for oxygen therapy or early hospitalization. 25 In this observational study, we described that SARS‐CoV‐2 infection in T2DM was associated with more severe inflammation, coagulation activation, myocardia injury, hepatic injury, and kidney injury. T2DM aggravated the clinical status of COVID‐19 patients and increased their critical illness rate and mortality. GC treatment and fasting blood glucose ≥11.1 mmol/L were found to be risk factors of fatality in these patients.
The prevalence of diabetes or the comorbidities of diabetes in COVID‐19 is from 5% to 20%. 2 , 3 , 4 , 5 , 6 , 7 During the early outbreak, hospitalizations consisted of severe cases of older patients among whom diabetes patients were well represented. Later, however, more mild cases were hospitalized due to expansion of medical services for COVID‐19 patients, and the proportion of diabetes patients started to reflect that of the general population. The most recent survey showed that the overall prevalence of diabetes in China is 12.8% with only half (6%) having a history of diabetes (self‐reported diabetes). 27 The overall prevalence of diabetes in those aged ≥40 years was 15.6%. 5 Our data show that the prevalence history of diabetes in patients with severe COVID‐19 is 31.3%, strongly suggesting that diabetes is a risk factor for more severe COVID‐19.
Our study also shows that a history of T2DM is a risk factor for the progression and prognosis of COVID‐19, 12 with a higher risk of severe pneumonia and higher chest CT scores. It also showed that T2DM patients had more severe organ impairments and worse inflammation. Moreover, diabetes patients have worse glycemic control which requires more antihyperglycemic treatment following COVID‐19 or hospitalization. Wu et al. 28 reported that diabetes was a risk factor of developing acute respiratory distress syndrome in COVID‐19. All of these indicate that diabetes status is a major risk factor for worse clinical outcomes/fatality in COVID‐19 patients. Indeed, based on our analysis of patients' clinical status, the survival curve showed that the prognosis for T2DM patients was worse. NEWS2 analysis on admission demonstrated that patients with a history of T2DM were generally sicker on admission and older which might be the explanation for the lower survival rate. The time of symptom onset to admission showed no difference between T2DM versus non‐DM patients. However, it has been reported that the SARS‐CoV‐2 infection was more likely to affect older men with diabetes. 6 As is widely reported, age is a strong indicator for COVID‐19 prognosis. Further logistic regression analysis showed that T2DM is an independent risk factor for severe status and fatality of COVID‐19 patients. Our data also show that SARS‐CoV‐2 infection affects men and women equally and indicates that gender may not be a risk factor for a poor outcome, in keeping with recent reports from China. 4 , 13
Huang et al 2 reported that SARS‐CoV‐2 infection in Wuhan caused multiple organ dysfunction and death in severe COVID‐19 patients. In our study, laboratory parameters associated with multiple organ function were tested on admission. The results showed abnormal or worse albumin, eGFR, WBC, CRP, IL‐6, AST, LDH, NT‐proBNP, CK‐MB, myoglobin, cTnI, and D‐dimer in COVID‐19 patients with T2DM. These data indicate that SARS‐CoV‐2 infection in T2DM is associated with more severe inflammation, coagulation activation, myocardia injury, hepatic injury, and kidney injury. Therefore, diabetes patients are more likely to develop multiple organ failures and die as a result. 4 ACE2, the SARS‐CoV‐2 receptor, is present in endothelial and smooth muscle cells in multiple organs, 29 suggesting that SARS‐CoV‐2 infection may induce multiple organ injury, possibly via ACE2. It is well recognized that viral infections can worsen blood glucose control. It has also been found that ACE2 protein shows strong immunostaining in islets, suggesting that SARS‐CoV‐2 may contribute to the development of diabetes or exacerbation of hyperglycemia by damaging pancreatic islets via ACE2. 30 Moreover, high levels of inflammatory cytokines such as IL‐6 and tumor necrosis factor alpha in diabetes patients and animal models suggests that diabetes may significantly promote the production of TLR4‐induced IL‐6 increase. 12 , 31 IL‐6‐dominated cytokine storms have been identified as one of the leading reasons for death from pneumonia caused by SARS‐CoV‐2 infection. 32 , 33
During the current pandemic of COVID‐19, systemic GC were used for their potent anti‐inflammatory properties. 34 The use of GC requires knowledge of the related side effects (eg, avascular necrosis, psychosis, and diabetes), their adequate prevention, and a prompt treatment if necessary. A randomized, controlled clinical trial in a preprint posted to medRxiv has found that dexamethasone with mechanic ventilation was shown to reduce deaths from severe COVID‐19. 35 However, whether GC should be used for the treatment of lung injury related to SARS‐CoV‐2 infection is still debatable. 36 In the past, steroid administration did not clearly improve the mortality rate of patients affected by SARS‐CoV and MERS‐CoV. 36 Our study found a higher risk of all‐cause fatality with GC treatment in severe COVID‐19 patients with T2DM. Although GC was more likely to be used in critical cases, our data indicated that GC treatment may be potentially harmful to T2DM patients with critical COVID‐19 if not properly used. First, the fatality of DM patients treated with GC was about twice that of non‐DM patients with GC treatment. Second, GC treatment was associated with increased IL‐6 levels in T2DM, but not in non‐DM patients. It suggested that we need to further define the indications for GC treatment and use it on the right patients.
The antihyperglycemic treatment approach for T2DM patients with COVID‐19 is uncertain because some oral drugs have not been recommended. 19 , 20 , 21 , 22 It seems that insulin may be the safest choice during this uncertainty. We analyzed the influences of antihyperglycemic drugs on the prognosis of COVID‐19 with T2DM. There were three types of insulin, with Aspart/Lispro/Human insulin being the most widely used, and four types of oral antihyperglycemic drugs, with acarbose being the most commonly used. Of these antihyperglycemic drugs, basal insulin, premix insulin, metformin, acarbose, sulfonylureas, insulin and OAH, and OAH alone showed lower fatality than diet treatment alone, despite their similar NEWS2 or clinical status on admission/at baseline. It seems to be consistent with their effects on improving fasting blood glucose metabolism. A recent study reported that a “good blood glucose control (3.9‐10moml/L)” was associated with less fatality. 37 Our study analyzed the outcome of patients with three groups of FPG and recommended to aim fasting blood glucose at (3.9‐11.0 mmol/L) for better survival.
We excluded patients with elevated glucose (FPG ≥6.1 mmol/L or random glucose ≥11.1 mmol/L) from the nondiabetic group in our analysis. However, they showed the same results as preexisting diabetes patients for those with GC treatment or FPG ≥11.1 mmol/L (Table S1). Moreover, elevated glucose was found in 182 patients (59 without GC treatment), indicating undiagnosed diabetes in these patients.
In summary, a history of T2DM aggravated the clinical status of COVID‐19 patients, increased their critical illness, and mortality rates. SARS‐CoV‐2 infection in diabetes led to more severe inflammation, coagulation activation, myocardia injury, hepatic injury, and kidney injury. GC treatment and FPG ≥11.1 mmol/L were found to be risk factors for fatality in diabetes patients with COVID‐19. There are some limitations of this study that took place within the context of an emergency outbreak including: lack of a control group—either as a matched control to compare patient groups or randomized control for the assessment of treatments. Besides, our study is based on small numbers of diabetic patients. However, our study provided evidence for informing clinical decisions.
CONFLICT OF INTERESTS
No conflicts of interest relevant to this article have been reported. The opinions and conclusions of this paper are solely the responsibility of the authors and do not necessarily represent the official positions of the Centers for Disease Control and Prevention.
Supporting information
Table S1 Demographic, fasting plasma glucose (FPG) and GC treatment on the prognosis of excluded patients with COVID‐19.
ACKNOWLEDGEMENTS
The authors thank all study participants.
Xu Z, Wang Z, Wang S, et al. The impact of type 2 diabetes and its management on the prognosis of patients with severe COVID‐19. Journal of Diabetes. 2020;12:909–918. 10.1111/1753-0407.13084
Solomon Tesfaye, Qingtao Meng, and Ling Gao contributed equally to this paper.
Funding information China Young Scientific Talent Research Fund for diabetes, Grant/Award Number: 2017; National Natural Science Foundation of China, Grant/Award Number: 81571376; Wuhan Young and Middle‐aged Medical Talent Award Plan, Grant/Award Number: 2018
REFERENCES
- 1. World Health Organization . Coronavirus disease 2019 (COVID‐19) Situation Report‐182. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200720-covid-19-sitrep-182.pdf. Accessed July 20, 2020.
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and Prediabetes in China in 2013. JAMA. 2017;317:2515‐2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short‐term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801‐2809. [DOI] [PubMed] [Google Scholar]
- 9. Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23:623‐628. [DOI] [PubMed] [Google Scholar]
- 10. Allard R, Leclerc P, Tremblay C, Tannenbaum TN. Diabetes and the severity of pandemic influenza A (H1N1) infection. Diabetes Care. 2010;33:1491‐1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van den Brand JM, Smits SL, Haagmans BL. Pathogenesis of Middle East respiratory syndrome coronavirus. J Pathol. 2015;235:175‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID‐19. Diabetes Metab Res Rev. 2020;e3319. https://doi:10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730‐1741. [DOI] [PubMed] [Google Scholar]
- 14. Schmid S, Molteni A, Fuchtenbusch M, et al. Reduced IL‐4 associated antibody responses to vaccine in early pre‐diabetes. Diabetologia. 2002;45:677‐685. [DOI] [PubMed] [Google Scholar]
- 15. Zhou J, Tan J. Diabetes patients with COVID‐19 need better blood glucose management in Wuhan, China. Metabolism. 2020;107:154216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367:1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xuan X, Gao F, Ma X, et al. Activation of ACE2/angiotensin (1‐7) attenuates pancreatic beta cell dedifferentiation in a high‐fat‐diet mouse model. Metabolism. 2018;81:83‐96. [DOI] [PubMed] [Google Scholar]
- 18. Takeda M, Yamamoto K, Takemura Y, et al. Loss of ACE2 exaggerates high‐calorie diet‐induced insulin resistance by reduction of GLUT4 in mice. Diabetes. 2013;62:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drucker DJ. Coronavirus infections and type 2 diabetes‐shared pathways with therapeutic implications. Endocr Rev. 2020;41:bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang P, Zhu L, Cai J, et al. Association of Inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126:1671‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hussain A, Bhowmik B, do Vale Moreira NC. COVID‐19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li J, Wang X, Chen J, Zhang H, Deng A. Association of Renin‐Angiotensin System Inhibitors with Severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Health Commission of the People's Republic of China. Diagnosis and treatment protocols of pneumonia caused by a novel coronavirus (trial version 7). 2020; http://www.nhc.gov.cn/xcs/zhengcwj/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
- 24.National Institute for Viral Disease Control and Prevention (China). Specific Primers and Probes for Detection 2019 Novel Coronavirus. 2020; http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html.
- 25. Cao B, Wang Y, Wen D, et al. A trial of Lopinavir‐ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams B. The National Early Warning Score 2 (NEWS2) in patients with hypercapnic respiratory failure. Clin Med. 2019;19:94‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maddaloni E, Buzzetti R. Covid‐19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020;e33213321. 10.1002/dmrr.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ascierto PA, Fox B, Urba W, et al. Insights from immuno‐oncology: the Society for Immunotherapy of cancer statement on access to IL‐6‐targeting therapies for COVID‐19. J Immunother Cancer. 2020;8:e000878. 10.1136/jitc-2020-000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Conti P, Ronconi G, Caraffa A, et al. Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by Coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): anti‐inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:1. 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 34. Shang L, Zhao J, Hu Y, du R, Cao B. On the use of corticosteroids for 2019‐nCoV pneumonia. Lancet. 2020;395:683‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID‐19: preliminary report. Preprint at medRxiv. 2020. 10.1101/2020.06.22.20137273. [DOI]
- 36. WHO (2020) Clinical management of severe acute respiratory infection when COVID‐19 is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed May 21, 2020.
- 37. Zhu L, She ZG, Cheng X, et al. Association of Blood Glucose Control and Outcomes in patients with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab. 2020;31:1068‐1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Demographic, fasting plasma glucose (FPG) and GC treatment on the prognosis of excluded patients with COVID‐19.


